Abstract
The intestinal mucosa provides a selective barrier between the anaerobic lumen and a highly metabolic lamina propria. A number of recent studies indicate that acute inflammation of the mucosa can result in tissue hypoxia and associated shifts in tissue metabolism. The activation of hypoxia-inducible factor (HIF) under these conditions has been demonstrated to function as an endogenous molecular cue to promote resolution of inflammation, particularly through the orchestration of barrier repair toward homeostasis. Given the central role of oxygen in tissue metabolism, ongoing studies have defined metabolic endpoints of HIF stabilization as important biomarkers of disease activity. Such findings make HIF and HIF-associated metabolic pathways particularly attractive therapeutic targets in inflammatory bowel disease (IBD). Here we review the recent literature related to tissue metabolism in IBD.
Keywords: hypoxia, inflammation, metabolite, microbiota, nucleotide
Introduction
The inflammatory bowel diseases (IBD), comprising Crohn's disease (CD) and ulcerative colitis (UC), affect approximately 1-2 per 1000 people in developed countries and result in significant morbidity and mortality. The current concept of IBD etiology denotes dysregulated intracellular sensing of low-level invasive bacteria and inappropriate propagation of intestinal immune responses in genetically susceptible individuals [1].
Tissues lined by epithelial cells, termed mucosal tissues, provide a selective barrier to the outside world. Of the mucosal tissues, the gastrointestinal (GI) tract constitutes the largest surface area found in multicellular organisms, covering an area of nearly 300m2 in adult humans. This epithelium comprises a highly dynamic barrier that is intricately regulated to both accommodate nutrient and fluid transport and to selectively exclude antigenic material from the luminal interface [2, 3]. As a result of this multidimensional functionality, the intestinal mucosa exhibits a unique metabolic profile that is regulated by a plethora of stimuli ranging from shifts in enteric microbiota to changes in intestinal perfusion, even under steady-state conditions [4]. It is also notable that this metabolic profile is prodigiously altered under conditions of active inflammation, such as those associated with inflammatory bowel disease (IBD), and has become an area of significant research interest. Work in the past decade has revealed that hypoxia-regulated pathways are strongly associated with barrier function in IBD, and may contribute to the resolution of ongoing mucosal inflammation [5]. In this mini-review, we highlight recent studies focused on the role tissue metabolism in mucosal inflammatory responses.
Energy metabolism within the intestinal mucosa
Even in the basal state, epithelial cells lining the mucosa exist in a relatively low oxygen tension environment, termed “physiologic hypoxia” [6]. This low basal pO2 has been attributed to a countercurrent oxygen exchange mechanism, where oxygen from arterial blood supplying the villi diffuses to adjacent venules, travelling from villous tip to base, resulting in graded hypoxia [6]. As a result, the intestinal epithelium has proven to be remarkably resistant to hypoxia, and even very low levels of oxygenation within this cell layer may be provide a regulatory adaptation for maintenance of barrier function and integrity[7].
It is likely that a shift in tissue metabolism and barrier maintenance contributes to the perpetuation of disease. For example, a role for epithelial barrier dysregulation in IBD is supported by observations of increased intestinal permeability in a subset of first-degree relatives of patients with Crohn's disease (CD) [8]. Likewise, studies with gnotobiotic mice have shown that enteric microbiota themselves influence epithelial cell metabolism, barrier function and survival [9]. As increased epithelial permeability and resultant mucosal inflammation and injury underpin the pathology of IBD, a fundamental understanding of microenvironmental metabolic factors that influence initiation, perpetuation and resolution of overt disease is central to defining potential therapeutic targets.
Hypoxia-inducible factor and mucosal tissue metabolism
Hypoxia-inducible factor (HIF) is a member of the Per-ARNT-Sim family of basic helix-loop-helix transcription factors that recognizes hypoxia response elements (HREs) at target gene loci under low oxygen conditions [10]. Functional HIF exists as an α/β heterodimer, comprised of both a constitutive subunit (HIF-1β), and a hypoxia-inducible ‘α’ component, stabilization of which is regulated in part by a family of oxygen- and iron-dependent prolyl hydroxylase (PHD) enzymes [11]. To date, three regulatory subunits have been identified, namely HIF-1α, HIF-2α, and HIF-3α. with the highest level of sequence homology conserved between HIF-1α and HIF-2α [12]. Evidence to date from genetic mouse models implies that HIF-1 and HIF-2 play non-redundant roles [10] despite their expression in many cell types, including intestinal epithelial cells [13].
In the context of mucosal inflammation, a protective role for HIF in regulation of intestinal epithelial barrier function has been strongly implicated [7]. Originally guided by microarray analysis of differentially expressed mRNA in cultured epithelial cells subjected to hypoxia[14], these studies have withstood the test of time and proven robust in a number of animal models and in human patients. Further interrogation of mechanisms related to HIF-dependent barrier protection has identified a number of target genes central to epithelial barrier properties, including mucin gene expression, tight junction genes and adherens junction gene regulation[7]. It is also notable that HIF-2α may preferentially regulate duodenal iron uptake via discrete regulation of DMT1 and Dcytb, rather than through basolateral iron transport [15]. These findings implicate HIF-2 as a significant molecular mechanism for communication of local changes in enterocyte iron or oxygen availability to altered duodenal iron absorption. Given that anemia one of the most prevalent extraintestinal manifestations of IBD [16], further elucidation of this mechanism may be warranted.
Tissue metabolism and acute inflammation
Sites of active mucosal inflammation are associated with profound metabolic shifts, wherein nutrients and local oxygen become rapidly depleted, resulting in hypoxia, hypoglycemia, lactate accumulation and acidosis [17]. Over the past decade, much work has focused on establishing the micro environmental metabolic cues for leukocyte recruitment to these sites, and the metabolic consequences therein. Adaptive immune responses to gut inflammation are characterized by high rates of local T- and B-cell proliferation, with marked demands for glucose, amino acids and lipids to fuel oxidative phosphorylation [18, 19]. Unlike resident lymphocytes, myeloid cells such as neutrophils (PMN), macrophages and dendritic cells are actively recruited to inflammatory lesions [20]. Cell migration to these lesions is triggered by orchestrated cytokine, chemokine and adhesion molecule expression.
A key effector of myeloid function is the generation of reactive oxygen species (ROS) in response to bacterial engulfment [21]. ROS are short-lived reactive molecules derived from the incomplete reduction of oxygen, such as superoxide anion, hydrogen peroxide and hydroxyl radical. Rapid generation of ROS by PMN and macrophages is mediated by a powerful respiratory or oxidative burst, commensurate with large increases in oxygen and glucose consumption that in turn trigger further ROS production [22]. Upon activation, it is estimated that PMN oxygen demands increase by as much as 50-fold and such profound oxygen consumption may be “sensed” as hypoxia by the surrounding parenchymal tissues. One study, for example, examined the impact of oxygen metabolism during PMN transepithelial migration across colonic epithelial cells [23]. In this study, the authors attributed gene expression changes within the epithelium to the massive consumption of local oxygen by PMN NADPH oxidase. These studies revealed that oxygen consumption by activated PMN resulted in the stabilization of HIF within the epithelium. In parallel, murine models of colitis demonstrated that both the presence of PMNs as well as PMN-elicited hypoxia were necessary for mucosal protection during inflammation. Depletion of PMNs led to exacerbated tissue destruction during colitis, providing an important link between antimicrobial defenses and parenchymal transcriptional signaling during ongoing inflammation. It is noteworthy that such signaling by NADPH oxidase may well extend beyond myeloid cells. For example, Bai, et al recently demonstrated that knockdown of NADPH oxidase in CD8+ T cells directly regulates signaling pathways that diminish IFNy production through the local generation of adenosine[24].
These findings that PMN generate a local hypoxic niche were also translated into human patients. Human IBD specimens containing crypt abscesses were assessed for the expression of the quintessential HIF gene target gene Glut-1. In this analysis, areas adjacent to the human crypt abscess revealed marked up regulation of Glut-1, indicative of stabilized HIF-1. It is notable that patients which lack a functional NADPH oxidase (i.e. chronic granulomatous disease, CGD) often present with an IBD-like syndrome [25]. CGD patients harbor congenital mutations in genes coding the subunits comprising the neutrophil NADPH oxidase complex (e.g. mutations in CYBA, CYBB, RAC1 and RAC2). Approximately 40% of CGD patients develop IBD-like symptoms [26].
There is also a body of literature implicating microvascular deficits in IBD that potentially contribute to mucosal hypoxia through diminished intestinal oxygen delivery. Notably, surgical specimens of inflamed colon from IBD patients revealed prominent immuno histochemical staining of the HIF-1 and HIF-2 [27]. Some staining differences were noted between HIF-1 and HIF-2 in CD and ulcerative colitis (UC). For example, while HIF-1 was expressed focally within various stromal cells, HIF-2 appeared to be expressed more diffusely in CD. These authors also noted that vascular density was significantly higher in CD and UC compared to normal tissue and that increased vascular density correlated with the expression of VEGF, a well-established HIF target gene [28].
Adenosine Metabolism and Intestinal Inflammation
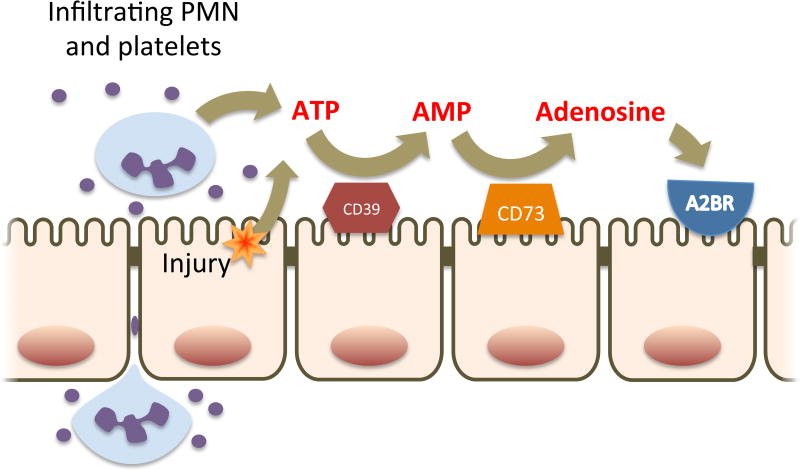
One of the most prominent pathways regulated by HIF is extracellular nucleotide metabolism and signaling [29, 30]. The metabolism of extracellular adenine nucleotides to adenosine is fundamental to the regulation of inflammation in the intestinal microenvironment in IBD. During intestinal inflammation, adenosine 5′-triphosphate (ATP) is released continuously into the extracellular space non-specifically as a result of direct cellular injury and specifically through hemi channels expressed by infiltrating inflammatory cells such as neutrophils (Fig. 1) [31]. The individual influences of extracellular ATP are specific to the tissue microenvironment in which they are released, acting through autocrine and paracrine mechanisms to respond to perceived injury. ATP acts as a classical damage-associated molecular pattern signal and is important for inflammasome activation by signaling through purinergic receptors such as P2X7 expressed on intestinal epithelial cells and lymphocytes [32]. In Crohn's Disease (CD) patients, P2X7 receptor expression is increased and P2X7 signaling contributes to elevated levels pro-inflammatory cytokines such as IL-1β, TNFα, and IL-17 in the tissue microenvironment [32].
Figure 1. Adenine nucleotide metabolism by intestinal epithelial cells.
During acute inflammation, PMN transmigration, platelet co-migration and direct cellular injury result in the accumulation of extracellular ATP on the luminal aspect of the intestinal mucosa. ATP is metabolized to AMP by ecto-nucleotidases (CD39) and then to adenosine by ecto-5′-nucleotidase (CD73). Extracellular adenosine then signals in an autocrine and paracrine manner through adenosine receptors expressed on the apical membrane of intestinal epithelial cells, the most prominent of which is Adora2B.
While ATP potently promotes inflammation in the intestinal mucosa, its downstream metabolite, adenosine, initiates resolution of inflammation and tissue repair. As previously stated ATP acts as a local signal of tissue injury Metabolism of extracellular ATP in the microenvironment, therefore, is important to prevent unchecked inflammation and occurs through the sequential activities of the ecto-enzymes CD39 and CD73 [33], both of which are abundantly expressed on the apical surface of intestinal epithelial cells (Fig. 1) [34-36]as well as by lymphocytes [37] in the intestinal mucosa. CD39, or ectonucleo side triphosphate diphosphohydrolase-1, catalyzes the conversion of ATP to adenosine-5′-monophosphate (5′-AMP) through two sequential phosphohydrolysis reactions. From animal models, CD39 expression is protective against chemically-induced colitis likely due to depletion of the proinflammatory ATP signaling [38]. In the same study, a polymorphism was identified in humans that correlates to CD39 expression. The allele associate with low CD39 expression was more prevalent in CD patients than controls, again suggesting CD39 is protective against IBD.
CD73, or ecto-5′-nucleotidase, is the terminal enzyme in the production of extracellular adenosine. In animal models, CD73 knockout or pharmacological inhibition of CD73 activity results in increased susceptibility to chemically-induced colitis and impaired resolution of colitis [39, 40]. Notably, in an experimental colitis model, CD73-/- mice were defective in the induction of IL-10 mRNA expression that is vital for the resolution of colitis, consistent with the known anti-inflammatory activity of adenosine. In human IBD, CD73 is upregulated and its expression appears to influence the expression of numerous genes involved in purine metabolism and purinergic signaling [41].
Adenosine is the ultimate product of the extracellular metabolism of adenine nucleotides in the intestinal microenvironment and possesses potent anti-inflammatory and tissue protective effects [42]. Multiple adenosine receptors are expressed by the intestinal epithelium, most prominently the G-protein coupled receptors, Adora2A and Adora2B. Through Adora2B signaling, adenosine is critical to restitution of intestinal barrier through a mechanism that involves activation of vasodilator-stimulated phosphoprotein (VASP) and ultimately tight-junction assembly [43, 44]. Adenosine also alters the intestinal microenvironment through the induction of electrogenic chloride secretion. Again, through a mechanism involving Adora2B signaling, cAMP-dependent chloride channels located in the apical membrane are activated resulting in chloride secretion [45]. The resulting osmotic gradient results in paracellular water transport across the epithelium in a basolateral to apical direction, which is thought to be an important flushing mechanism for the clearance of enteric pathogens as well as transmigrated inflammatory cells. Activation of Adora2b receptors also inhibits NF-κB-mediated signaling by reducing proteasomal degradation of IκB through a mechanism involving deneddylation of cullin-1 [46]. These actions result in diminished pro-inflammatory cytokine expression.
Previous studies have clearly demonstrated a role for adenosine signaling in adaptive immunity. Many of these responses have been mapped to HIF-1 signaling and the T cell Adora2A receptor [47, 48]. These studies have indicated that in addition to suppression of immune responses, adenosine signals as a “metabokine” to functionally re-direct the immune response through the T cell Adora2A receptors. Multiple lines of evidence are provided that elevations in intracellular cyclic AMP in coordination with HIF-1 stabilization are necessary to drive such re-direction of the immune response[49]. Adenosine signaling by T cells has been demonstrated to significantly influence intestinal inflammatory responses. Targeted deletion of the ENTPDase7 member of the CD39 family of enzymes was shown to increase small intestinal ATP levels that resulted in the selective increase in Th17 cells and resistance to Citrobacter rodentium infection [50]. Likewise, studies in RAG1-deficient T cell transfer models have indicated that Adora2A expression on both CD45RBhi and CD45RBlo cells are essential for control of colitic responses [51] and that Adora2A signaling by multiple cell types contribute to appropriate inflammatory resolution [52]. Collectively, these studies point to purine nucleotide metabolism as a key metabolic pathway in the regulation of inflammation in the intestinal microenvironment.
Tryptophan Metabolism and Intestinal Inflammation
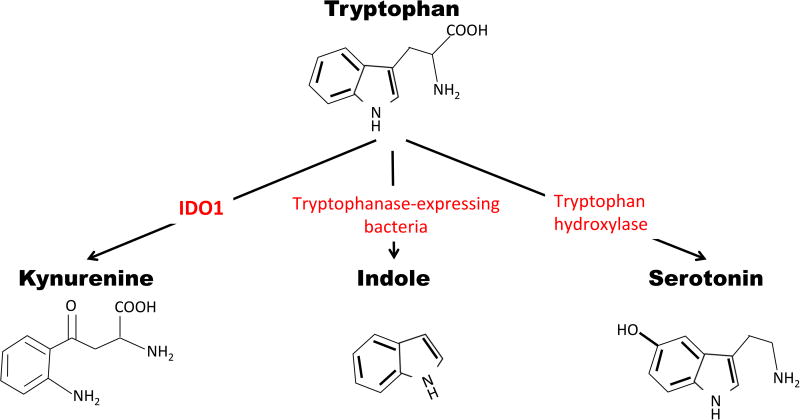
Tryptophan metabolism in the GI tract is a major source of immunosuppressive signaling, promoting tolerance and tissue homeostasis. As an essential amino acid, humans must obtain all tryptophan from the diet for synthesis into protein or conversion to a number of critical signaling metabolites. Tryptophan is the precursor of three distinct metabolic pathways within the gut: kynurenine, serotonin, and indole (exclusively mediated by the resident gut microbes) (Fig. 2). The metabolic pathway leading to kynurenine is the most prevalent, accounting for up to 90% of tryptophan catabolism [53].
Figure 2. Summary of the tryptophan (Trp) metabolism pathway including the enzymes involved in the primary metabolism of Trp.
From left to right: The enzyme indoleamine 2,3-dioxygenase-1 (IDO1) converts Trp to kynurenine (Kyn), host microbes producing tryptophanases catabolize Trp into indole metabolites, and Trp hydroxylase produces serotonin from Trp.
Within the intestine, indoleamine 2,3 dioxygenase-1 (IDO1) is the predominant enzyme that catalyzes the degradation of tryptophan (Trp) into kynurenine (Kyn). IDO1 is widely expressed throughout the gut, in the mucosa as well as mononuclear cells, and expression levels are sensitive to inflammatory stimuli such as IFN-γ signaling. Intestinal levels of IDO1 are high in patients with IBD, and localized Trp depletion inhibits T-cell proliferation and causes growth arrest of Trp-dependent microorganisms. This increase in IDO1 expression is most prominent around areas of ulceration suggesting that IDO1 expression may be important in wound healing. The expression of IDO1 in intestinal epithelial cells also correlates with anti-inflammatory properties of dendritic cells, inducing colonic lymphocytes to secrete IFN-γ, triggering IDO1 expression in intestinal epithelial cells [54-58], and reducing the severity of colitis in mice [57]. The role of IDO1 in colitis is somewhat controversial; studies using pharmacological inhibitors of IDO1 or whole body Ido1 knockout mice indicate that IDO1 is necessary for protection from murine TNBS colitis [59, 60], yet Ido1 gene deficiency seems to play a protective role in both DSS and Citrobacter rodentium induced colitis [61, 62]. Stimulation of IDO1 through TLR-9, however, lessens disease severity in both TNBS and DSS colitis.
The primary pathway of IDO1 induced immune suppression is through the production of Kyn, which functions as an endogenous ligand of the aryl hydrocarbon receptor (AHR) [63, 64] which shares the p-chain signaling component with HIF, namely HIF-1β (also called ARNT). The AHR is a ligand activated transcription factor of the PER-ARNT-SIM class 1 basic helix loop helix family of transcription factors that has been historically studied in the context of the synthetic dioxin based compound TCDD [65, 66]. In this context, exposure to TCDD and other polycyclic hydrocarbons bind AHR, which controls the expression of genes in the cytochrome P oxidase family such as Cyp1A1 [67, 68]. While AHR response to dioxin is known as a toxic response, AHR is also responsive to endogenous ligands such as Kyn in order to internally regulate systemic inflammation and tolerance [64].
Within the gut, AHR stimulation by Kyn and other Trp metabolites can promote the differentiation of Tregs[69, 70], down regulate IL-7 production by epithelial cells[71], and regulate Th17 development[72-74]. Th17 cells are pro-inflammatory T helper cells that produce IL-17 and are associated with many autoimmune disorders such as systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, and IBD. However, Th17 cells also contribute to bacterial clearance at mucosal surfaces and help maintain mucosal barriers through epithelial proliferation and mucus production via IL-22. In colitis, these lymphocytes can then be transdifferentiated back toward Th1 cell types by Kyn signaling through AHR and contribute to the resolution of inflammation [75, 76]. Further contributing to resolution is the AHR induced expression of the IL-10 receptor (IL-10R1) on colonic epithelial cells [77]. IL-10 signaling is critical for colonic homeostasis and IL-10R1 expression on colonic epithelial cells helps regulate barrier formation [78]. Recent evidence demonstrates that exogenous Kyn enhances wound healing in an IL-10 dependent manner in vitro and alleviates DSS colitis in vivo [77].
Host-microbial metabolism and intestinal inflammation
The GI tract of mammals is host to trillions of microbes. This finely tuned host-microbe relationship exists on the surface of the intestinal mucosa, where microbes are essential for host health, and under some circumstances serve to initiate disease [79]. Collectively termed the microbiota, these microbes aid in digestion, produce a number of vitamins and benefit the host through the local synthesis of 2, 3 and 4 carbon short-chain fatty acids (SCFAs), namely acetate, propionate and butyrate, respectively.
Primarily derived from digestion resistant starches, SCFAs are end products of bacterial fermentation [80]. Members of the anaerobic phylum Firmicutes, [81] produce butyrate at high levels through the conversion of microbial acetyl-CoA to the butyryl-CoA via fatty acid β-oxidation. The final conversion from butyryl-CoA to butyrate is either catalyzed by butyryl-CoA:acetate CoA transferase or butyrate kinase. Due the presence of highly conserved regions these enzymes can be used for the identification of butyrate-producing bacterial communities in molecular analyses [82-84]. In the healthy gut, butyrate concentrations can exceed 30mM [85], where it serves as a primary metabolic fuel for colonic epithelial cell metabolism. For this reason, shifts in microbial compositions can result in abnormal colonocyte function. It is recently appreciated, for example, that the dysbiosis in IBD is characterized by reduced abundance of butyrate-producing organisms (e.g., certain Faecalibacterium and Roseburia genera) and lower concentrations of luminal SCFA, particularly butyrate [86].
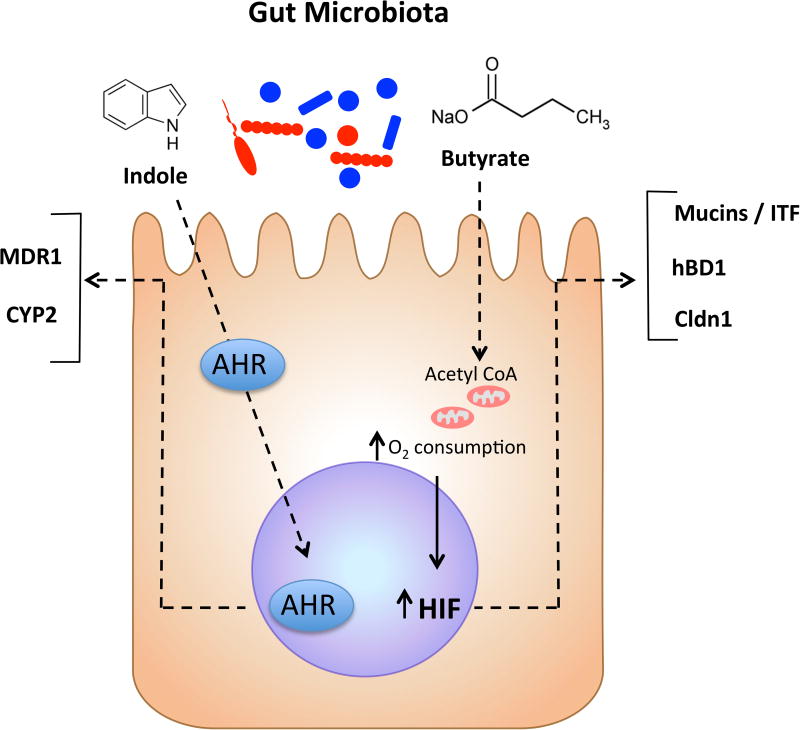
It is likely that butyrate influences HIF expression in the colon. For example, it was recently shown that butyrate metabolism directly influences epithelial oxygen consumption and may shift intracellular oxygen availability. Intestinal epithelial cell lines stimulated with butyrate exhibit increased and sustained rates of oxygen consumption to the extent that HIF is stabilized and transactivates a number of HIF target genes (Fig. 3) [87]. Antibiotic depletion of the microbiota in vivo resulted in increased detectable pO2 within the colonic epithelium. Parallel studies in germ free mice revealed that deficiency of the microbiota results in higher pO2 within the colonic epithelium compared with controls [87]. Restoring luminal butyrate with tributyrin in antibiotic treated mice reconstituted the physiologic low pO2 of the colonic epithelium and HIF-dependent signatures [87]. Such studies provide further rationale for responsible antibiotic stewardship and provide potentially new strategies to improve patient care. It is interesting to speculate that the promotion of butyrate-producing strains or the selective elimination of butyrate-depleting strains might serve the overall intestinal health of IBD patients.
Figure 3. Microbiota derived metabolites act as signaling molecules in colonic epithelial cells.
Butyrate produced by anaerobic bacteria increases epithelial oxygen consumption, stabilizes HIF, and activates HIF dependent gene transcription. Indole metabolites are produced by a variety of enteric bacteria and can alter colonic epithelial cell signaling through activation of AHR.
Finally, as mentioned above, the intestinal microbiota plays an important role in the proper development and maintenance of the immune system in the GI tract, and patients with IBD have profound shifts in the bacterial populations present in the colon [88]. Resident microbes are responsible for the production of a variety of indole compounds from the catabolism of tryptophan including 3-indoleacetic acid and indole-3-aldehyde. These indole metabolites have been shown to activate AHR in the mucosa leading to resolution of inflammation (Fig. 3) [89, 90]. Further, treatment of human intestinal epithelial cells with indole itself has been shown to increase barrier function and decrease inflammatory markers [91]. These studies suggest that microbial tryptophan metabolites provide significant benefit to the host.
Conclusions
The microenvironment of the IBD mucosa represents a unique setting to study metabolic changes commensurate with disease. In this review, we have summarized recent literature related to host and microbial metabolism as important signaling mechanisms within the intestinal mucosa. Studies from cultured cell systems, animal models and human IBD patients have revealed a plethora of new insight into the role of the inflammatory microenvironment is disease progression and resolution. Moreover, studies to date in animal models of intestinal inflammation have demonstrated an almost uniformly beneficial influence of HIF stabilization on disease outcomes [92]. Ongoing studies to define the similarities and differences between innate and adaptive immune responses as well as acute and chronic inflammation will be insightful. Continued focus in this area will undoubtedly provide new targets as templates for the development of therapies for human IBD.
Acknowledgments
This work was supported by NIH grants DK50189 / DK104713 / DK095491 and VA Merit Award 1I01BX002182.
Footnotes
Contributions: JML, drafted manuscript content, reviewed manuscript
DJK, drafted manuscript content, reviewed manuscript
EEA, drafted manuscript content, reviewed manuscript
SPC, drafted manuscript content, reviewed manuscript
References
- 1.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2008;14:401–407. doi: 10.3748/wjg.14.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 4.Colgan SP. Taylor CT Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glover LE, Lee JS, Colgan SP. Oxygen metabolism and barrier regulation in the intestinal mucosa. J Clin Invest. 2016;126:3680–3688. doi: 10.1172/JCI84429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng L, Kelly CJ, Colgan SP. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am J Physiol Cell Physiol. 2015;309:C350–360. doi: 10.1152/ajpcell.00191.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colgan SP, Campbell EL, Kominsky DJ. Hypoxia and Mucosal Inflammation. Ann Rev Pathol. 2016;11:77–100. doi: 10.1146/annurev-pathol-012615-044231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 9.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 10.Ratcliffe PJ. HIF-1 and HIF-2: working alone or together in hypoxia? J Clin Invest. 2007;117:862–865. doi: 10.1172/JCI31750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 12.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 13.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. 2009;119:1159–1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson RJ, McKie AT. Regulation of intestinal iron absorption: the mucosa takes control? Cell Metab. 2009;10:84–87. doi: 10.1016/j.cmet.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Stein J, Hartmann F. Dignass AU Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. doi: 10.1038/nrgastro.2010.151. DOI. [DOI] [PubMed] [Google Scholar]
- 17.Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med. 2007;85:1295–1300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- 18.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 19.Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol. 184:4062–4068. doi: 10.4049/jimmunol.0903002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE. Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukoc Biol. 1999;66:889–900. doi: 10.1002/jlb.66.6.889. [DOI] [PubMed] [Google Scholar]
- 21.El-Benna J, Dang PM, Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. 2008;30:279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 22.Gabig TG, Bearman SI, Babior BM. Effects of oxygen tension and pH on the respiratory burst of human neutrophils. Blood. 1979;53:1133–1139. [PubMed] [Google Scholar]
- 23.Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, Bayless AJ, Scully M, Saeedi BJ, Golden-Mason L, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai A, Moss A, Rothweiler S, Longhi MS, Wu Y, Junger WG, Robson SC. NADH oxidase-dependent CD39 expression by CD8(+) T cells modulates interferon gamma responses via generation of adenosine. Nat Commun. 2015;6:10. doi: 10.1038/ncomms9819. 1038/ncomms9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang JS, Noack D, Rae J, Ellis BA, Newbury R, Pong AL, Lavine JE, Curnutte JT, Bastian J. Chronic granulomatous disease caused by a deficiency in p47(phox) mimicking Crohn's disease. Clin Gastroenterol Hepatol. 2004;2:690–695. doi: 10.1016/s1542-3565(04)00292-7. [DOI] [PubMed] [Google Scholar]
- 26.Werlin SL, Chusid MJ, Caya J, Oechler HW. Colitis in chronic granulomatous disease. Gastroenterology. 1982;82:328–331. [PubMed] [Google Scholar]
- 27.Giatromanolaki A, Sivridis E, Maltezos E, Papazoglou D, Simopoulos C, Gatter KC, Harris AL, Koukourakis MI. Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol. 2003;56:209–213. doi: 10.1136/jcp.56.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colgan SP, Fennimore B, Ehrentraut SF. Adenosine and gastrointestinal inflammation. J Mol Med (Berl) 2013;91:157–164. doi: 10.1007/s00109-012-0990-0. doi:110.1007/s00109-00012-00990-00100. Epub 02013 Jan 00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colgan SP, Eltzschig HK. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol. 2012;74:153–175. doi: 10.1146/annurev-physiol-020911-153230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eltzschig HK, Eckle T, Mager A, Küper N, Karcher C, Weissmüller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP Release From Activated Neutrophils Occurs via Connexin 43 and Modulates Adenosine-Dependent Endothelial Cell Function. Circulation Research. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 32.Neves AR, Castelo-Branco MTL, Figliuolo VR, Bernardazzi C, Buongusto F, Yoshimoto A, Nanini HF, Coutinho CMLM, Carneiro AJV, Coutinho-Silva R, et al. Overexpression of ATP-activated P2X7 receptors in the intestinal mucosa is implicated in the pathogenesis of Crohn's disease. Inflammatory Bowel Diseases. 2014;20:444–457. doi: 10.1097/01.MIB.0000441201.10454.06. [DOI] [PubMed] [Google Scholar]
- 33.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic Signaling during Inflammation. New England Journal of Medicine. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strohmeier GR, Lencer WI, Patapoff TW, Thompson LF, Carlson SL, Moe SJ, Carnes DK, Mrsny RJ, Madara JL. Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. Journal of Clinical Investigation. 1997;99:2588–2601. doi: 10.1172/JCI119447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson LF, Eltzschig HK, Ib la JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weissmüller T, Campbell EL, Rosenberger P, Scully M, Beck PL, Furuta GT, Colgan SP. PMNs facilitate translocation of platelets across human and mouse epithelium and together alter fluid homeostasis via epithelial cell-expressed ecto-NTPDases. Journal of Clinical Investigation. 2008;118:3682–3692. doi: 10.1172/JCI35874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai A, Moss A, Kokkotou E, Usheva A, Sun X, Cheifetz A, Zheng Y, Longhi MS, Gao W, Wu Y, et al. CD39 and CD161 modulate Th17 responses in Crohn's disease. Journal of Immunology (Baltimore, Md: 1950) 2014;193:3366–3377. doi: 10.4049/jimmunol.1400346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedman DJ, Künzli BM, A-Rahim YI, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC. CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proceedings of the National Academy of Sciences. 2009;106:16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bynoe MS, Waickman AT, Mahamed DA, Mueller C, Mills JH, Czopik A. CD73 Is Critical for the Resolution of Murine Colonic Inflammation. BioMed Research International. 2012;2012 doi: 10.1155/2012/260983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Louis NA, Robinson AM, Macmanus CF, Karhausen J, Scully M, Colgan SP. Control of IFN-{alpha{A by CD73: Implications for mucosal inflammation. J Immunol. 2008;180:4246–4255. doi: 10.4049/jimmunol.180.6.4246. [DOI] [PubMed] [Google Scholar]
- 41.Rybaczyk L, Rozmiarek A, Circle K, Grants I, Needleman B, Wunderlich JE, Huang K, Christofi FL. New bioinformatics approach to analyze gene expressions and signaling pathways reveals unique purine gene dysregulation profiles that distinguish between CD and UC. Inflammatory Bowel Diseases. 2009 2009 Jul;15:971–984. doi: 10.1002/ibd.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colgan SP, Fennimore B, Ehrentraut SF. Adenosine and gastrointestinal inflammation. Journal of Molecular Medicine. 2013;91:157–164. doi: 10.1007/s00109-012-0990-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aherne C, Saeedi B, Collins C, Masterson J, McNamee E, Perrenoud L, Rapp C, Curtis V, Bayless A, Fletcher A, et al. Epithelial-specific A2B adenosine receptor signaling protects the colonic epithelial barrier during acute colitis. Mucosal immunology. 2015;8:1324–1338. doi: 10.1038/mi.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawrence DW, Comerford KM, Colgan SP. Role of VASP in reestablishment of epithelial tight junction assembly after Ca2+ switch. American Journal of Physiology Cell Physiology. 2002;282:C1235–1245. doi: 10.1152/ajpcell.00288.2001. [DOI] [PubMed] [Google Scholar]
- 45.Strohmeier GR, Reppert SM, Lencer WI, Madara JL. The A Adenosine Receptor Mediates cAMP Responses to Adenosine Receptor Agonists in Human Intestinal Epithelia. Journal of Biological Chemistry. 1995;270:2387–2394. doi: 10.1074/jbc.270.5.2387. [DOI] [PubMed] [Google Scholar]
- 46.Amir RE, Iwai K, Ciechanover A. The NEDD8 Pathway Is Essential for SCFβ-TrCP-mediated Ubiquitination and Processing of the NF-κB Precursor p105. Journal of Biological Chemistry. 2002;277:23253–23259. doi: 10.1074/jbc.M200967200. [DOI] [PubMed] [Google Scholar]
- 47.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 48.Sitkovsky M, Lukashev D, Deaglio S, Dwyer K, Robson SC, Ohta A. Adenosine A2A receptor antagonists: blockade of adenosinergic effects and T regulatory cells. Br J Pharmacol. 2008;153:S457–464. doi: 10.1038/bjp.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sitkovsky MV. T regulatory cells: hypoxia-adenosinergic suppression and redirection of the immune response. Trends Immunol. 2009;30:102–108. doi: 10.1016/j.it.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Kusu T, Kayama H, Kinoshita M, Jeon SG, Ueda Y, Goto Y, Okumura R, Saiga H, Kurakawa T, Ikeda K, et al. Ecto-nucleoside triphosphate diphosphohydrolase 7 controls Th17 cell responses through regulation of luminal ATP in the small intestine. J Immunol. 2013;190:774–783. doi: 10.4049/jimmunol.1103067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 52.Kurtz CC, Drygiannakis I, Naganuma M, Feldman S, Bekiaris V, Linden J, Ware CF, Ernst PB. Extracellular adenosine regulates colitis through effects on lymphoid and nonlymphoid cells. Am J Physiol Gastrointest Liver Physiol. 2014;307:G338–346. doi: 10.1152/ajpgi.00404.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sainio EL, Pulkki K, Young SN. L-Tryptophan: Biochemical, nutritional and pharmacological aspects. Amino Acids. 1996;10:21–47. doi: 10.1007/BF00806091. [DOI] [PubMed] [Google Scholar]
- 54.Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, Chieppa M, Rescigno M. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A. 2010;107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 57.Ciorba MA, Bettonville EE, McDonald KG, Metz R, Prendergast GC, Newberry RD, Stenson WF. Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis. J Immunol. 2010;184:3907–3916. doi: 10.4049/jimmunol.0900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muzaki AR, Tetlak P, Sheng J, Loh SC, Setiagani YA, Poidinger M, Zolezzi F, Karjalainen K, Ruedl C. Intestinal CD103CD11b dendritic cells restrain colitis via IFN-gamma-induced anti-inflammatory response in epithelial cells. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takamatsu M, Hirata A, Ohtaki H, Hoshi M, Hatano Y, Tomita H, Kuno T, Saito K, Hara A. IDO1 plays an immunosuppressive role in 2,4,6-trinitrobenzene sulfate-induced colitis in mice. J Immunol. 2013;191:3057–3064. doi: 10.4049/jimmunol.1203306. [DOI] [PubMed] [Google Scholar]
- 60.Gurtner GJ, Newberry RD, Schloemann SR, McDonald KG, Stenson WF. Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. Gastroenterology. 2003;125:1762–1773. doi: 10.1053/j.gastro.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 61.Shon WJ, Lee YK, Shin JH, Choi EY, Shin DM. Severity of DSS-induced colitis is reduced in Ido1-deficient mice with down-regulation of TLR-MyD88-NF-κB transcriptional networks. Sci Rep. 2015;5:17305. doi: 10.1038/srep17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrington L, Srikanth CV, Antony R, Rhee SJ, Mellor AL, Shi HN, Cherayil BJ. Deficiency of indoleamine 2,3-dioxygenase enhances commensal-induced antibody responses and protects against Citrobacter rodentium-induced colitis. Infect Immun. 2008;76:3045–3053. doi: 10.1128/IAI.00193-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 64.Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EM, Macchiarulo A, Vacca C, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flaveny CA, Murray IA, Chiaro CR, Perdew GH. Ligand selectivity and gene regulation by the human aryl hydrocarbon receptor in transgenic mice. Mol Pharmacol. 2009;75:1412–1420. doi: 10.1124/mol.109.054825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bacsi SG, Reisz-Porszasz S, Hankinson O. Orientation of the heterodimeric aryl hydrocarbon (dioxin) receptor complex on its asymmetric DNA recognition sequence. Mol Pharmacol. 1995;47:432–438. [PubMed] [Google Scholar]
- 67.Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140:173–179. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- 68.Gielen JE, Goujon FM, Nebert DW. Genetic regulation of aryl hydrocarbon hydroxylase induction. II. Simple Mendelian expression in mouse tissues in vivo. J Biol Chem. 1972;247:1125–1137. [PubMed] [Google Scholar]
- 69.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, Siddiqui S, Basso AS, Otterbein LE, Pardoll DM, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat Med. 2015;21:638–646. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ji T, Xu C, Sun L, Yu M, Peng K, Qiu Y, Xiao W, Yang H. Aryl Hydrocarbon Receptor Activation Down-Regulates IL-7 and Reduces Inflammation in a Mouse Model of DSS-Induced Colitis. Dig Dis Sci. 2015;60:1958–1966. doi: 10.1007/s10620-015-3632-x. [DOI] [PubMed] [Google Scholar]
- 72.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 73.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 74.Duarte JH, Di Meglio P, Hirota K, Ahlfors H, Stockinger B. Differential influences of the aryl hydrocarbon receptor on Th17 mediated responses in vitro and in vivo. PLoS One. 2013;8:e79819. doi: 10.1371/journal.pone.0079819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One. 2011;6:e23522. doi: 10.1371/journal.pone.0023522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limon P, Paiva RS, Ching T, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lanis JM, Alexeev EE, Curtis VF, Kitzenberg DA, Kao DJ, Battista KD, Gerich ME, Glover LE, Kominsky DJ, Colgan SP. Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol. 2017 doi: 10.1038/mi.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kominsky DJ, Campbell EL, Ehrentraut SF, Wilson KE, Kelly CJ, Glover LE, Collins CB, Bayless AJ, Saeedi B, Dobrinskikh E, et al. IFN-gamma-mediated induction of an apical IL-10 receptor on polarized intestinal epithelia. J Immunol. 2014;192:1267–1276. doi: 10.4049/jimmunol.1301757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davila AM, Blachier F, Gotteland M, Andriamihaja M, Benetti PH, Sanz Y, Tome D. Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol Res. 2013;68:95–107. doi: 10.1016/j.phrs.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 81.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x.Epub02009Feb01513. [DOI] [PubMed] [Google Scholar]
- 82.Herrmann G, Jayamani E, Mai G, Buckel W. Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. J Bacteriol. 2008;190:784–791. doi: 10.1128/JB.01422-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Louis P, Flint HJ. Development of a semiquantitative degenerate real-time pcr-based assay for estimation of numbers of butyryl-coenzyme A (CoA) CoA transferase genes in complex bacterial samples. Appl Environ Microbiol. 2007;73:2009–2012. doi: 10.1128/AEM.02561-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vital M, Penton CR, Wang Q, Young VB, Antonopoulos DA, Sogin ML, Morrison HG, Raffals L, Chang EB, Huffnagle GB, et al. A gene-targeted approach to investigate the intestinal butyrate-producing bacterial community. Microbiome. 2013;1:1–8. doi: 10.1186/2049-2618-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 86.Miquel S, Martin R, Rossi O, Bermudez-Humaran LG, Chatel JM, Sokol H, Thomas M, Wells JM, Langella P. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013;16:255–261. doi: 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 87.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berry D, Reinisch W. Intestinal microbiota: a source of novel biomarkers in inflammatory bowel diseases? Best Pract Res Clin Gastroenterol. 2013;27:47–58. doi: 10.1016/j.bpg.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 89.Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A, Chapkin RS, Alaniz R, Safe S. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol. 2014;85:777–788. doi: 10.1124/mol.113.091165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D'Angelo C, Massi-Benedetti C, Fallarino F, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 91.Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci U S A. 2010;107:228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov. 2014;13:852–869. doi: 10.1038/nrd4422. [DOI] [PMC free article] [PubMed] [Google Scholar]