Abstract
Polymerization of deoxy sickle cell hemoglobin (HbS) is well recognized as the primary event that triggers the classic cycles of sickling/unsickling of patients red blood cells (RBCs). RBCs are also subjected to continuous endogenous and exogenous oxidative onslaughts resulting in hemolytic rate increases which contribute to the evolution of vasculopathies associated with this disease. Compared to steady-state conditions, the occurrences of vaso-occlusive crises increase the levels of both RBC-derived microparticles as well as extracellular Hb in circulation. Common byproduct resulting from free Hb oxidation and from Hb-laden microparticles is heme (now recognized as damage associated molecular pattern (DAMP) molecule) which has been shown to initiate inflammatory responses. This review provides new insights into the interplay between microparticles, free Hb and heme focusing on Hb’s pseudoperoxidative activity that drives RBC’s cytosolic, membrane changes as well as oxidative toxicity towards the vascular system. Emerging antioxidative strategies that include the use of protein and heme scavengers in controlling Hb oxidative pathways are discussed.
Keywords: sickle cell hemoglobin, microparticles, pseudoperoxidase, heme oxidation, ferryl hemoglobin
2. Sickle cell anemia, a molecular disease of hemoglobin
Sickle cell disease (SCD) affects over 100,000 people in the United States and millions of people worldwide. Linus Pauling was first to coin the term “molecular disease” describing how this hemoglobinopathy originated from a single amino acid substitution [1]. This disease (caused by a mutation at the β6 position of Hb (β6Glu→Val)) results in the creation of a hydrophobic (sticky) patch (Val6) on the surface of one deoxy molecule in close proximity to another molecule with hydrophobic amino acids (e.g. Phe85 and Leu88). The primary molecular event is polymerization of these deoxyHbS molecules and subsequent aggregation into long fibers that leads to hemolytic anemia [2]. RBCs in hypoxic regions undergo the classic sickle cell shape change, and after several cycles of sickling and unsickling, they rupture releasing a mixture of Hb fibers and Hb molecules to circulation. SCD is characterized by chronic hemolysis, inflammation, vaso-occlusion, and ischemia-reperfusion injury leading to strokes and organ infarctions (3). Vascular endothelial cell activation (a critical component of microvascular responses) plays a significant part in the development of the vaso-occlusive crises, the hallmarks of the disease. Ischemia reperfusion injury is characterized by intermittent cessation (and restoration) of blood flow and the production of reactive species (ROS); these ischemic events collectively contribute to the oxidative stress implicated in the SCD pathogenesis [4].
During its short life span, the SS RBC undergoes several cytosolic and membrane transformations that result in alterations to the proteome, metabolome, redox state, and rheological properties [5]. Altered cytosolic composition (in particular antioxidant enzymes) impacts the overall redox state of the cell. In addition to changes in the oxidative milieu, RBC hemolysis vasculopathy also results in the toxic accumulation of heme and Hb in the plasma [6]. It is well recognized that plasma levels of free Hb can be as high as 25μM during sickle cell crisis, with basal Hb levels at 5–10 μM in sickle cell patients [7]. Free heme exerts multiple adverse effects, including leukocyte activation/migration, cytokine up-regulation, and oxidant production [8].
In spite of our increased knowledge of the molecular basis of SCD, no effective therapy has yet been found. Since the discovery of this condition, therapeutic efforts have primarily focused on preventing HbS polymerization (either directly or indirectly) and subsequent sickling of the RBC. It is difficult to separate the impact of the polymerization process from other alterations, including peroxidative stress and redox changes in the Hb molecule and within the RBCs [9]. These changes bring about Hb instability which impacts the oxidative environment by triggering oxidative stress both intra- and extracellularly. The focus of this review is to describe SCD oxidative events (driven primarily by Hb) and their potential impact on the vascular system. Also reviewed in this article are recent attempts by many researchers to explore antioxidant strategies that are designed to control Hb-mediated redox activity in circulation.
3. Lessons learned from cell-free hemoglobin developed as blood substitutes
The development of Hb-based oxygen carriers (HBOCs) as viable oxygen therapeutics has been hampered by several safety concerns and adverse events associated with the infusion of HBOCs that include transient hypertension, gastrointestinal symptoms, pancreatic and liver enzyme, myocardial infarction, cardiac arrhythmias, renal injury and death in humans [10].
HBOCs are generally found in circulation (~250 μM-1000 μM (heme)) after infusion in humans and animals, with half-lives ranging from 8–17 hours. This extended persistence in circulation is due to the fact that these Hbs have been chemically modified in a tetrameric or polymeric forms thus avoiding rapid clearance by kidneys [11]. Three major biochemical mechanisms were put forward by researchers to explain the basis of free Hb-mediated toxicity that would otherwise have been suppressed inside RBCs. These are: (1) scavenging of endothelial derived nitric oxide (NO), a vasodilator; (2) oversupply of oxygen; and (3) heme-mediated oxidative reactions (for review see [12, 13]). Hemodynamic imbalances (as manifested in blood pressure elevation) in response to HBOC infusion are primarily due to NO scavenging by Hb. An alternative mechanistic explanation to NO scavenging is the hypothesis of premature oversupply of oxygen to tissues. This results in autoregulatory vaso-constriction and/or through the formation of reactive oxygen species (ROS) and local destruction of NO [14]. Additionally, other less-studied enzymatic activities initiated by endogenous oxidants (as they react with the heme moiety of Hb) may have more lasting tissue-damaging effects than the other two mechanisms [11, 15]. Hb oxidative toxicity and the consequences of these redox side reactions are difficult to study in living systems but animal studies have recently confirmed the involvement of oxidation reactions in the initiation of inflammatory responses [11, 16]. Hb undergoes oxidation, in which the oxygen-bound ferrous (FeII) heme iron atom oxidizes non-enzymatically to the ferric/metHb (FeIII) state (autooxidation), initially generating a mixture of protonated and anionic superoxide radicals (Figure 1). H2O2 is produced during Hb autooxidation by spontaneous (kauto) and enzymatic dismutation of superoxide (kd). Hb autooxidation is associated with subsequent globin dysfunction and instability due to H2O2 generation resulting from dismutation of the initial superoxide products [17]. When H2O2 is in excess i.e., under oxidative stress conditions, a pseudoperoxidase catalytic cycle of Hb begins with three distinct steps in which H2O2 is ultimately and completely consumed (Figure 1): (1) initial ferrous (HbFeII) oxidation to a higher oxidation ferryl Hb(HbFeIV) (k1); (2) autoreduction of the ferryl intermediate to ferric (HbFeIII) (k2); and (3) reaction of HbFeIII(metHb) with an additional H2O2 molecule to regenerate the ferryl intermediate/ferryl protein radical (·HbFeIV=O) (k3) (Figure 1)). This radical may migrate and further damage the protein, including the irreversible oxidation of βCys93 and other “hotspots” amino acids [18, 19]. These internal reactions if not controlled also result in the modification of heme and its attachment to nearby amino acids. Irreversible oxidation of βCys93 to cysteic acid results in Hb dissociation into dimers, higher autooxidation rates, and rapid heme loss. Whereas, the Hb ferryl state and its associated radicals are more damaging than the ferric form, we have recently shown that heme loss from the ferric rather than the ferryl is at higher rates [20].
Figure 1. Oxygenation and the pseudoperoxidase pathways in sickle cell hemoglobin.
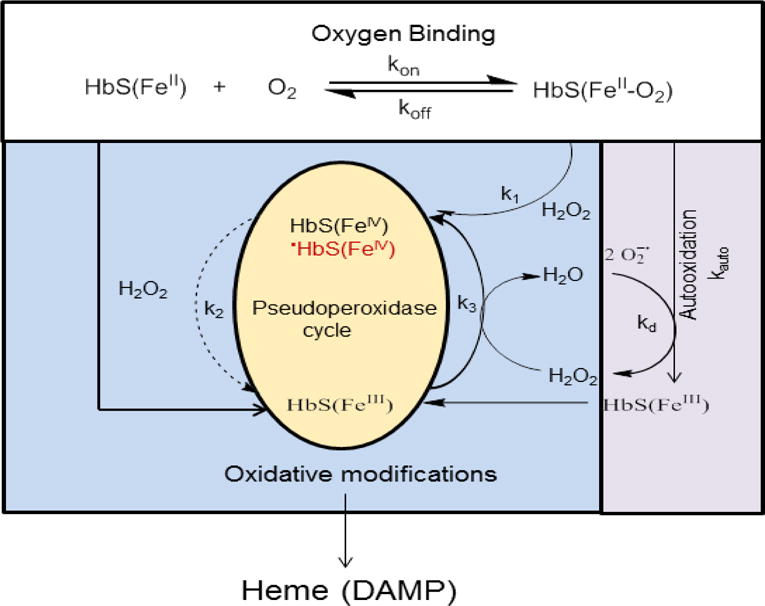
Hemoglobin (HbFeII) reversibly binds oxygen (koff/kon), and spontaneously oxidizes at a slow rate (kauto) to the non-functional ferric/metHb (HbFeIII) and superoxide (O2·−) that dismutate (kd) to give peroxide (H2O2). In the presence of this and/or exogenous H2O2, a catalytic cycle between the ferric (HbFeIII) and the ferryl (HbFeIV) hemes is initiated, in which H2O2 is eliminated in a peroxidase-like manner. However, in the case of HbS the autoreduction of ferryl back to ferric heme is slower (dotted line) than that of normal HbA, leading to a longer lived and more damaging ferrylHb [19]. If H2O2 reacts with the ferric form of Hb a protein radical is produce (.HbIV). The radical escapes through βCys93 resulting in its irreversible oxidation and the collapse of the β subunits. These oxidative changes then lead to unfolding and denaturation of the protein and heme loss. Heme is recognized as damage associated molecular pattern (DAMP) molecule able to trigger inflammation.
Due to the nature of chemical and/or genetic modifications employed in first-generation HBOCs, heme iron autooxidation and subsequent oxidative changes have been observed to occur at higher rates than those of unmodified Hb [21]. Lowered oxygen affinities (due to these modifications) have also been shown to enhance autooxidation rates [22], redox potential [23], and heme loss [24]. These issues have led many researchers to design countermeasures that can retard and/or control iron/heme oxidation in HBOCs. This ranged from either directly adding antioxidants (or reductants) to the HBOC solutions or even chemically crosslinking some of these antioxidants to the protein [21].
Measuring autooxidation and oxidative changes of HBOCs in circulation is difficult to monitor and is dependent on animal species used in these investigations. For example, the in vitro oxidation measurements of some HBOCs have been reported to be less of a predictor of the in vivo oxidation of Hb in rats (commonly used animal model) [25], whereas in sheep almost 30–40% metHb was accumulated after infusion of HBOCs and subsequent oxidation in circulation [26].
Guinea pigs, on the other hand, have been successfully used as a model for examining Hb oxidative processes because they (similar to humans and unlike rats) lack the enzymatic ability to produce ascorbate, a powerful reductant capable of controlling intravascular Hb oxidation [16]. It has also been demonstrated in this model that autooxidation after infusion of Oxyglobin™ (a bovine Hb polymerized with gltutaraldehyde approved by the FDA for veterinary use) can compromise the ability of Hb to carry oxygen, as reflected by the suppression of hypoxia inducible factor (HIF-1α) (an oxygen sensor molecule) in kidney tissues for the first 4–6 after infusion [27]. Furthermore, renal HO-1 induction and L-ferritin expression were accompanied by significant iron deposition after Oxyglobin infusion. In a follow up experiment, evidence was presented to show that Oxyglobin transfusion suppressed renal antioxidant enzyme expression at the gene and protein level, possibly through epigenetic alterations involving DNA methylation [28]. In massive transfusion of stored blood (~10 units), it was also reported that Hb-driven pathologies as consequence of the RBC storage lesions were seen in guinea pigs that were attenuated by co-infusion of haptoglobin (Hp) [29].
A recent case of compassionate use of HBOC-201 (human analogue of Oxyglobin) was reported in a severely injured Jehovah’s Witness patient, for whom survival was considered unlikely. Severe anemia and cardiac hypoxia were reversed after slow co-infusion of this Hb with ascorbic acid, a powerful reducing agent of Hb (1 g twice daily). No vasoactive side effects were associated with the treatment, possibly due to the slow infusion, and the patient survived [30].
4. Oxidative environment of sickle cells and the redox state of hemoglobin
It is well established that the vicious cycles of sickling/unsickling result in the production of lipid oxidation products and greater (almost twofold) amounts of ROS (mainly O2·−, and H2O2) that accumulate within SS RBCs [31, 32]. Consequently, RBC antioxidative enzymes activities (primarily SOD and catalase) increase in response to ROS induced oxidative stress [33]. Recent studies have also revealed the presence of up-regulated NADPH oxidase catalytic subunits which contribute to the SS RBC defective oxidative environment. As a result of this increased expression, NADPH oxidase-derived ROS may also cause direct oxidative damage to a variety of subcellular structures which ultimately leads to increased RBC fragility and hemolysis. Moreover, NADPH oxidase activity may deplete the cellular pool of NADPH, thus impairing the ability of the RBC to maintain its antioxidant defenses [34]. Another hallmark of SS RBCs that leads to a defective oxidative environment is the significant decrease in glutathione levels. Glutathione (GSH) is an important scavenger of free radicals and a potent endogenous antioxidant, which helps to protect cells from oxidative injury. In some studies, GSH has been found to be 32–36% lower in SCD RBCs compared to controls. In addition, catalase, several other proteins are involved in antioxidant protection and H2O2 elimination such glutathione peroxidase1 (GPX1) and peroxiredoxin within RBCs were found to be significantly impaired [35].
The redox balance within SS RBCs (in the face of the continuous reversible Hb O2 binding and generation of ROS) is brought about in large part by these enzymatic and non-enzymatic antioxidative activities. Decreased antioxidant defenses overtime in SCD are eventually coupled by an increase in Hb non-enzymatic oxidation (autooxidation) and pseudoperoxidase activities (in the presence of oxidants such H2O2). This cumulative internal oxidative stress induces membrane (particularly those with high phosphatidylserine composition) instability, contributing to accelerated intravascular hemolysis [4]. The unique oxidative environment within SS RBCs has been found to disturb HbS’s own normal conformational dependent interactions with band 3 membrane proteins (which regulate glycolysis in RBCs) and compromise RBCs antioxidant defenses during hypoxia [36]. Accelerated redox reactions associated with the HbS protein have been reported during malarial infection [37]. Ferryl HbS has been found to inhibit actin polymerization in malaria-infected HbS RBCs, thereby preventing the malarial parasites from creating their own actin cytoskeleton within the host cell cytoplasm. Although this mechanism appears to explain how HbS confers protection against malaria, it also serves to show that Hb oxidative reactions, including the formation of ferryl Hb can indeed be detected in RBCs in spite of the presence of antioxidative enzymes [37].
Limited numbers of clinical trials mostly on patients in clinical steady-state conditions have been reported in literature that describe the role of oxidative stress in disease complications [38]. A recent perspective study, in which 32 sickle patients were monitored during vaso-occlusive crisis showed exacerbated oxidative stress including the accumulation of harmful RBC-derived microparticles (MPs). Oxidative biomarkers assessed in this study included free heme in plasma, advanced oxidation protein products, myeloperoxidase, glutathione content, RBC caspase-3 activity, total neutrophil- and RBC-derived MPs. The formation of RBC-derived MPs (associated with reduced anti-band 3 autoantibodies levels) may be both related to the recruitment of oxidized band 3 into membrane aggregates [39].
5. Mechanisms of the pseudoperoxidase reactions of sickle cell hemoglobin
It has been recognized for some time that the unique oxidative environment of SS RBCs becomes an incubator for oxidized Hb and other redox forms [40]. Because of the catalytic nature of some of these reactions, Hb participates inside and outside RBCs in radical enzymatic and pseudoenzymatic side reactions. Other non-oxygen carrying activities that have attracted recent research interest in Hb include nitric oxide (NO) dioxygenase, nitrite reductase, and the non-enzymatic S-nitrosylation. Accordingly Hb has been described as an “honorary enzyme” and in some cases, Hb has been referred to as the “rogue” enzyme [17].
An important aspect of the hemolysis associated with sickling crises is the release of large quantities of Hb in circulation which can contribute to the complications of blood vessel injury and inflammation. Hebbel and coworkers have demonstrated that HbS is oxidatively less stable in vitro upon exposure to heat, oxidants, and mechanical shaking than HbA [31]. These oxidation-related mechanisms have been suggested to contribute to the pathophysiology of the disease. HbS under aerobic conditions autoxidizes at faster rates than HbA and has greater affinity than HbA to react with membrane aminophospholipids; these consequential membrane interactions lead to metHb conversion and the concomitant generation of ROS via superoxide ions (O2−), and peroxide H2O2) [31]. This, results in greatly enhanced Hb denaturation and partitioning of the released heme to the membrane bilayer [7]. In the presence of H2O2 Hb (unlike classic peroxidases such as cytochrome oxidase and prostaglandin H synthase) is unable to harness its own radicals as it transitions to a higher oxidation states [41]; Hb therefore has been classified as a pseudoperoxidase enzyme (Figure 2).
Figure 2. Proposed erythrocyte internal oxidative changes that trigger membrane alternations and microparticles formation.
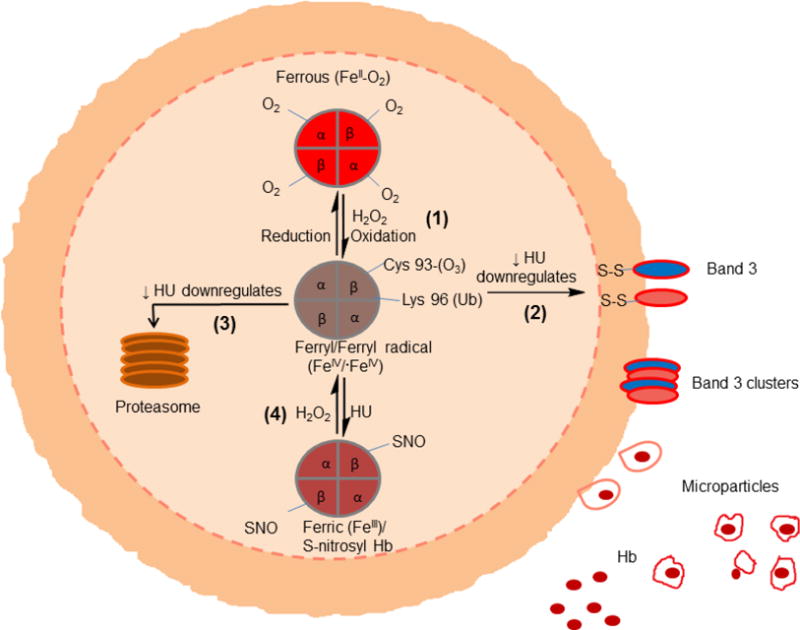
Oxidative reactions within RBCs include the following pathways: (1) Ferrous hemoglobin (FeII-O2) undergoes spontaneous oxidation (autooxidation) to form ferric (FeIII) and ferryl forms (FeIV). Ferryl species has been shown to form in sickle cells where antioxidative mechanisms are compromised. Ferryl Hb once formed undergoes oxidative changes including irreversible oxidation of βCys93 and ubiquitination of lysine 96. Ferryl Hb can oxidatively target band 3 leading to clustering of band3 and microparticles formation (2) or denaturation and proteasome degradation (3) some ferryl Hb revert back to ferric by a process of autoreduction (4). Hydroxyurea has been shown to have an antioxidant effects by minimizing the consequences of these processes.
When HbS is compared with HbA (under the same experimental conditions) a unique pseudoperoxidative activity is observed [19]. Specifically, as HbS recycles between ferric and ferryl iron (in the presence of H2O2) it tends to remain longer in the ferryl state due to slower autoreduction (Figure 1). Thus, slower autoreduction means greater distribution of the damaging ferryl form and its final reduction, whether through autoreduction or through reduction by a cellular component. However a faster autoreduction rate results in lesser damage to the protein and exogenous species. We have recently shown that a persistent ferryl HbS not only induces self-mediated oxidative changes but also promotes cell and bioenergetics changes in epithelial lung cells [19]. This suggests that mitochondrial dysfunction is triggered by Hb oxidation and likely contributes to SCD-induced vascular pathology. In summary, a persistent ferryl heme and its radical create an oxidative milieu leading to the following: (a) heme attachment to nearby amino acids; (b) irreversible oxidation of hot spot amino acids particularly the βCys93 side chain; (c) structural instability that leads to heme loss, and finally (d) oxidative damage to other biological entities such as the mitochondria [19].
These Hb oxidative pathways have also been monitored in transgenic SCD mice venules as they undergo stasis (little or no blood flow) in the microcirculation [42]. Infusion of Hb or heme triggered vaso-occlusion in SCD (but not control) mice. MetHb also induced vaso-occlusion, indicating heme liberation was necessary. This has been further substantiated by the fact that Hb-induced vaso-occlusion was blocked by the metHb-reducing agent methylene blue, haptoglobin (Hp), and the heme-binding protein, hemopexin (HPX). Additionally, free heme (released from Hb) elicited vaso-occlusion (stasis) in transgenic SCD mice by binding to the endothelial Toll-like receptor-4 (TLR4); this heme-TLR4 complex is known to activate NF-κB and thus trigger vaso-occlusion through Weibel-Palade body degranulation and adhesion molecule expression [42]. In a follow up study, low dose heme caused acute intravascular hemolysis and autoamplification of extracellular heme in transgenic sickle mice, but not in sickle-trait littermates. However, pharmacologic inhibition of TLR4 and hemopexin replacement therapy (prior to heme infusion) has been shown to protect sickle mice from developing acute chest syndrome (ACS) [6]. Total plasma heme and plasma free hemes have been recently quantified in children with SCD. In this observational clinical study, an association between plasma free heme and incidence of vaso-occlusive episodes and acute chest has been reported [43].
6. Sickle cell derived microparticles delivers highly oxidized hemoglobin and heme to the vasculature
SCD RBCs lose a substantial amount of Hb during their lifespan in circulation through frequent hemolysis and the formation of RBC-derived microparticle (MPs). Several MP subtypes have been observed in in sickle cell blood, namely those derived from RBCs, platelets, endothelial cells, and monocytes. MPs increase in number as they bud from RBCs after cycles of sickling/unsickling [44, 45]. MPs are sub-micron, unilamellar vesicles possessing a lipid bilayer and proteins derived from the plasma membrane of their parent cells-of-origin. These RBC-derived MPs (isolated from the plasma of freshly drawn blood) have been shown to contain all Hb components in a pattern similar to that of parent RBCs [46]. Approximately 20–30% of the MP’s content consists of Hb; however these vesicles also contain enzymes, miRNA and other multi-functional biologic mediators [46].
Recent investigations have revealed that MPs (consisting of high levels of phosphatidylserine (PS)) are 3- to 10-fold higher in SCD patients during steady state than in healthy control subjects, and further increased up to 3-fold during vasooclusive crisis. Moreover, MPs derived from sickle RBCs have been shown to be important delivery vehicles for Hb and heme [47]. This suggests that Hb oxidative pathways that lead to heme loss are likely occurring within circulating particles. It has been shown that in SCD, MPs can carry 20% to 35% of their Hb content in oxidized (met) form. This contradicts earlier reports on normal but aged RBC-derived MPs in which it has been suggested that Hb contained in these MPs was predominantly in the ferrous form [48]. Heme-laden MPs transfer heme to vascular endothelium which contributes to the overall pathophysiology of vaso-occlusion crisis by increasing oxidative stress and vascular dysfunction. In transgenic SAD mice, infusion of heme-laden MPs has also been shown to trigger rapid vaso-occlusions in kidneys and compromised microvascular dilation ex vivo. These vascular effects were largely blocked by heme-scavenging HPX and by the PS antagonist annexin-a5, in vitro and in vivo [49].
We have recently investigated the Hb oxidation kinetics within MPs in blood from SCD mice and human patients in order to accurately monitor how HbS transforms to ferric and ferryl redox states (unpublished data). These studies showed that the Hb oxidation time course (during 30 hour incubations) were biphasic, starting out with initial levels of oxidized (ferric) Hb of up to (30 to 45%), in agreement with work by Campus et al [49]. Oxidized Hb was slightly reduced within the first ~10 hours, likely due to the presence of RBC residual reductive enzymes within MPs. This was followed by a second phase in which Hb oxidation (ferric Hb) increased linearly and uncontrollably to 65 to 75% of total Hb. SCD MP’s contained highly reactive ferryl Hb intermediates, carbonylated membrane proteins, and phosphorylated band 3 proteins (unpublished data).
Quantitative proteomic analysis of MPs (from SCD mice and from SCD patients) also indicated a higher level of protein oxidation of βCys93 and other “hotspot” amino acids. Intriguingly, HbS β subunits from SCD MPs were ubiquitinated (barely detected in HbA β subunits) and MPs isolated from SCD patients had several-fold higher ubiquitination levels than hydroxyurea-treated SCD patients. The presence of this degradative PTM on HbS support our earlier observations showing HbS β subunits to be oxidatively unstable and more subceptible to turnover and concomitant heme release [19]. Additionally, proteomic analysis has also revealed the presence of Band 3 phosphorylation in SCD MPs. Band 3 phosphorylation has been previously identified to be involved in microparticle formation [46] (Figure 2). Compared to respective control MPs, incubation of either mice or human SCD MPs with human endothelial cells (HUVEC) activated apoptotic pathways and impacted cellular bioenergetic parameters by lowering mitochondrial oxygen consumption rates to a greater degree in a manner that was correlated with the redox state of Hb iron within MPs. Human endothelial cells incubated with SCD MPs showed greater intracellular ROS production and heme oxygenase-1 induction. When incubated with endothelial cells, MPs led to mitochondrial dysfunction and apoptotic cell death (unpublished data). These mechanistic analyses of RBC-derived SCD microparticles suggest potential anti-oxidative reducing modalities that may interrupt MP heme-mediated pathophysiology in patients with SCD.
7. Antioxidants that target the oxidation of hemoglobin in sickle cell disease
7.1. Haptoglobin and hemopexin, the first line of defense against hemoglobin and heme
Nature has provided a multitude of protective mechanisms that can effectively detoxify decompartmentalized Hb under normal physiological conditions. Chief amongst them is haptoglobin (Hp) which chaperones Hb subunits (αβ dimers) to macrophages for safe degradation through the CD163 receptors on the macrophage membrane. Complete degradation of heme into carbon monoxide (CO), bilirubin and biliverdin byproducts occurs in these cells [50]. The Hp protein consists of four chains; two α-chains each about approximately 9 kDa and two β chains of approximately 33 kDa molecular weights. In mammals, the α-chains and the β-chains are linked by a disulfide bond and in most species, a disulfide bond crosslinks the α-chain of the two αβ subunits. The β-chain is also a serine protease (SP) that has the ability to proteolyze proteins bound to it. The binding between Hp and Hb is among the strongest non-covalent interactions known in biological systems. The binding of Hp 1-1 to human dimeric Hb for instance is reported to occur with high affinity (k = 5.5 × 105 M−1 s− 1) and slow dissociation rate, while Hp 2-1 and Hp 2-2 bind Hb dimers with lesser affinity [51]. When release in plasma, Hb is instantly captured with high avidity by Hp to form the Hb–Hp complex. This strong binding detoxifies Hb and prevents Hb’s peroxidative side reactions known to be toxic to tissues. Moreover, the Hb–Hp complex impairs filtration and clearance of Hb dimers by the kidney, and instead directs Hb to CD163 on macrophages for a process of endocytosis and final degradation [for review see 52].
Our recent work on the interactions of Hp with Hb may explain the molecular basis of Hp action in protecting Hb against its own oxidative toxicity in circulation. Apart from site specific protection of Hb’s hotspots amino acids (i.e. βCys93) that are amenable to oxidation, Hp has been shown to allow the heme active site to operate unhindered leading to the elimination of oxidants [52, 53]. As the inner-sphere oxidative attack by H2O2 proceeds, Hp also diffuses the radical chemistry emanating from the heme as a consequence of this reaction. Recent mutagenesis studies have confirmed that a tyrosine residue at the Hb α subunit (αTyr42) acts as a conduit for electron transfer to and from the heme which facilitates the autoreduction of the ferrylHb [54]. EPR studies have also shown that this electron transfer is diverted to another Tyr residue namely, βTyr145 when Hb is complexed with Hp in the presence of H2O2 in a process that stabilizes the ferryl-induced free radicals on βTyr145. This radical reactivity may ultimately be directed to the Hp molecule resulting in a safer redox inactive Hb molecule [55].
Hemopexin (HPX) is an acute phase plasma protein, and its plasma levels vary from 8 mM to 21 mM [56]. HPX serves as a specific carrier of plasma heme and participates in clearance by transporting it to the liver and thus like Hp, HPX functions as a key plasma protector against Hb oxidation. Importantly, the Heme–HPX complex is completely inactive as an oxidant; unlike the Hp-Hb complex, however, the heme-HPX complex is unable to bind or consume ligands such as NO, O2 and H2O2 (52). This is because the heme in HPX is in a hexacoordinated configuration with the low spin iron (FeIII), thus unable to bind ligands.
When compared with other plasma heme scavengers (such as albumin, high and low density lipoproteins and α1-microglobin), HPX has the tightest linkage (Ka~1.9×1013 M−1) with heme via a bis-His complex that is stabilized by hydrophobic and electrostatic interactions within the heme pocket. The affinity of albumin towards heme is much lower (Ka~5×107 to 2×109 M−1); however, this is compensated by a remarkable abundance of albumin in plasma [57]. Additionally, HPX also competes with LDL for heme; by reducing the amount of available heme for LDL binding HPX effectively plays a regulatory role by reducing Hb oxidative activity (42). After HPX transports the heme through a receptor on parenchymal cells, intra- cellular HPX is recycled to its intact free form and released into the blood stream [56]. The crystal structure of the equimolar heme-HPX complex [58] revealed a unique coordination of the heme with the protein which contributes to one of the highest affinities known (Kd less than pM) [59]. Thus, HPX is the key defense against the deleterious effects of heme on cells, particularly hepatic, immune system and endothelial cells [56].
Understanding the role protein and heme scavengers play in controlling Hb oxidative side reactions and the mechanisms of Hb-induced toxicity may also provide new therapeutic avenues against hemolytic diseases like SCD in particular. Endogenous Hb/heme scavenging proteins are increasingly being investigated for their roles in ameliorating Hb/heme-induced toxicities. For example, our recent study revealed that Toll-like receptor (TLR4) antagonists inhibit vaso-occlusion in a SCD model [42]. Similarly, HO-1 overexpression reduced hypoxia-reoxygenation induced stasis and inflammation [60]. Endogenous Hb/heme scavenging proteins are increasingly being investigated for their roles in ameliorating Hb/heme-induced toxicities. For example, Hp reduced acellular Hb-induced renal damage in multiple animal models predominantly by promoting Hb clearance and metabolism [61]. Moreover, recent in vitro and in vivo experiments indicated that Hp shields Hb from peroxidative modifications and consequent tissue damage. In one study where infusion of HbA (32 μM heme/kg) induced HO-1 expression in kidneys of SCD mice exogenous Hp attenuated this HO-1 expression [62]. This study therefore has demonstrated that Hb-mediated oxidative toxicity may contribute to renal damage in SCD and that Hp treatment reduces heme/iron toxicity in the kidneys following hemolysis [62].
Although single Hp infusions have been shown to ameliorate vaso-occlusion in SCD mouse models, the therapeutic benefit of Hp dosing has not been explored until recently. The effect of Hp treatment over a 3-month period at two dosing regimens: the first at a moderate dose of 200 mg/kg three times weekly and the second at a higher dose of 400 mg/kg 3 times weekly were recently investigated in sickle mice. It was found that only the higher dosing regimen resulted in increased HO-1 and heavy chain ferritin (H-ferritin) expression and decreased iron deposition in the kidney. Despite decreased kidney iron deposition, there was no significant improvement in kidney function; however, there was a decrease in liver infarction [63]. Interestingly, when Hp and HPX were infused (either individually or in combination) in the same sickle cell model both proteins have been found to be equally effective. It was suggested that Hp may have ameliorated Hb-induced toxicity by reducing heme overload in kidney or by modulating HO-1 expression as part of a well-developed anti-inflammatory response. In a recent in vitro experiment, co-incubation of ferric Hb with the 3 major Fractions of Hp were found to be equally effective in inhibiting heme loss to below detectable levels. The most intriguing finding was that Hp binding almost completely inhibited heme dissociation on relevant physiological time scales (i.e., 1 to 24 h) [24]. This may also explain the effectiveness of Hp in inhibiting heme toxicity in SCD as noted above. Both Hp and Hpx plasma levels are generally depleted and the total binding capacity of both scavengers in conditions with intravascular hemolysis is reduced, suggesting that supplementation of the plasma proteins might provide some benefit to patients [7, 56]. Accordingly, it has been recently suggested that quantification of plasma heme, HPX, Hb-Hp, heme-HXP, and heme-albumin levels in hemolytic anemias is a crucial first step in developing targeted plasma protein supplementation or “replenishment” therapies for patients with hemolytic disease [56].
7.2. Reducing oxidized hemoglobin by ascorbate
Vitamin C (ascorbic acid) and vitamin E supplementation in SCD patients has been reported to have conflicting beneficial outcomes [40]. However, an ex vivo study and a pilot clinical trial, has demonstrated that a cocktail consisting of daily doses of ascorbic acid may be beneficial to SCD patients. Ascorbic acid supplementation efficiently decreases ROS production, increase GSH concentration, and prevents H2O2-induced hemolysis [64]. These supplements have been shown to inhibit dense RBC formation and decrease lipid peroxidation levels in SCD patients [65, 66, and 67].
Ascorbate is among the most important antioxidants (inside the RBC) that protect Hb against autooxidation by reducing both ferric and ferryl heme iron [68]. This has been substantiated by photometric and EPR studies characterizing ascorbate as an active reductant capable of controlling Hb oxidative toxicity. Furthermore, these studies also indicated that ascorbate removes key precursors to Hb oxidative damage in vitro and in vivo [69]. Specifically it was found later that ascorbate reduces the ferryl iron through interactions with the redox active α Tyr-42 [70]. However, dehydroascorbic acid, an oxidizing species formed as a result of this one electron reduction step of Hb is efficiently re-reduced by the erythrocyte membrane-bound reductase donor [69]. Therefore extra precaution must be taken into account when these oxidative intermediates are formed as a result of ascorbate infusion to ensure the presence of sufficient healthy RBCs of the receipt subjects in order to rejuvenate the added ascorbate.
7.3. Antioxidant effects of hydroxyurea
Hydroxyurea (HU) (also known as hydroxycarbamide) is the only drug approved by the FDA for use in SCD; HU has been shown to increase the synthesis of fetal Hb (HbF) believed to be one of the main effects of this drug on the sickling process [71]. HU presumably increases HbF expression by acting on the enzyme ribonucleotide reducatase which consequently perturbs subsequent S-phase cell cycle arrest. However, HbF containing cells (F-Cells) (in sickle cell patients treated with this drug) vary considerably in the percentage of HbF they contain [72]. Since HbF does not co-polymerize with HbS it is thought that this “sparing” effect may impact the gelation delay times. However, recent research has shown that HU’s effect goes above and beyond its impact on the polymerization process (73). General nonspecific antioxidative effects by HU have also been reported. This includes increases in catalase activity, GSH levels and decreased lipid peroxidation; in addition HU has been shown to directly protect the RBC membrane when exposed to oxidizing agents by reducing lipid peroxidation [74].
Furthermore, HU has also been reported to have a capacity as a NO donor, to enhance NO metabolite levels as well as cGMP in SCD patients within 1–2 h after HU administration [72]. By reducing vaso-occlusion and hemolysis alone, HU administration could have a major impact on overall oxidative stress in SCD. At the molecular level, HU can act as a biological NO donor in a site specific manner by binding to either oxy or metHb. In the case of the reaction of HU with oxyHb, the binding sites consist of several hydrophobic residues, specifically His58 that act as a ligand entry gate to the oxyheme to yield HU nitroxide radical. In the case of the ferric iron, a low-spin metHb-HU complex is formed within the heme pocket [75]. The proximity of HU to the Hb heme group and subsequent electron transfer mechanisms between HU may explain the reported antioxidant effects of HU. We have recently obtained data to show that HU (when added to sickle cell patient’s hemolysates and RBC derived microparticles) reduces Hb oxidation considerably by shielding βCys93 though an S-nitrosylation mechanism (unpublished data). HU therapy appears to also reduce other posttranslational modifications such as Hb ubiquitination and Band 3 phosphorylation (Figure 1).
7.4. Hemoglobin-based oxygen carriers ligated with carbon monoxide
In spite of potential detrimental effects, the use of CO gas and CO-releasing molecules (CO-RMs) as controlled pharmacological agents (to regulate important signaling mechanisms) has recently been explored. Additionally, CO is recognized as a cell signaling molecule with cytoprotective, anti-inflammatory and vasoldialtory properties [76]. Several hemoglobin-based oxygen carriers (HBOC) products that have been chemically and/or genetically modified to therapeutically correct oxygen deficiency have in recent years been considered for a new clinical indication in conditions as complex as the SCD. In some cases these HBOCs have been ligated with CO. By virtue of their higher oxygen affinity (P50) (R-state), and smaller size HBOCs may be able to reach the microvasculature unload of oxygen to reverse the cycles of sickling/unsickling of the deoxyHbS (T-state), thus preventing vaso-occlusion, a central event in SCD pathophysiology. CO if ligated to Hb, can retard Hb oxidation through its binding to heme iron and slow down the autooxidation process, once released from Hb it can also act as an anti-inflammatory agent as well [77].
MP4 (Hemospan), is human Hb that has been pegylated with PEG-50 to generate MP4 or Hemospan manufactured by Sangart Inc. (San Diego, CA, USA) [78]. This particular Hb unlike other HBOCs had a very high oxygen affinity (P50 = 4.0 mmHg). MP4 was indicated for use in elective surgery and had gone through clinical trials in in orthopedic patients as an oxygen carrier [79]. Initially Hemospan (MP4) was developed as an oxygen carrier however has recently been re-evaluated as a CO carrier (CO-MP4). In a rat model of myocardial infract (in contrast to oxy unliganded MP4) CO-MP4 reduced infract size when administrated prior to the induction of ischemia [80]. CO-MP4 was found to modulate heme oxygenase-1 (HO-1) expression, inflammation and vaso-occlusion in transgenic sickle cell mice. These effects were mediated by Nrf2, an important transcriptional regulator of HO-1 [81].
Another purified bovine Hb manufactured by Prolong Pharmaceuticals, known as Sanguinate is conjugated with 5000 molecular weight of PEG residues on the surface lysines and is ligated to CO [82]. The unligated form of this HBOC has a high oxygen affinity (a P50 of approximately 11 mmHg). In a topload transfusion rat model, PEG-CO (<1g/dl) produced noticeable reduction in infarct volume [83]. In a Phase 1 trial, three cohorts of eight healthy volunteers received single ascending doses of Sanguinate (80, 120, or 160 mg/kg of) that were well tolerated. Phase 1b studies have been completed in stable patients with sickle cell disease, but no published data was available in the open literature [84].
7.5. Nitric Oxide therapeutics
The use of nitric oxide (NO) donors or the manipulation of the NO synthetic pathways are among the most common methods employed by researchers to control hemodynamic imbalances after infusion of HBOCs or when Hb is free in hemolytic anemias such as SCD [77]. These measures have been implemented primarily to counter NO scavenging by free Hb and to maintain NO bioavailability in circulation. Such short-term strategies to control blood pressure elevation, include the transformation of the Hb into an NO carrier (S-nitrosylation of βCys93 residue) or enzymatically transforming Hb in the presence of nitrite into a source for NO (nitrite reductase) [85, 86]. However, these approaches have failed to resolve long-term toxicities associated with HBOCs or to minimize the consequences of hyperhemolysis in SCD (87). A case in point is the recent co-infusion of nitrite with an HBOC in which a profound cytotoxicity in the lungs of a swine animal model was observed [88]. Although vasocontrictive effects of Hb were ameliorated, an increase in pulmonary complications (in a dose-dependent fashion independent of the hemodilution effect) was observed [88]. Co-infusion of nitrite with Hb in a guinea pig model has also been shown recently to accelerate Hb oxidation and inducing profound oxidative tissue toxicity [89].
Chronic pulmonary complications such as chronic dyspnea and pulmonary hypertension are common in patients with SCD. A major risk factor for development of chronic vasculopathy and pulmonary hypertension in patients with SCD is hemolytic anemia, caused by the oxidative and inflammatory effects of plasma cell free Hb [90]. Attempts to control pulmonary blood pressure (triggered by free Hb) in humans have resulted in disappointing outcome in a clinical trial involving NO modulating strategy for treating SCD patients [91]. NO Inhalational (at low concentration) has however been shown in initial studies to improve HbS oxygen affinity by stabilizing the High oxygen affinity R state. Despite initial enthusiasm for treating sickle cell crisis patients with inhaled NO, results from a large multicenter study showed no reduction of pain scores [92]. Pulmonary hypertension lowering effects were seen in these patients as a result of NO inhalation; it was not however recommended as a therapy for treatment of painful crises in SCD [93].
NO reacts with RBC’s oxyHb and more avidly with free Hb to form stoichiometric nitrate and oxidized ferric (met) Hb. This dioxygenase reaction is critical to human physiology that involves NO metabolism, signaling, and toxicity. DeoxyHb forms NO heme-bound nitrosylHb with several oxidative intermediates, including ferrylHb [94]. Increasing HbS oxygen affinity by exposure to sodium cyanate or carbon monoxide CO and NO has been used effectively to reduce HbS RBC sickling in vitro. However, these agents are too toxic for clinical use (77). In vitro experiments in which a variety of NO donor agents were used resulted in a right shifted oxygen equilibrium curve (OEC) of SS cells due to NO heme reactions (nitrosylation or oxidation) and by direct S-nitrosation of Hb on the β-globin (Cys93) [95]. However, when heme iron was blocked with either CO or CN, S-nitrosation of βCys93 still occurred excluding any potential intramolecular transfer of NO from heme to this residue as previously reported [96].
Although NO and its metabolites (nitrite, peroxynitrite) are oxidizing species, NO releasing compounds, such as HU (section 7.3) can have beneficial antioxidant effects on Hb. Since we have shown that the target of Hb oxidation is βCys93, irreversible oxidation and subsequent protein destabilization can be reversed by HU as it protects this residue by converting it to an SNO species (unpublished data) (Figure 2). Additionally, a variety of slow NO releasing compounds can be used to reduce the free radical electrophilic centers of the ferryl radicals thus protecting against oxidative damage. This effect is due to the two-electron reduction of the ferryl iron at the oxoferryl center rather than to nitrosylation of the tyrosine residues [97].
7.6. Agents with dual antioxidant and antisickling properties
It has been well established that Hb oxidative side reactions are fuelled by biological oxidants such H2O2 (or lipid peroxides) and that these reactions can trigger oxidative cascades that result in self inflecting damages onto the Hb molecule, primarily the β subunits. These reactions are more exaggerated with the HbS and other hemoglobinopathies such as HbE [98].
This oxidative toxicity is primarily due to the formation of ferryl heme and its associated protein cation radicals with mid-point redox potentials (Eo ½~1.0 V), among the highest among biological radicals [17]. These internal reactions result in irreversible oxidation of amino acids, in the protein specifically in the area known as the “oxidative hotspots” (specifically the β Cys93 side chain) that cause the collapse of β subunits, unfolding and degradation of Hb and the ultimate release of heme [19].
The amino acid βCys93 occupies an important and crucial position at the β/α interface and is critically involved in the Hb R to T transition. It is also known that many amino acids of proteins are susceptible to oxidation by various forms of reactive oxygen species, during the aging process and oxidative stress. Methionine and cysteine residues in proteins are particularly sensitive to oxidation by reactive oxygen species. However, unlike the oxidation of other amino acid residues, βCys-93 has been shown to be extensively and irreversibly oxidized to cysteic acid in the presence of relatively low levels of H2O2. Moreover, among the amino acids that make the bulk of the oxidative “hotspot” area in Hb, βCys93 is consistently more exposed to the surface of the protein in the oxy and deoxy conformation than other residues, and thus βCys93 is more amenable to oxidative attach by H2O2 [18].
Since ferryl Hb and protein radicals also target βCys93 [18], reagents that can block the cysteine thiol group (and in the same time have an allosteric effect) are currently been evaluated in our laboratory. Examples of such βCys93 binding reagents that allosterically alter Hb oxygen affinity and possibly exhibit anti ferryl activities include DT-1 (di(5-(2,3-dihydro-1,4-benzodioxin-2-yl)-4H-1,2,4-triazol-3-yl)disulfide) [99] and hydroxyurea. These reagents will be compared with those representing reagents that bind outside the “hotspot” area (such hydroxyfurfural and GBT440 (2-hydroxy-6 ((2-(1-isopropyl-1H-pyrazol-5-yl) pyridin-3-yl) methoxy) benzaldehyde)) and are currently under clinical evaluation by the FDA (www.clinicaltrials.gov) [100]. These investigations may ultimately suggest potential therapeutic modalities that can interrupt heme-mediated inflammation in patients with SCD. It has also recently been suggested that treating SCD by targeting HbS polymerization may ultimately require administering therapeutic interventions of combination drugs, of non-competitive nature acting on different molecular targets [101].
8. Concluding remarks
Recent in vitro and in vivo studies have shown unequivocally that HbS oxidizes at faster rates than normal Hb and undergoes oxidative changes dues to the inability of the protein to enzymatically deal with its own as well as outside oxidants. These unique oxidative pathways result in irreversible Hb oxidation and ultimate heme loss to the vasculature aggravating the pathophysiology of the disease. Several intervention strategies have been suggested to control these reactions including the design of reagents that have dual antioxidant and antisickling properties that can be added to our antisickling armaments to defeat this disease.
Acknowledgments
This work was supported by National Institutes of Health NHLBI Grant P01-HL110900 and grants from the United States Food and Drug Administration (MODSCI). The author acknowledges the assistance of Dr. Tigist Kassa in constructing Figure 1 and 2, and Dr. Michael Brad Strader for reading the manuscript.
Abbreviations
- TLR4
Toll-like receptor
- HO-1
heme oxygenase
- SS RBCs
homozygous sickle cells
- PS
phosphatidylserine
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pauling L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science. 1949;110:543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- 2.Dickerson RE, Gies I. Hemoglobin: structure, function, evolution, and pathology, The Benjamin/Cummings Publishing Company, California. 1983 [Google Scholar]
- 3.Hebbel RP. Ischemia-reperfusion injury in sickle cell anemia: relationship to acute chest syndrome, endothelial dysfunction, arterial vasculopathy, and inflammatory pain. Hematol Oncol Clin North Am. 2014;28:181–198. doi: 10.1016/j.hoc.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Wood KC, Granger DN. sickle cell disease: role for reactive oxygen and nitrogen metabolites. Clin Exp Pharm Physiol. 2007;34:926–932. doi: 10.1111/j.1440-1681.2007.04639.x. [DOI] [PubMed] [Google Scholar]
- 5.Connes PI, Lamarre Y, Waltz X, Ballas SK, Lemonne N, et al. Haemolysis and abnormal haemorheology in sickle cell anaemia. Br J Haematol. 2014;165:564–572. doi: 10.1111/bjh.12786. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh S, Adisa OA, Chappa P, Tan F, Jackson KA, et al. Extracellular hemin crisis triggers acute chest syndrome in sickle mice. J Clin Invest. 2013;123:4809–4820. doi: 10.1172/JCI64578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaer DJ, Buehler PW, Alayash AI, Belcher DJ, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121:1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hebbel RP, Morgan WT, Eaton JW, Hedlund BE. Accelerated autoxidation and heme loss due to instability of sickle hemoglobin. Proc Natl Acad Sci USA. 1988;85:237–241. doi: 10.1073/pnas.85.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuypers FA. Hemoglobin S polymerization and red cell membrane changes. Hematol Oncol Clin N Am. 2014;28:155–179. doi: 10.1016/j.hoc.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Silverman TA, Weiskopf RB. Hemoglobin-based oxygen carriers: current status and future directions. Transfusion. 2009;49:2495–515. doi: 10.1111/j.1537-2995.2009.02356.x. [DOI] [PubMed] [Google Scholar]
- 11.Buehler PW, D’Agnillo F, Schaer DJ. Hemoglobin-based oxygen carriers: From mechanisms of toxicity and clearance to rational drug design. Trends Mol Med. 2010;10:447–457. doi: 10.1016/j.molmed.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Alayash AI. Setbacks in blood substitutes research and development: a biochemical perspective. Clin Lab Med. 2010;30:381–389. doi: 10.1016/j.cll.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Weiskopf RB. Hemoglobin-based oxygen carriers: disclosed history and the way ahead: the relativity of safety. Anesth Analg. 2014;119:758–760. doi: 10.1213/ANE.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 14.Winslow RM. Oxygen: the poison is in the dose. Transfusion. 2013;53:424–437. doi: 10.1111/j.1537-2995.2012.03774.x. [DOI] [PubMed] [Google Scholar]
- 15.Alayash AI. Oxygen therapeutics: can we tame haemoglobin? Nat Rev Drug Discov. 2004;3:152–159. doi: 10.1038/nrd1307. [DOI] [PubMed] [Google Scholar]
- 16.Buehler PW, D’Agnillo F, Hoffman V, Alayash AI. Effects of endogenous ascorbate on oxidation, oxygenation, and toxicokinetics of cell-free modified hemoglobin after exchange transfusion in rat and guinea pig. J Pharmacol Exp Ther. 2007;323323:49–60. doi: 10.1124/jpet.107.126409. [DOI] [PubMed] [Google Scholar]
- 17.Reeder BJ. The redox activity of hemoglobins: from physiologic functions to pathologic mechanisms. Antioxid Redox Signal. 2010;13:1087–123. doi: 10.1089/ars.2009.2974. [DOI] [PubMed] [Google Scholar]
- 18.Jia Y, Buehler PW, Boykins RA, Venable RM, Alayash AI. Structural basis of peroxide-mediated changes in human hemoglobin: a novel oxidative pathway. J Biol Chem. 2007;282:4894–4907. doi: 10.1074/jbc.M609955200. [DOI] [PubMed] [Google Scholar]
- 19.Kassa T, Jana S, Strader MB, Meng F, Jia Y, et al. Sickle Cell Hemoglobin in the ferryl state promotes βCys-93 oxidation and mitochondrial dysfunction in epithelial lung cells (E10) J Biol Chem. 2015;290:27939–27958. doi: 10.1074/jbc.M115.651257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kassa T, Jana S, Meng F, Alayash AI. Differential heme release from various hemoglobin redox states and the upregulation of cellular heme oxygenase-1. FEBS Open Bio. 2016;6:876–884. doi: 10.1002/2211-5463.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonaventura C, Henkens R, Alayash AI, Crumbliss AL. Allosteric effects on oxidative and nitrosative reactions of cell-free hemoglobins. IUBMB Life. 2007;59:498–505. doi: 10.1080/15216540601188546. [DOI] [PubMed] [Google Scholar]
- 22.Nagababu E, Ramasamy S, Rifkind JM, Jia Y, Alayash AI. Site-specific cross-linking of human and bovine hemoglobins differentially alters oxygen binding and redox side reactions producing rhombic heme and heme degradation. Biochemistry. 2002;41:7407–7415. doi: 10.1021/bi0121048. [DOI] [PubMed] [Google Scholar]
- 23.Bonaventura C, Henkens R, Alayash AI, Banerjee S, Crumbliss AL. Molecular controls of the oxygenation and redox reactions of hemoglobin. Antioxid Redox Signal. 2013;18:2298–2313. doi: 10.1089/ars.2012.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mollan TL, Jia Y, Banerjee S, Wu G, Kreulen RT, et al. Redox properties of human hemoglobin in complex with fractionated dimeric and polymeric human haptoglobin. Free Radic Biol Med. 2014;69:265–277. doi: 10.1016/j.freeradbiomed.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandegriff KD, Malavalli A, Minn C, Jiang E, Lohman J, et al. Oxidation and haem loss kinetics of poly(ethylene glycol)-conjugated haemoglobin (MP4): dissociation between in vitro and in vivo oxidation rates. Biochem J. 2006;3:463–471. doi: 10.1042/BJ20060809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee R, Neya K, Svizzero TA, Vlahakes GJ. Limitations of the efficacy of hemoglobin-based oxygen-carrying solutions. J Appl Physiol. 1985;79:236–242. doi: 10.1152/jappl.1995.79.1.236. [DOI] [PubMed] [Google Scholar]
- 27.Manalo DJ, Buehler PW, Baek JH, Butt O, D’Agnillo F, et al. Acellular haemoglobin attenuates hypoxia-inducible factor-1alpha (HIF-1alpha) and its target genes in haemodiluted rats. Biochem J. 2008;414:461–469. doi: 10.1042/BJ20080313. [DOI] [PubMed] [Google Scholar]
- 28.Rentsendorj O, Zhang X, Williams MC, Buehler PW, D’Agnillo F. Transcriptional suppression of renal antioxidant enzyme systems in guinea pigs exposed to polymerized cell-free hemoglobin. Toxics. 2016;46 doi: 10.3390/toxics4010006. pii. Epub 2016 Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baek JH, D’Agnillo F, Vallelian F, Pereira CP, Williams MC, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzgerald MC, Chan JY, Ross AW, Liew SM, Butt WW, et al. A synthetic haemoglobin-based oxygen carrier and the reversal of cardiac hypoxia secondary to severe anaemia following trauma. Med J Aust. 2011;194:471–473. doi: 10.5694/j.1326-5377.2011.tb03064.x. [DOI] [PubMed] [Google Scholar]
- 31.Hebbel RP, Ney PA, Foker W. Autoxidation, dehydration, and adhesivity may be related abnormalities of sickle erythrocytes. Am J Physiol. 1989;56:C579–C583. doi: 10.1152/ajpcell.1989.256.3.C579. [DOI] [PubMed] [Google Scholar]
- 32.Marva E, Hebbel RP. Denaturing interaction between sickle hemoglobin and phosphatidylserine liposomes. Blood. 1994;83:242–249. [PubMed] [Google Scholar]
- 33.Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life. 2012;64:72–80. doi: 10.1002/iub.584. [DOI] [PubMed] [Google Scholar]
- 34.George A, Pushkaran S, Konstantinidis DG, Koochaki S, Malik P, et al. Erythrocyte NADPH oxidase activity modulated by Rac GTPases, PKC, and plasma cytokines contributes to oxidative stress in sickle cell disease. Blood. 2013;121:2099–2109. doi: 10.1182/blood-2012-07-441188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gizi A, Papassotiriou I, Apostolakou F, Lazaropoulou C, Papastamataki M, et al. Assessment of oxidative stress in patients with sickle cell disease: The glutathione system and the oxidant–antioxidant status. Blood Cells Mol Dis. 2011;46:220–225. doi: 10.1016/j.bcmd.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Rogers JG, Ross SC, d’Avignon A, Gibbons LB, Gazit V, et al. Sickle hemoglobin disturbs normal coupling among erythrocyte O2 content, glycolysis, and antioxidant capacity. Blood. 2013;121:1651–1662. doi: 10.1182/blood-2012-02-414037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cyrklaff M, Sanchez CP, Kilian N, Bisseye C, Simpore J, et al. Hemoglobins S and C interfere with actin remodeling in Plasmodium falciparum-infected erythrocytes. Science. 2011;334:1283–1286. doi: 10.1126/science.1213775. [DOI] [PubMed] [Google Scholar]
- 38.Nur E, Brandjes DP, Schnog JJ, Otten HM, Fijnvandraat K, et al. Plasma levels of advanced glycation end products are associated with haemolysis-related organ complications in sickle cell patients. Br J Haematol. 2010;151:62–69. doi: 10.1111/j.1365-2141.2010.08320.x. [DOI] [PubMed] [Google Scholar]
- 39.Hierso R, Lemonne N, Villaescusa R, Lalanne-Mistrih ML, Charlot K. Exacerbation of oxidative stress during sickle vaso-occlusive crisis is associated with decreased anti-band 3 autoantibodies rate and increased red blood cell-derived microparticle level: a prospective study. Br J Haematol. 2016 Dec 16; doi: 10.1111/bjh.14476. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Silva DGH, Belini E, Jr, de Almeida E Alves, Regina Bonini-Domingos CCR. Oxidative stress in sickle cell disease: An overview of erythrocyte redox metabolism and current antioxidant therapeutic strategies. Free Rad Biol Med. 2013;65:1101–1109. doi: 10.1016/j.freeradbiomed.2013.08.181. [DOI] [PubMed] [Google Scholar]
- 41.Stubbe J, Riggs-Gelasco P. Harnessing free radicals: formation and function of the tyrosyl radical in ribonucleotide reductase. TIBS. 1998;23:434–443. doi: 10.1016/s0968-0004(98)01296-1. [DOI] [PubMed] [Google Scholar]
- 42.Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123:377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adisa OA, Yijuan H, Ghosh1 S, Aryee D, Ifeyinwa O, et al. Association between plasma free haem and incidence of vasoocclusive episodes and acute chest syndrome in children with sickle cell disease. Br J Haematol. 2013;162:702–705. doi: 10.1111/bjh.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allan D, Limbrick AR, Thomas P, Westerman MP. Release of spectrin-free spicules on reoxygenation of sickled erythrocytes. Nature. 1982;295:612–613. doi: 10.1038/295612a0. [DOI] [PubMed] [Google Scholar]
- 45.Willekens FL, Werre JM, Kruijt JK, Roerdinkholder-Stoelwinder B, Groenen-Dopp YA, et al. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood. 2005;105:2141–2145. doi: 10.1182/blood-2004-04-1578. [DOI] [PubMed] [Google Scholar]
- 46.Hebbel RP, Key NS. Microparticles in sickle cell anaemia: promise and pitfalls. Br J Haem. 2016;174:16–29. doi: 10.1111/bjh.14112. [DOI] [PubMed] [Google Scholar]
- 47.Vercellotti GM. Special delivery: microparticles convey heme. Blood. 2015;125:3677–3678. doi: 10.1182/blood-2015-04-639484. [DOI] [PubMed] [Google Scholar]
- 48.Azarov I, Liu C, Reynolds H, Tsekouras Z, Lee JS, et al. Mechanisms of slower nitric oxide uptake by red blood cells and other hemoglobin-containing vesicles. J Biol Chem. 2011;286:33567–33579. doi: 10.1074/jbc.M111.228650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Camus SM, De Moraes JO, Bonnin P, Abbyad P, Le Jeune S, et al. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease Lambry,3 Dominique Charue. Blood. 2015;125:3805–3814. doi: 10.1182/blood-2014-07-589283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wicher K, Fries E. Evolutionary aspects of hemoglobin scavenger. Antioxid Redox Signal. 2010;12:249–59. doi: 10.1089/ars.2009.2760. [DOI] [PubMed] [Google Scholar]
- 51.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. 1996;42:589–600. [PubMed] [Google Scholar]
- 52.Alayash AI. Haptoglobin: Old protein with new functions. Clin Chim Acta. 2011;412:493–498. doi: 10.1016/j.cca.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Alayash AI, Andersen CB, Moestrup SK, Bülow L. Haptoglobin: the hemoglobin detoxifier in plasma. Trends Biotechnol. 2013;31:2–3. doi: 10.1016/j.tibtech.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Reeder BJ, Grey M, Silaghi-Dumitrescu RL, Svistunenko DA, Bülow L, et al. Tyrosine residues as redox cofactors in human hemoglobin: implications for engineering nontoxic blood substitutes. J Biol Chem. 2008;283:30780–3087. doi: 10.1074/jbc.M804709200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooper CE, Schaer DJ, Buehler PW, Wilson MT, Reeder BJ. Haptoglobin binding stabilizes hemoglobin ferryl iron and the globin radical on tyrosine β145. Antioxid Redox Signal. 2013;18:2264–2267. doi: 10.1089/ars.2012.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith A, McCulloh RJ. Hemopexin and haptoglobin: allies against heme toxicity from hemoglobin not contenders. Front Physiol. 2015;6:187. doi: 10.3389/fphys.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buehler PW, Karnaukhova E, Gelderman MP, Alayash AI. Blood aging, safety, and transfusion: capturing the “radical” menace. Antioxid Redox Signal. 2011;14:1713–1728. doi: 10.1089/ars.2010.3447. [DOI] [PubMed] [Google Scholar]
- 58.Paoli M, Anderson BF, Baker HM, Morgan WT, Smith A, et al. Crystal structure of hemopexin reveals a novel high-affinity heme site formed between two beta-propeller domains. Nat Struct Biol. 1999;6:926–931. doi: 10.1038/13294. [DOI] [PubMed] [Google Scholar]
- 59.Hrkal Z, Vodrázka Z, Kalousek I. Transfer of heme from ferrihemoglobin and ferrihemoglobin isolated chains to hemopexin. Eur J Biochem. 1974;43:73–78. doi: 10.1111/j.1432-1033.1974.tb03386.x. [DOI] [PubMed] [Google Scholar]
- 60.Vercellotti GM, Zhang P, Nguyen J, Abdulla F, Chen C, et al. Overexpression of hemopexin inhibits inflammation and vascular stasis in murine models of sickle cell disease. Mol Med. 2016;22:437–451. doi: 10.2119/molmed.2016.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schaer DJ, Buehler PW. Cell-free hemoglobin and its scavenger proteins: new disease models leading the way to targeted therapies. Cold Spring Harb Perspect Med. 2013 Jun 1;3 doi: 10.1101/cshperspect.a013433. 6 or replace0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chintagari NR, Nguyen J, Belcher JD, Vercellotti GM, Alayash AI. Haptoglobin attenuates hemoglobin-induced heme oxygenase-1 in renal proximal tubule cells and kidneys of a mouse model of sickle cell disease. Blood Cells Mol Dis. 2015;54:302–306. doi: 10.1016/j.bcmd.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi PA, Choi E, Chintagari NR, Nguyen J, Guo X, et al. Sustained treatment of sickle cell mice with haptoglobin increases HO-1 and H-ferritin expression and decreases iron deposition in the kidney without improvement in kidney function. Br J Haematol. 2016;175:714–723. doi: 10.1111/bjh.14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amer J, Ghoti H, Rachmilewitz E, Koren A, Levin C, et al. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. Br J Haematol. 2006;132:108–113. doi: 10.1111/j.1365-2141.2005.05834.x. [DOI] [PubMed] [Google Scholar]
- 65.Marangon K, Devaraj S, Tirosh O, Packer L, Jialal I. Comparison of the effect of α-lipoic acid and α-tocopherol supplementation on measures of oxidative stress. Free Radic Biol Med. 1999;27:1114–1121. doi: 10.1016/s0891-5849(99)00155-0. [DOI] [PubMed] [Google Scholar]
- 66.Arruda MM, Mecabo G, Rodrigues CA, Matsuda SS, Rabelo IB, et al. Antioxidant vitamins C and E supplementation increases markers of haemolysis in sickle cell anaemia patients: a randomized, double-blind, placebo-controlled trial. Br J Haematol. 2013;160:688–700. doi: 10.1111/bjh.12185. [DOI] [PubMed] [Google Scholar]
- 67.Muskiet FA, Muskiet FD, Meiborg G, Schermer JG. Supplementation of patients with homozygous sickle cell disease with zinc, alpha-tocopherol, vitamin C, soybean oil, and fish oil. Am J Clin Nutr. 1991;54:736–744. doi: 10.1093/ajcn/54.4.736. [DOI] [PubMed] [Google Scholar]
- 68.Dunne J, Caron A, Menu P, Alayash AI, Buehler PW, et al. Ascorbate removes key precursors to oxidative damage by cell-free haemoglobin in vitro and in vivo. Biochem J. 2006;399:513–524. doi: 10.1042/BJ20060341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cooper CE, Silaghi-Dumitrescu R, Rukengwa M, Alayash AI, Buehler PW. Peroxidase activity of hemoglobin towards ascorbate and urate: a synergistic protective strategy against toxicity of Hemoglobin-Based Oxygen Carriers (HBOC) Biochim Biophys Acta. 2008;1784:1415–1420. doi: 10.1016/j.bbapap.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 70.Silkstone GG, Silkstone RS, Wilson MT, Simons M, Bülow L, et al. Engineering tyrosine electron transfer pathways decreases oxidative toxicity in hemoglobin: implications for blood substitute design. Biochem J. 2016;473:3371–3383. doi: 10.1042/BCJ20160243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kato GJ. New insights into sickle cell disease: mechanisms and investigational therapies. Curr Opin Hematol. 2016;23:224–232. doi: 10.1097/MOH.0000000000000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinberg MH, Lu ZH, Barton FB, Terrin ML, Charache S, Dover GJ. Fetal hemoglobin in sickle cell anemia: determinants of response to hydroxyurea. Multicenter Study of Hydroxyurea. Blood. 1997;89:1078–1088. [PubMed] [Google Scholar]
- 73.Ferrone FA. Sickle cell disease: Its molecular mechanism and the one drug that treats it, Inter. J Biol Macromol. 2016;93:1168–1173. doi: 10.1016/j.ijbiomac.2016.09.073. [DOI] [PubMed] [Google Scholar]
- 74.Silva DG, Belini E, Jr, Torres Lde SE, Ricci O, Jr, Lobo C, et al. Relationship between oxidative stress, glutathione S-transferase polymorphisms and hydroxyurea treatment in sickle cell anemia. Blood Cells Mol Dis. 2011;47:23–28. doi: 10.1016/j.bcmd.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 75.Vankayala SL, Hargis JC, Woodcock HL. Unlocking the binding and reaction mechanism of hydroxyurea substrates as biological nitric oxide donors. J Chem Inf Model. 2012;52:1288–1297. doi: 10.1021/ci300035c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Araujo JA. HO-1 and CO: Fighters vs sickle cell disease? Blood. 2013;122:2535–2536. doi: 10.1182/blood-2013-08-521922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alayash AI. Hemoglobin-Based Blood Substitutes and the Treatment of Sickle Cell Disease: More Harm than Help? Biomolecules. 2017;7:E2. doi: 10.3390/biom7010002. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winslow RM. MP4, a new nonvasoactive polyethylene glycol-hemoglobin conjugate. Artif Organs. 2004;9:800–806. doi: 10.1111/j.1525-1594.2004.07392.x. [DOI] [PubMed] [Google Scholar]
- 79.Olofsson AZ, Górecki CI, Dirksen R, Kofranek I, Majewski JA, et al. Evaluation of MP4OX for prevention of perioperative hypotension in patients undergoing primary hip arthroplasty with spinal anesthesia: A randomized, double-blind, multicenter study. Anesthesiology. 2011;114:1048–1063. doi: 10.1097/ALN.0b013e318215e198. [DOI] [PubMed] [Google Scholar]
- 80.Vandegriff KD, Young MA, Lohman J, Bellelli A, Samaja M, et al. CO-MP4, a polyethylene glycol-conjugated haemoglobin derivative and carbon monoxide carrier that reduces myocardial infarct size in rats. Br J Pharmacol. 2008;154:1649–1661. doi: 10.1038/bjp.2008.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Belcher JD, Young M, Chen C, Nguyen J, Burhop K, et al. MP4CO, a pegylated hemoglobin saturated with carbon monoxide, is a modulator of HO-1, inflammation, and vaso-occlusion in transgenic sickle mice. Blood. 2013;122:2757–2764. doi: 10.1182/blood-2013-02-486282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nho K, Glower D, Bredehoeft S, Shankar H, Shorr RA, et al. PEG-bovine hemoglobin: Safety in a canine dehydrated hypovolemic-hemorrhagic shock model. Biomater Artif Cells Immobil Biotechnol. 1992;20:511–524. doi: 10.3109/10731199209119677. [DOI] [PubMed] [Google Scholar]
- 83.Zhang J, Cao S, Kwansa H, Crafa D, Kibler KK, et al. Transfusion of hemoglobin-based oxygen carriers in the carboxy state is beneficial during transient focal cerebral ischemia. J Appl Physiol. 2012;113:1709–1717. doi: 10.1152/japplphysiol.01079.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abuchowski A. PEGylated Bovine Carboxyhemoglobin (SANGUINATE™): Results of Clinical Safety Testing and Use in Patients. Adv Exp Med Biol. 2016;876:461–467. doi: 10.1007/978-1-4939-3023-4_58. [DOI] [PubMed] [Google Scholar]
- 85.Zhang R, Hess DT, Qian Z, Hausladen A, Fonseca F, R, et al. Hemoglobin βCys93 is essential for cardiovascular function and integrated response to hypoxia. Proc Natl Acad Sci USA. 2015;112:6425–6430. doi: 10.1073/pnas.1502285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim-Shapiro DB, Gladwin MT. Mechanisms of nitrite bioactivation. Nitric Oxide. 2014;38:58–68. doi: 10.1016/j.niox.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bunn HF, Nathan DG, Dover RP, Hebbel GJ, Platt OS, et al. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 2010;116:687–692. doi: 10.1182/blood-2010-02-268193. [DOI] [PubMed] [Google Scholar]
- 88.Moon-Massat P, Scultetus A, Arnaud F, Brown A, Haque A, et al. Effect of HBOC-201 and sodium nitrite resuscitation after uncontrolled haemorrhagic shock in swine. Injury. 2012;43:638–647. doi: 10.1016/j.injury.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 89.Baek JH, Zhang X, Williams MC, Hicks PW, Buehler W, et al. Sodium nitrite potentiates renal oxidative stress and injury in hemoglobin exposed guinea pigs. Toxicology. 2015;333:89–99. doi: 10.1016/j.tox.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 90.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest. 2017;127:750–760. doi: 10.1172/JCI89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Machado RF, Barst RJ, Yovetich NA, Hassell KL, Kato GJ, et al. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011;118:855–864. doi: 10.1182/blood-2010-09-306167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Head A, Brugnara C, Martinez-Ruiz MRR, Kacmarek RM, Kenneth R, et al. Low concentrations of nitric oxide increase oxygen affinity of sickle erythrocytes in vitro and in vivo. J Clin Invest. 1997;100:1193–1198. doi: 10.1172/JCI119631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rossaint OR, Lewandowski K, Zapol WM. Our paper 20 years later: Inhaled nitric oxide for the acute respiratory distress syndrome—discovery, current understanding, and focused targets of future applications Intensive. Care Med. 2014;40:1649–1658. doi: 10.1007/s00134-014-3458-6. [DOI] [PubMed] [Google Scholar]
- 94.Keszler A, Piknova B, Schechter AN, Hogg N. The reaction between nitrite and oxyhemoglobin: a mechanistic study. J Biol Chem. 2008;283:9615–9622. doi: 10.1074/jbc.M705630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hrinczenko BW, Alayash AI, Wink DA, Gladwin MT, Rodgers GP, et al. Effect of nitric oxide and nitric oxide donors on red blood cell oxygen transport. Br J Haematol. 2000;110:412–419. doi: 10.1046/j.1365-2141.2000.02203.x. [DOI] [PubMed] [Google Scholar]
- 96.Hrinczenko BW, Schechter AN, Wojtkowski TL, Pannell LK, Cashon RE, et al. Nitric oxide-mediated heme oxidation and selective beta-globin nitrosation of hemoglobin from normal and sickle erythrocytes. Biochem Biophys Res Commun. 2000;275:962–967. doi: 10.1006/bbrc.2000.3413. [DOI] [PubMed] [Google Scholar]
- 97.Gorbunov NV, Osipov AN, Day BW, Zayas-Rivera B, Kagan VE, et al. Reduction of ferrylmyoglobin and ferrylhemoglobin by nitric oxide: a protective mechanism against ferryl hemoprotein-induced oxidations. Biochemistry. 1995;34:6689–6699. doi: 10.1021/bi00020a014. [DOI] [PubMed] [Google Scholar]
- 98.Strader MB, Kassa T, Meng F, Wood FB, Hirsch RE, et al. Oxidative instability of hemoglobin E (β26 Glu→Lys) is increased in the presence of free α subunits and reversed by α-hemoglobin stabilizing protein (AHSP): Relevance to HbE/β-thalassemia. Redox Biol. 2016;8:363–374. doi: 10.1016/j.redox.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakagawa A, Lui FE, Wassaf D, Yefidoff-Freedman R, Dominick C, et al. Identification of a small molecule that increases hemoglobin oxygen affinity and reduces SS erythrocyte sickling. ACS Chem Biol. 2014;9:2318–2325. doi: 10.1021/cb500230b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lia Q, Henry RER, Hofrichter J, Jeffrey F, Smith F, et al. Kinetic assay shows that increasing red cell volume could be a treatment for sickle cell disease. Proc Natl Acad Sci U S A. 2017;114:E689–E696. doi: 10.1073/pnas.1619054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eaton WA, Bunn HF. Treating sickle cell disease by targeting HbS polymerization. Blood. 2017 Apr 6; doi: 10.1182/blood-2017-02-765891. pii. blood-2017-02-765891. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


