Abstract
In this report, we present a case study involving an older, female patient with a history of pediatric TBI. Magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) volumes were acquired from the volunteer in question, her brain volumetrics and morphometrics were extracted, and these were then systematically compared to corresponding metrics obtained from a large sample of older healthy control (HC) subjects as well as from subjects in various stages of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD). Our analyses find the patient’s brain morphometry and connectivity most similar to patients classified as having early-onset MCI, in contrast to HC, late MCI, and AD samples. Our examination will be of particular interest to those interested in assessing the clinical course in older patients having suffered TBI earlier in life, in contradistinction to those which experience incidents of head injury during aging.
Keywords: Traumatic brain injury, mild cognitive impairment, aging
Graphical Abstract
We report on a study of a 67-year old patient who sustained TBI as a young child. Her 3T MRI data was subjected to cortical partitioning and analysis of inter-regional DTI white matter fiber connectivity as compared to a cohort of healthy older adults (HC), those with early mild cognitive impairment (eMCI), late mild cognitive impairment (lMCI), and a diagnosis of Alzheimer's Disease (AD) was conducted. “Goodness-of-Fit” assessment of the subject's brain morphometry and connectivity indicated probable group membership with early MCI. Implications for this patient as well as for other case-study investigations of TBI earlier in life as patients experience aging are discussed.
Introduction
Despite reasonable chances of recovery and frequent evidence for cognitive reserve (Bigler and Stern 2015), traumatic brain injury (TBI) experienced early in life may result in late-life cognitive decline and dementia (Plassman and Grafman 2015; LoBue, Wadsworth et al. 2016; LoBue, Wilmoth et al. 2016). However, a direct cause-and-effect relationship has yet to be firmly established between pediatric TBI and early-onset Alzheimer’s Disease (AD) (Mendez, Paholpak et al. 2015). Reports suggest that TBI during childhood may not necessarily cause dementia later in life per se, but may result in earlier onset (Li, Risacher et al. 2016). For example, in those who are genetically predisposed to dementia, the occurrence of TBI early in life may hasten AD onset. In contrast, neuropsychological studies of older adults who had recently suffered a TBI did not show any more nor less amnesia or cognitive impairment (CI) than similarly-aged patients suffering from mild CI (MCI) alone (Rapoport, Wolf et al. 2008). In other words, late-life TBI may not necessarily result in MCI or AD. Additionally, TBI as a risk factor for MCI may also be confounded by other factors such as sex and clinical depression (LoBue, Denney et al. 2016). Nevertheless, neuroimaging of older subjects who experienced TBI during their youth can provide valuable insights with potential diagnostic utility.
MRI measurements of brain structure have been shown to demonstrate brain atrophy (which correlates with neuronal loss) in MCI and AD as well as increasing rates of brain atrophy as MCI/AD patients become more impaired (Thompson, Hayashi et al. 2007; Hua, Leow et al. 2008; Morra, Tu et al. 2008; Nestor, Rupsingh et al. 2008; Hua, Gutman et al. 2011; Wolz, Julkunen et al. 2011). For this reason, structural MRI can be used to quantify the rate of disease progression, and possibly as a measure of treatment effect, in AD treatment trials (Poulin and Zakzanis 2002; Van Horn and Toga 2009). Moreover, interest exists in using neuroimaging data resources as a basis for comparison in studies of potentially degenerative syndromes (Van Horn and Toga 2009), including TBI (Weiner, Friedl et al. 2013; Li, Risacher et al. 2016).
In this report, we present a case study involving an older, female patient with a history of pediatric TBI. Magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) volumes were acquired from the volunteer in question, her brain volumetrics and morphometrics were extracted, and these were then systematically compared to corresponding metrics obtained from a large sample of older healthy control (HC) subjects as well as from subjects in various stages of MCI and AD.
Methods
Subject
Patient “IH” is a 67 year-old female who experienced a severe head injury in childhood (at 5 years of age) subsequent to a moving-vehicle traffic accident. After successful clinical recovery, the patient led an exemplary adult life which involved higher-education achievements, philanthropy, and community service. When the patient was in her early 60s, however, her family members reported to clinicians that she experiencing memory loss and other cognitive deficits.
The patient underwent clinical assessment at the University of California Los Angeles, where the following examinations were administered: Mini-Mental State Exmaination (MMSE); Wechsler Test of Adult Reading (WTAR); Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) selected subtests; California Verbal Learning Test – II (CLVT-II); Wechsler Memory Scale – 3rd Edition (WMS-III) selected subtests; Trailmaking A & B; Stroop Color-Word Interference Test; Boston Naming Test (BNT); Rey-Osterrieth Complex Figure Test (ROCFT); Controlled Oral Word Association Test (FAS, animals); Geriatric Depression Scale (GDS), and the Wisconsin Card Sorting Test (WCST).
Genetic screening (30X, Illumina HQ, San Diego, CA, 2013) indicated that the patient was APOE-negative, although her genome did feature single nucleotide polymorphisms (SNPs) associated with presenilin-encoding genes (PSEN1, PSEN2), which are recognized as having a putative role in familial AD (Mathews, Cataldo et al. 2000; Lalli, Cox et al. 2014). Clinical abnormalities were also observed on resting and task-oriented electroencephaolographic (EEG) recordings, suggestive of remnant morphological damage. Prior neuroimaging assessment using 1.5 T MRI over two occasions (Table 1) showed that Patient IH had experienced considerable percentage increases in the volume of the ventricular system over the roughly three year period during which symptoms of cognitive decline became noticeable by her close relatives.
Table 1.
Longitudinal Gross Morphological Changes in the Brain of Patient IH
| Volumes (mm3) | ||||
|---|---|---|---|---|
| Structure | 5/27/2011 | 2/18/2014 | %Change | %Annual |
| Total gray matter | 624,685 | 629,350 | −0.096 | −0.035 |
| Total cortical gray matter | 452,898 | 453,531 | 0.507 | 0.186 |
| Total cortical white matter | 436,302 | 419,036 | 4.578 | 1.674 |
| Intracranial Volume | 1,482,111 | 1,491,752 | ||
| Left-Thalamus-Proper | 6,272 | 6,198 | 1.819 | 0.665 |
| Right-Thalamus-Proper | 6,421 | 6,140 | 4.994 | 1.827 |
| Left-Lateral-Ventricle | 16,282 | 18,480 | −12.766 | −4.670 |
| Right-Lateral-Ventricle | 16,067 | 17,167 | −6.156 | −2.251 |
| 3rd-Ventricle | 1,384 | 1,566 | −12.419 | −4.542 |
| 4th-Ventricle | 1,221 | 1,541 | −25.392 | −9.287 |
Neuroimaging
Patient IH provided informed consent and underwent brain imaging using the Alzheimer’s Disease Neuroimaging Initiative 2 (ADNI-2) neuroimaging protocol in a General Electric 3 T MRI scanner located at the Keck School of Medicine of USC. The MRI protocol consisted of four sequences: (1) inversion-recovery spoiled gradient echo (IR SPGR) T1-weighted structural MRI, (2) fluid-attenuated inversion recovery (FLAIR) MRI, (3) gradient recalled echo (GRE) T2* MRI and (4) 64-direction DTI. Resting-state functional MRI was also collected but whose analysis we have chosen to defer to a later exploration. Here, we focus specifically on the analyses of the T1 structural and DTI imaging data. The details of the ADNI-2 neuroimaging protocol may be found described in Jack et al. (2010), while various applications and a review of results obtained from this multi-site trial are given in Wiener et al. (2012).
Analyses
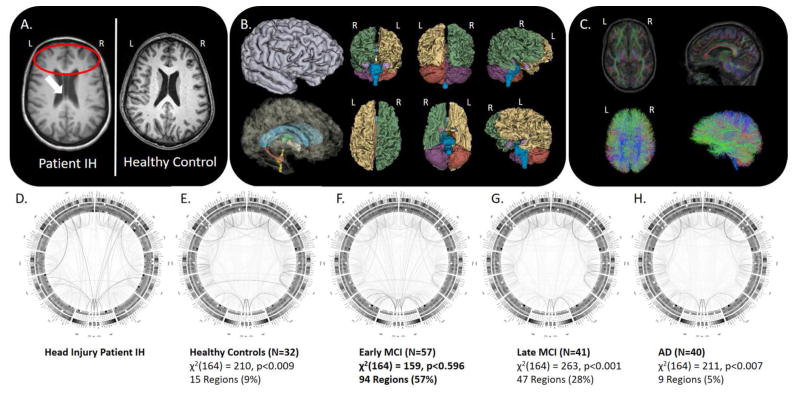
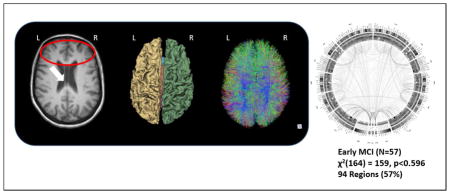
Tissue classification and anatomical parcellation of T1-weighted volume—together with DTI processing and connectogram creation (see Figure 1 D–H)—were performed as described elsewhere (Irimia, Chambers et al. 2012). We identified patients from the ADNI-2 cohort of similarly aged females. These included HCs (N=32); early MCI (EMCI, N=57); late MCI (LMCI, N=41) and AD (N=40) with mean (±SD) ages of 75.00±6.06, 75.00±7.74, 73.87±6.05, and 75.32±8.28, respectively. In instances where multiple MRI scan session data were available from these subjects, we selected the scan session representing the date closest in age to Patient IH. We then assessed the overall pattern of connectivity density across the brain of Patient IH against these groups using a posteriori Bayesian probabilities of group membership (see Rencher 2002, for description). Finally, we sought to assign Patient IH to most likely group via Chi-Squared “Goodness-of-Fit” test to enable us to classify her into one or more of the ADNI-2 patient cohorts and, thus, infer upon her a computational “diagnosis”. In “Goodness-of-Fit” testing, one seeks the smallest χ2 statistic when comparing across classes, thus, the largest probability for a particular comparison based upon probabilistic similarity. In other words, this reflects the probability of a subject “belonging” to that particular group. This is in contrast to the more familiar seeking of a large χ2 statistic against the associated degrees-of-freedom (and small p-value) suggesting departure from theoretical expectation (Kvalseth 2004; Johnston, Berry et al. 2006).
Figure 1.
A) MRI of Patient IH relative to an age-matched healthy control subject; B) cortical parcellation and volumetric modeling; C) diffusion imaging tractography; D–H) group-specific connectograms for Patient IH, Healthy Older Adults, Early MCI, Late MCI, and AD, respectively, with accompanying “Goodness-of-Fit” assessment of Patient IH’s group membership.
Results
The results of her neuropsychological evaluation revealed no areas of significant cognitive or intellectual impairment (Table 2). Given her estimated high premorbid level of functioning, relative weaknesses were noted selected aspects of processing speed (e.g. rapid number sequencing), as well as aspects of executive functioning, including mental flexibility, divided attention, and word generation. Patient IH did report some family-related stress which could have adversely impacted cognitive function and which might have contributed to her more subjective complaints. However, many individuals demonstrate areas of relative weakness due to normal variability and aging which are not pathological in nature, and her performance on other measures in these domains were intact. Despite her childhood injury and reported declines in memory, her performance on measures of both verbal and non-verbal memory were intact and comparable to younger adults.
Table 2.
The Results of Neuropsychological Testing
| Neuropsychological Assessment | Score/Outcome (Percentile) | Comments: |
|---|---|---|
| Mini-Mental State Examination (MMSE) | 30/30 | Suggesting intact cognitive functioning |
| Wechsler Test of Adult Reading (WTAR) | Verbal IQ = 122 (93rd) | In the superior range |
| Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) selected subtests | Digit Span = 7 forward, 5 backward (63rd) Digit-Symbol = 84th percentile Picture Completion = 91st percentile Block Design = 84th percentile |
Average range |
| California Verbal Learning Test – II (CLVT-II) |
|
Demonstrated a positive learning curve High average range for her age |
| Wechsler Memory Scale – 3rd Edition (WMS-III) selected subtests | Logical Memory I = 55, (95th) Logical Memory II = 37, (98th), (95% retention) Visual Reproduction I = 78, (50th) Visual Reproduction II = 61, (75th), 78% retention |
Average to superior range of memory |
| Trail Making A & B | Trails A = 14th percentile Trails B = 71 seconds, (36th), 0 errors |
Average mental flexibility and divided attention |
| Stroop Color-Word Interference Test | Word reading = 95th percentile Color naming = 83rd percentile |
In the superior range |
| Boston Naming Test (BNT) | 3 min delay = 17.5, (58th) | In the average range |
| Rey-Osterrieth Complex Figure Test (ROCFT) | ROCFT Copy = 33, (>16th) | Within limits, demonstrating overall gestalt. |
| Controlled Oral Word Association Test (FAS, animals) | Animals = 19, (58th) FAS = 39, (40th) |
In the average range for her age |
| Geriatric Depression Scale (GDS) | GDS = 4 | In the normal range for mood |
| Wisconsin Card Sorting Test (WCST) | Categories = 6, (>16th) Perseverative Errors = 6, (61st) |
In the average range |
The brain imaging from Patient IH, however, was noted to exhibit a cavum septum pellucidum (Figure 1A), where the septal lamina have become detached and separated – a condition often associated with head trauma (see, for instance, the study of American football players by Gardner, Hess et al. 2016). Also present was a concave deformation of the lateral grey matter surface extending over the antero-superior portion of the temporal lobe (inferior, middle and superior temporal gyri) and over the inferior portion of right pre-frontal cortex. No similar deformation was observed in the corresponding region of the right hemisphere. Patient IH’s brain also exhibited reduced white matter volume in the right hemisphere in contrast to that of the left hemisphere. Interestingly, the left pallidum was noted to be ~47% larger than the right pallidum; such a large asymmetry is rare in healthy adults though it has been observed frequently in patients with chronic TBI and dementia (Gooijers, Chalavi et al. 2016). More specifically, examining inter-regional brain connectivity (Figure 1B–C), we measured the multivariate Mahalanobis distance and a posteriori probabilities of group memberships between the connectogram-based patterns white matter fiber density in Patient IH versus the same in the HC, EMCI, LMCI, and AD groups from the ADNI-2 cohort (Figure 1D–H). Patient IH’s overall pattern of brain connectivity was most probabilistically different from AD Patients, LMCI Subjects, and HC subjects. Patient IH did, however, show a pattern of brain connectivity probabilistically most similar, via Chi-Squared goodness-of-fit test (df = 164), to the EMCI patients in the ADNI-2 cohort (see Figure 1F).
Discussion
In this brief article, we present a case study examination of a female sexagenarian patient who experienced TBI as a child, presented an admirable recovery, spending her life conducting philanthropic work before suffering cognitive decline. Neuropsychological assessments indicated an intelligent woman presenting no major deficits for her age but with minor weaknesses in executive function and divided attention, which might simply reflect normal age- or stress-related variation. Using structural and diffusion neuroimaging, we parcellated the brain and compute its inter-regional DTI connectivity. Our analyses find Patient IH’s brain morphometry and connectivity most similar to patients from the ADNI cohort classified as having early MCI. Several points of discussion are worth considering relevant to Patient IH and our analyses of her neuroimaging data:
Altered Brain Morphometry in Patient IH
Patient IH was noted here to exhibit cavum septum pellucidum, altered left temporal grey matter, frontal lobe white volume asymmetry, and a greater volume of the left than right pallidum. Cavum septum pellicidum (CSP) variations are common in neurological patients (Akinola, Idowu et al. 2014) and have been intermittently reported in retired professional athletes (Bogdanoff and Natter 1989; Bodensteiner and Schaefer 1997; Casson, Viano et al. 2014). In a study by Silk et al. (2013), there was no difference in presence of CSP between TBI patients and controls; however, there was larger and more severely graded CSP in the patient group, with the size of the CSP correlated positively with injury severity. Patient IH was unconscious for two weeks following her injury suggesting a particular and sufficient level of severity. Greater degenerative white matter alterations have been reported in pediatric TBI patients (Keightley, Sinopoli et al. 2014), where lowered white matter integrity may be more important in the pathophysiology of brain injury than indices of gray matter change, macroscopic lesions, and injury severity (Max, Wilde et al. 2012). MRI findings frequently identify frontal and temporal alterations in pediatric TBI group relative to comparison samples (Wilde, Hunter et al. 2005), however, temporal lobe injuries may not be associated with greater levels of anxiety (Vasa, Grados et al. 2004). Finally, subcortical volumetric alterations are frequently reported in TBI (Gooijers, Chalavi et al. 2016), are notable in MRI of pediatric head injury cases (Wilde, Bigler et al. 2007), and which can affect cognitive processes such as proactive inhibition (Hermans, Beeckmans et al. 2016). Such alterations in this case, thus, are not uncommon with regard to other reported TBI studies in young and adult samples and suggest her injury as the initiation of a process culminating in eventual cognitive decline later in life.
Age as an Important Factor in TBI Recovery but Eventual Cognitive Decline
Outcome from TBI is a function of age at injury (Testa, Malec et al. 2005), with younger subjects tending to recover more fully than older individuals. Indeed, early intervention, surgical treatment, and/or intensive care for patients has been reported to produce excellent clinical results up to the age of 59 years, with favorable outcomes still possible for 39% of cases aged 60–69 years, without an excessive burden of severely disabled patients (Stocchetti, Paterno et al. 2012). Children suffering TBI, however, show cortical thickness changes measured using MRI at three and eighteen months following injury in contrast to children with orthopedic injuries (Wilde, Merkley et al. 2012). TBI in animal models has suggested increased motor and cognitive deficits in rats suffering injuries earlier in development, in contrast to greater anxiety in rats injured during adulthood (Rowe, Ziebell et al. 2016). So while structural and connectomic injuries may be clinically recoverable, their residual effects may be expressed later in life as cognitive decline. As such, an improved integration of major clinical and scientific effort needs to be made to improve any potential for post-traumatic recovery after TBI in neonates and young children (Maxwell 2012).
Brain Reserve Capacity (BRC)
Patient IH did not present pathological levels of cognitive decline despite subjective reports from herself and her family. Indeed, she showed above average to superior performance on many of the measures assessed. BRC refers to pre-injury quantitative measures such as brain size that relate to outcome (Bigler and Stern 2015). Higher degrees of BRC imply threshold differences when clinical deficits will become apparent after injury, where those individuals with higher BRC require more pathology to reach that threshold. Cognitive reserve (CR), more specifically, pertains to how flexibly and efficiently the individual makes use of available brain resources even in the presence of advancing age (Whalley, Deary et al. 2004). The CR model suggests the brain actively attempts to cope with brain damage resulting from TBI by using pre-existing cognitive processing approaches or by enlisting compensatory approaches (Nunnari, Bramanti et al. 2014). Standard contributors to CR include education and IQ (Carnero Pardo and del Ser 2007; Elkana, Eisikovits et al. 2016; Kowoll, Degen et al. 2016), including literacy, occupational attainment, engagement in leisure activities, and the integrity of social networks (Henderson 2014; Sakka 2015; Cheng 2016; Moussard, Bermudez et al. 2016). Most research on BRC and CR has taken place in aging and degenerative disease (Sobral, Pestana et al. 2015) but these concepts likely apply to the effects of TBI, especially with regards to recovery (Scheibel, Newsome et al. 2009; Miller, Colella et al. 2013; Sumowski, Chiaravalloti et al. 2013; Bigler 2014; Schneider, Sur et al. 2014). Since increased incidence of TBI occurs in those under age 35, both CR and BRC factors likely relate to how the individual copes with TBI over the lifespan. Mild to moderate TBI during childhood, during a time of maximal neural plasticity, may show sufficient recovery when exposed to highly educational and psychosocial encouraging environments (Max, Roberts et al. 1999; Beauchamp and Anderson 2013). Such factors may be particularly relevant to the timing of cognitive decline an the individual who has sustained a TBI earlier in life, in contrast to an older adult suffering affected cognition as a consequence of head injury.
Presence of Presenilin Proteins
AD patients with an inherited form of the disease may carry mutations in the presenilin proteins (PSEN1; PSEN2) or the amyloid precursor protein (APP). These disease-linked mutations result in increased production of the longer form of amyloid beta (Aβ). Presenilins are believed to regulate APP processing through their effects on gamma secretase, an enzyme that cleaves APP (Cruchaga, Chakraverty et al. 2012). Moreover, presenilins contribute to the cleavage of the Notch receptor (Newman, Wilson et al. 2014). They either directly regulate gamma secretase activity or themselves are protease enzymes. A number of alternatively spliced transcript variants have been identified for this gene, the full-length natures of only a handful have been determined (Bennet, Reynolds et al. 2011). The study of patients having PSEN1/2 protein mutations provide unique opportunities to examine AD biomarkers in persons in whom the diagnosis is certain, as in the present case of Patient IH.
In a particular example, Ringman et al. (2011) describe a 55 year-old female AD patient having a PSEN1 mutation who underwent genetic, clinical, biochemical and magnetic resonance and nuclear imaging assessments. They also explored neuropathological findings in her similarly affected male sibling. Neuropsychological testing confirmed deficits in memory, visuospatial, and language function. CSF-based t-tau and p-tau were markedly elevated and Aβ levels reduced. FDG-PET revealed hypo-metabolism in the left parieto-temporal cortex. FDDNP-PET revealed greater tracer binding in medial temporal and parietal lobes, in the head of the caudate nucleus, and the anterior putamen bilaterally. Neuropathological examination of her brother showed the typical findings of AD and the striatum demonstrated amyloid pathology and marked neurofibrillary pathology beyond that typically seen in late-onset AD. A novel S212Y substitution in PSEN1 was present in the index patient and her affected brother but not in an older unaffected sister. An in vitro assay in which the S212Y mutation was introduced in cell culture confirmed that it was associated with increased production of Aβ. Their study helps to validate the pathogenicity of this mutation as an index used to assess familial AD.
While a causal relationship due to the presence of PSEN gene variants is difficult, if not impossible, to establish, a potential linkage between genetic susceptibility and Patient IH’s head injury early in life is compelling. Patient IH was determined here to have cortical anatomy and white matter connectedness most similar to the ADNI MCI cohort, however, it remains to be seen if she might later convert to full AD, and a more detailed examination of her family history would be warranted.
Statistical Similarity
We have systematically compared Patient IH’s brain morphometry and connectivity to the distributions of the various cohorts of the ADNI-2 sample via multivariate modeling, a posteriori probabilistic classification, and goodness-of-fit assessment. This approach is distinguished from machine learning classification since we wish to compute a ‘distance’ between the observed values obtained from Patient IH against those expected from each ADNI patient cohort relative to underlying random classification error to obtain a probabilistic statement about group membership (Choi and McHugh 1989; Reijneveld 1990; Boyle, Flowerdew et al. 1997; Fisher, Marshall et al. 2011). Related methods have been widely applied in neuroimaging voxel pattern analysis (Walther, Nili et al. 2016), structural connectivity (Gupta, Mayer et al. 2015), as well as in functional MRI time series data (Friston, Chu et al. 2008). While machine learning methods might also have been appropriate here (for instance, Apostolova, Hwang et al. 2010), this approach serves a parsimonious purpose - showing the degree of similarity of Patient IH to each of the ADNI patient groups. It is unclear if the computational efforts of a machine learning approach would provide additional information about Patient IH’s similarity to the MCI group, per se. Regardless, the availability of the ADNI neuroimaging dataset as a basis for comparison represents a highly useful resource for computationally assessing the status of independently collected data in patient samples in health as well as in disease.
In general, Patient IH’s brain morphometry and white matter connectivity density were determined to be statistically most similar to that of patients having been diagnosed with MCI as indicated under the ADNI-2 study protocol. This agrees with what has been documented about MCI (Celsis 2000) and its potential connections with TBI earlier in life (Plassman and Grafman 2015). Still, the use of computational approaches for subject classification in this manner has important implications for the proactive utilization of neuroimaging data from leading databases for such purposes. It is important to note, however, that while the role of early-life TBI on neurocognitive changes in later-life is an important topic, a number factors beyond Patient IH’s injury could also be at play over her lifetime which complicate making broad sweeping statement about her experience to that of other TBI sufferers. However, such subject classification approaches may be useful for providing key insights into the distinctions between patients having early versus later-life TBI and the clinical outcomes thereof.
Conclusions
Examining the long term effects of early life TBI, especially in cases of apparent recovery, may take on particular importance as the brain ages. This may be particularly true in military veterans (Weiner, Veitch et al. 2014), as well as in former professional and collegiate athletics (Hart, Kraut et al. 2013). In the case presented here, Patient IH presented suffering from symptoms and a pattern of brain degeneration consistent with EMCI with suggestive link to head injury during youth. While a causal relationship between the presence of the PSEN gene variants is difficult to establish, a potential linkage between genetic susceptibility and Patient IH’s head injury early in life is possible. Most likely, her injury during childhood likely began a degenerative process sufficient to culminate in cognitive decline and MCI in later adulthood. Our examination will be of particular interest to those assessing the clinical importance of patients who are “aging after having suffered TBI” in contradistinction to those which experience “incidents of TBI during aging” (Peters 2016).
Significance Statement.
Structural and diffusion measurements of the brain have been shown to demonstrate brain atrophy in mild cognitively impaired (MCI) and Alzheimer’s Disease (AD) as well as increasing rates of brain atrophy as MCI/AD patients become more impaired. Examining the long term effects of pediatric TBI, especially in cases of successful recovery, using neuroimaging may take on unique importance in the aging brain. Comparison of individual cases having suffered TBI against aging-specific disease cohorts can contribute insights into the clinical picture of early-life brain injury.
Acknowledgments
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: JVH. Acquisition of data: JVH. Analysis and interpretation of data: JVH and AA. Drafting of the manuscript: JVH. Statistical analysis: CMT, JVH, and AA. Obtained funding: JVH. Administrative, technical, and material support: CMT, ZJ, AB, and PMV. Study supervision: JVH. The authors report no conflicts of interest related to this work. The authors wish to express our sincere gratitude to Dr. Rand McClain and Robert Harding as well as the staff of the USC Mark and Mary Stevens Neuroimaging and Informatics Institute. This work was supported by NIH grant R44 NS081792-03A1 to JVH.
Footnotes
Data Accessibility
Data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) is freely available, with permission, from http://adni.loni.usc.edu/. As a multi-site consortia, ADNI researchers systematically obtain, rigorously validate, and promote the utilization of MRI and PET images, genetics, cognitive tests, CSF, and blood biomarkers as predictors from patients with the disease. Data from the North American ADNI’s study participants, including Alzheimer’s disease (AD) patients, mild cognitive impairment (MCI) subjects and healthy elderly controls (HC), are available from this website. Neuroimaging and other meta-data from Patient IH are available upon written request to the lead author of this study.
References
- Akinola RA, Idowu OE, Nelson-Paseda AO. Caval variations in neurologically diseased patients. Acta Radiol Short Rep. 2014;3(5):2047981614530288. doi: 10.1177/2047981614530288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Hwang KS, Babakchanian S, Green RC, Sun D, Zoumalan C, Kenani R, Hana G, Wang L, Cummings JL, Toga AW, Thompson PM. Statistical Map-based Classification of MCI and Alzheimer’s disease using machine learning. Organization for Human Brain Mapping. Barcelona; Spain: 2010. [Google Scholar]
- Beauchamp MH, Anderson V. Cognitive and psychopathological sequelae of pediatric traumatic brain injury. Handb Clin Neurol. 2013;112:913–920. doi: 10.1016/B978-0-444-52910-7.00013-1. [DOI] [PubMed] [Google Scholar]
- Bennet AM, Reynolds CA, Eriksson UK, Hong MG, Blennow K, Gatz M, Alexeyenko A, Pedersen NL, Prince JA. Genetic association of sequence variants near AGER/NOTCH4 and dementia. J Alzheimers Dis. 2011;24(3):475–484. doi: 10.3233/JAD-2011-101848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED. Comment: importance of cognitive reserve in traumatic brain injury. Neurology. 2014;82(18):1641. doi: 10.1212/WNL.0000000000000395. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Stern Y. Traumatic brain injury and reserve. Handb Clin Neurol. 2015;128:691–710. doi: 10.1016/B978-0-444-63521-1.00043-1. [DOI] [PubMed] [Google Scholar]
- Bodensteiner JB, Schaefer GB. Dementia pugilistica and cavum septi pellucidi: born to box? Sports Med. 1997;24(6):361–365. doi: 10.2165/00007256-199724060-00002. [DOI] [PubMed] [Google Scholar]
- Bogdanoff B, Natter HM. Incidence of cavum septum pellucidum in adults: a sign of boxer's encephalopathy. Neurology. 1989;39(7):991–992. doi: 10.1212/wnl.39.7.991. [DOI] [PubMed] [Google Scholar]
- Boyle P, Flowerdew R, Williams A. Evaluating the goodness of fit in models of sparse medical data: a simulation approach. Int J Epidemiol. 1997;26(3):651–656. doi: 10.1093/ije/26.3.651. [DOI] [PubMed] [Google Scholar]
- Carnero Pardo C, del Ser T. Education provides cognitive reserve in cognitive deterioration and dementia. Neurologia. 2007;22(2):78–85. [PubMed] [Google Scholar]
- Casson IR, Viano DC, Haacke EM, Kou Z, LeStrange DG. Is There Chronic Brain Damage in Retired NFL Players? Neuroradiology, Neuropsychology, and Neurology Examinations of 45 Retired Players. Sports Health. 2014;6(5):384–395. doi: 10.1177/1941738114540270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celsis P. Age-related cognitive decline, mild cognitive impairment or preclinical Alzheimer's disease? Ann Med. 2000;32(1):6–14. doi: 10.3109/07853890008995904. [DOI] [PubMed] [Google Scholar]
- Cheng ST. Cognitive Reserve and the Prevention of Dementia: the Role of Physical and Cognitive Activities. Curr Psychiatry Rep. 2016;18(9):85. doi: 10.1007/s11920-016-0721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, McHugh RB. A reduction factor in goodness-of-fit and independence tests for clustered and weighted observations. Biometrics. 1989;45(3):979–996. [PubMed] [Google Scholar]
- Cruchaga C, Chakraverty S, Mayo K, Vallania FL, Mitra RD, Faber K, Williamson J, Bird T, Diaz-Arrastia R, Foroud TM, Boeve BF, Graff-Radford NR, St Jean P, Lawson M, Ehm MG, Mayeux R, Goate AM. Rare Variants in APP, PSEN1 and PSEN2 Increase Risk for AD in Late-Onset Alzheimer's Disease Families. PLoS ONE. 2012;7(2):e31039. doi: 10.1371/journal.pone.0031039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkana O, Eisikovits OR, Oren N, Betzale V, Giladi N, Ash EL. Sensitivity of Neuropsychological Tests to Identify Cognitive Decline in Highly Educated Elderly Individuals: 12 Months Follow up. J Alzheimers Dis. 2016;49(3):607–616. doi: 10.3233/JAD-150562. [DOI] [PubMed] [Google Scholar]
- Fisher MJ, Marshall AP, Mitchell M. Testing differences in proportions. Aust Crit Care. 2011;24(2):133–138. doi: 10.1016/j.aucc.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Friston K, Chu C, Mourao-Miranda J, Hulme O, Rees G, Penny W, Ashburner J. Bayesian decoding of brain images. Neuroimage. 2008;39(1):181–205. doi: 10.1016/j.neuroimage.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Gardner RC, Hess CP, Brus-Ramer M, Possin KL, Cohn-Sheehy BI, Kramer JH, Berger MS, Yaffe K, Miller B, Rabinovici GD. Cavum Septum Pellucidum in Retired American Pro-Football Players. J Neurotrauma. 2016;33(1):157–161. doi: 10.1089/neu.2014.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooijers J, Chalavi S, Beeckmans K, Michiels K, Lafosse C, Sunaert S, Swinnen SP. Subcortical Volume Loss in the Thalamus, Putamen, and Pallidum, Induced by Traumatic Brain Injury, Is Associated With Motor Performance Deficits. Neurorehabil Neural Repair. 2016;30(7):603–614. doi: 10.1177/1545968315613448. [DOI] [PubMed] [Google Scholar]
- Gupta A, Mayer EA, Sanmiguel CP, Van Horn JD, Woodworth D, Ellingson BM, Fling C, Love A, Tillisch K, Labus JS. Patterns of brain structural connectivity differentiate normal weight from overweight subjects. Neuroimage Clin. 2015;7:506–517. doi: 10.1016/j.nicl.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J, Jr, Kraut MA, Womack KB, Strain J, Didehbani N, Bartz E, Conover H, Mansinghani S, Lu H, Cullum CM. Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: a cross-sectional study. JAMA Neurol. 2013;70(3):326–335. doi: 10.1001/2013.jamaneurol.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW. Education and cognitive reserve. Climacteric. 2014;17(4):510–511. doi: 10.3109/13697137.2014.931618. [DOI] [PubMed] [Google Scholar]
- Hermans L, Beeckmans K, Michiels K, Lafosse C, Sunaert S, Coxon JP, Swinnen SP, Leunissen I. Proactive Response Inhibition and Subcortical Gray Matter Integrity in Traumatic Brain Injury. Neurorehabil Neural Repair. 2016 doi: 10.1177/1545968316675429. [DOI] [PubMed] [Google Scholar]
- Hua X, Gutman B, Boyle C, Rajagopalan P, Leow AD, Yanovsky I, Kumar AR, Toga AW, Jack CR, Jr, Schuff N, Alexander GE, Chen K, Reiman EM, Weiner MW, Thompson PM. Accurate measurement of brain changes in longitudinal MRI scans using tensor-based morphometry. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Leow AD, Lee S, Klunder AD, Toga AW, Lepore N, Chou YY, Brun C, Chiang MC, Barysheva M, Jack CR, Jr, Bernstein MA, Britson PJ, Ward CP, Whitwell JL, Borowski B, Fleisher AS, Fox NC, Boyes RG, Barnes J, Harvey D, Kornak J, Schuff N, Boreta L, Alexander GE, Weiner MW, Thompson PM The Alzheimer's Disease Neuroimaging I. 3D characterization of brain atrophy in Alzheimer's disease and mild cognitive impairment using tensor-based morphometry. Neuroimage. 2008;41(1):19–34. doi: 10.1016/j.neuroimage.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia A, Chambers MC, Torgerson CM, Van Horn JD. Circular representation of human cortical networks for subject and population-level connectomic visualization. Neuroimage. 2012;60(2):1340–1351. doi: 10.1016/j.neuroimage.2012.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Borowski BJ, Gunter JL, Fox NC, Thompson PM, Schuff N, Krueger G, Killiany RJ, Decarli CS, Dale AM, Carmichael OW, Tosun D, Weiner MW. Update on the magnetic resonance imaging core of the Alzheimer's disease neuroimaging initiative. Alzheimers Dement. 2010;6(3):212–220. doi: 10.1016/j.jalz.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JE, Berry KJ, Mielke PW., Jr Measures of effect size for chi-squared and likelihood-ratio goodness-of-fit tests. Percept Mot Skills. 2006;103(2):412–414. doi: 10.2466/pms.103.2.412-414. [DOI] [PubMed] [Google Scholar]
- Keightley ML, Sinopoli KJ, Davis KD, Mikulis DJ, Wennberg R, Tartaglia MC, Chen JK, Tator CH. Is there evidence for neurodegenerative change following traumatic brain injury in children and youth? A scoping review. Front Hum Neurosci. 2014;8:139. doi: 10.3389/fnhum.2014.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowoll ME, Degen C, Gorenc L, Kuntzelmann A, Fellhauer I, Giesel F, Haberkorn U, Schroder J. Bilingualism as a Contributor to Cognitive Reserve? Evidence from Cerebral Glucose Metabolism in Mild Cognitive Impairment and Alzheimer's Disease. Front Psychiatry. 2016;7:62. doi: 10.3389/fpsyt.2016.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvalseth TO. On a general class of chi-squared goodness-of-fit statistics. Percept Mot Skills. 2004;98(3 Pt 1):967–970. doi: 10.2466/pms.98.3.967-970. [DOI] [PubMed] [Google Scholar]
- Lalli MA, Cox HC, Arcila ML, Cadavid L, Moreno S, Garcia G, Madrigal L, Reiman EM, Arcos-Burgos M, Bedoya G, Brunkow ME, Glusman G, Roach JC, Hood L, Kosik KS, Lopera F. Origin of the PSEN1 E280A mutation causing early-onset Alzheimer's disease. Alzheimers Dement. 2014;10(5 Suppl):S277–S283. e210. doi: 10.1016/j.jalz.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Risacher SL, McAllister TW, Saykin AJ. Traumatic brain injury and age at onset of cognitive impairment in older adults. J Neurol. 2016;263(7):1280–1285. doi: 10.1007/s00415-016-8093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoBue C, Denney D, Hynan LS, Rossetti HC, Lacritz LH, Hart J, Womack KB, Woon FL, Cullum CM. Self-Reported Traumatic Brain Injury and Mild Cognitive Impairment: Increased Risk and Earlier Age of Diagnosis. J Alzheimers Dis. 2016;51(3):727–736. doi: 10.3233/JAD-150895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoBue C, Wadsworth H, Wilmoth K, Clem M, Hart J, Jr, Womack KB, Didehbani N, Lacritz LH, Rossetti HC, Cullum CM. Traumatic brain injury history is associated with earlier age of onset of Alzheimer disease. Clin Neuropsychol. 2016:1–14. doi: 10.1080/13854046.2016.1257069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoBue C, Wilmoth K, Cullum CM, Rossetti HC, Lacritz LH, Hynan LS, Hart J, Jr, Womack KB. Traumatic brain injury history is associated with earlier age of onset of frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2016;87(8):817–820. doi: 10.1136/jnnp-2015-311438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews PM, Cataldo AM, Kao BH, Rudnicki AG, Qin X, Yang JL, Jiang Y, Picciano M, Hulette C, Lippa CF, Bird TD, Nochlin D, Walter J, Haass C, Levesque L, Fraser PE, Andreadis A, Nixon RA. Brain expression of presenilins in sporadic and early-onset, familial Alzheimer's disease.[In Process Citation] Mol Med. 2000;6(10):878–891. [PMC free article] [PubMed] [Google Scholar]
- Max JE, Roberts MA, Koele SL, Lindgren SD, Robin DA, Arndt S, Smith WL, Jr, Sato Y. Cognitive outcome in children and adolescents following severe traumatic brain injury: influence of psychosocial, psychiatric, and injury-related variables. J Int Neuropsychol Soc. 1999;5(1):58–68. doi: 10.1017/s1355617799511089. [DOI] [PubMed] [Google Scholar]
- Max JE, Wilde EA, Bigler ED, Thompson WK, MacLeod M, Vasquez AC, Merkley TL, Hunter JV, Chu ZD, Yallampalli R, Hotz G, Chapman SB, Yang TT, Levin HS. Neuroimaging correlates of novel psychiatric disorders after pediatric traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 2012;51(11):1208–1217. doi: 10.1016/j.jaac.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell WL. Traumatic brain injury in the neonate, child and adolescent human: an overview of pathology. Int J Dev Neurosci. 2012;30(3):167–183. doi: 10.1016/j.ijdevneu.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Paholpak P, Lin A, Zhang JY, Teng E. Prevalence of Traumatic Brain Injury in Early Versus Late-Onset Alzheimer's Disease. J Alzheimers Dis. 2015;47(4):985–993. doi: 10.3233/JAD-143207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LS, Colella B, Mikulis D, Maller J, Green RE. Environmental enrichment may protect against hippocampal atrophy in the chronic stages of traumatic brain injury. Front Hum Neurosci. 2013;7:506. doi: 10.3389/fnhum.2013.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra JH, Tu Z, Apostolova LG, Green AE, Avedissian C, Madsen SK, Parikshak N, Toga AW, Jack CR, Jr, Schuff N, Weiner MW, Thompson PM. Automated mapping of hippocampal atrophy in 1-year repeat MRI data from 490 subjects with Alzheimer's disease, mild cognitive impairment, and elderly controls. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussard A, Bermudez P, Alain C, Tays W, Moreno S. Life-long music practice and executive control in older adults: An event-related potential study. Brain Res. 2016;1642:146–153. doi: 10.1016/j.brainres.2016.03.028. [DOI] [PubMed] [Google Scholar]
- Nestor SM, Rupsingh R, Borrie M, Smith M, Accomazzi V, Wells JL, Fogarty J, Bartha R. Ventricular enlargement as a possible measure of Alzheimer's disease progression validated using the Alzheimer's disease neuroimaging initiative database. Brain. 2008;131(Pt 9):2443–2454. doi: 10.1093/brain/awn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M, Wilson L, Verdile G, Lim A, Khan I, Moussavi Nik SH, Pursglove S, Chapman G, Martins RN, Lardelli M. Differential, dominant activation and inhibition of Notch signalling and APP cleavage by truncations of PSEN1 in human disease. Hum Mol Genet. 2014;23(3):602–617. doi: 10.1093/hmg/ddt448. [DOI] [PubMed] [Google Scholar]
- Nunnari D, Bramanti P, Marino S. Cognitive reserve in stroke and traumatic brain injury patients. Neurol Sci. 2014;35(10):1513–1518. doi: 10.1007/s10072-014-1897-z. [DOI] [PubMed] [Google Scholar]
- Peters ME. Traumatic brain injury (TBI) in older adults: aging with a TBI versus incident TBI in the aged. Int Psychogeriatr. 2016;28(12):1931–1934. doi: 10.1017/S1041610216001666. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Grafman J. Traumatic brain injury and late-life dementia. Handb Clin Neurol. 2015;128:711–722. doi: 10.1016/B978-0-444-63521-1.00044-3. [DOI] [PubMed] [Google Scholar]
- Poulin P, Zakzanis KK. In vivo neuroanatomy of Alzheimer's disease: evidence from structural and functional brain imaging. Brain Cogn. 2002;49(2):220–225. [PubMed] [Google Scholar]
- Rapoport M, Wolf U, Herrmann N, Kiss A, Shammi P, Reis M, Phillips A, Feinstein A. Traumatic brain injury, Apolipoprotein E-epsilon4, and cognition in older adults: a two-year longitudinal study. J Neuropsychiatry Clin Neurosci. 2008;20(1):68–73. doi: 10.1176/jnp.2008.20.1.68. [DOI] [PubMed] [Google Scholar]
- Reijneveld SA. The choice of a statistic for testing hypotheses regarding seasonality. Am J Phys Anthropol. 1990;83(2):181–184. doi: 10.1002/ajpa.1330830206. [DOI] [PubMed] [Google Scholar]
- Rencher AC. Methods of Multivariate Analysis. New York, NY: John Wiley & Sons, Inc; 2002. [Google Scholar]
- Ringman JM, Gylys KH, Medina LD, Fox M, Kepe V, Flores DL, Apostolova LG, Barrio JR, Small G, Silverman DH, Siu E, Cederbaum S, Hecimovic S, Malnar M, Chakraverty S, Goate AM, Bird TD, Leverenz JB. Biochemical, neuropathological, and neuroimaging characteristics of early-onset Alzheimer's disease due to a novel PSEN1 mutation. Neurosci Lett. 2011;487(3):287–292. doi: 10.1016/j.neulet.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe RK, Ziebell JM, Harrison JL, Law LM, Adelson PD, Lifshitz J. Aging with Traumatic Brain Injury: Effects of Age at Injury on Behavioral Outcome following Diffuse Brain Injury in Rats. Dev Neurosci. 2016;38(3):195–205. doi: 10.1159/000446773. [DOI] [PubMed] [Google Scholar]
- Sakka P. Brain reserve and cognitive training in the elderly. Adv Exp Med Biol. 2015;821:125–126. doi: 10.1007/978-3-319-08939-3_16. [DOI] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Troyanskaya M, Steinberg JL, Goldstein FC, Mao H, Levin HS. Effects of severity of traumatic brain injury and brain reserve on cognitive-control related brain activation. J Neurotrauma. 2009;26(9):1447–1461. doi: 10.1089/neu.2008.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider EB, Sur S, Raymont V, Duckworth J, Kowalski RG, Efron DT, Hui X, Selvarajah S, Hambridge HL, Stevens RD. Functional recovery after moderate/severe traumatic brain injury: a role for cognitive reserve? Neurology. 2014;82(18):1636–1642. doi: 10.1212/WNL.0000000000000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk T, Beare R, Crossley L, Rogers K, Emsell L, Catroppa C, Beauchamp M, Anderson V. Cavum septum pellucidum in pediatric traumatic brain injury. Psychiatry Res. 2013;213(3):186–192. doi: 10.1016/j.pscychresns.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Sobral M, Pestana MH, Paul C. Cognitive reserve and the severity of Alzheimer's disease. Arq Neuropsiquiatr. 2015;73(6):480–486. doi: 10.1590/0004-282X20150044. [DOI] [PubMed] [Google Scholar]
- Stocchetti N, Paterno R, Citerio G, Beretta L, Colombo A. Traumatic brain injury in an aging population. J Neurotrauma. 2012;29(6):1119–1125. doi: 10.1089/neu.2011.1995. [DOI] [PubMed] [Google Scholar]
- Sumowski JF, Chiaravalloti N, Krch D, Paxton J, Deluca J. Education attenuates the negative impact of traumatic brain injury on cognitive status. Arch Phys Med Rehabil. 2013;94(12):2562–2564. doi: 10.1016/j.apmr.2013.07.023. [DOI] [PubMed] [Google Scholar]
- Testa JA, Malec JF, Moessner AM, Brown AW. Outcome after traumatic brain injury: effects of aging on recovery. Arch Phys Med Rehabil. 2005;86(9):1815–1823. doi: 10.1016/j.apmr.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Dutton RA, Chiang MC, Leow AD, Sowell ER, De Zubicaray G, Becker JT, Lopez OL, Aizenstein HJ, Toga AW. Tracking Alzheimer's disease. Ann N Y Acad Sci. 2007;1097:183–214. doi: 10.1196/annals.1379.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn JD, Toga AW. Databasing the Aging Brain. In: Jagust W, D'Esposito M, editors. Imaging the Aging Brain. Oxford: Oxford University Press; 2009. [Google Scholar]
- Van Horn JD, Toga AW. Multisite neuroimaging trials. Curr Opin Neurol. 2009;22(4):370–378. doi: 10.1097/WCO.0b013e32832d92de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa RA, Grados M, Slomine B, Herskovits EH, Thompson RE, Salorio C, Christensen J, Wursta C, Riddle MA, Gerring JP. Neuroimaging correlates of anxiety after pediatric traumatic brain injury. Biol Psychiatry. 2004;55(3):208–216. doi: 10.1016/s0006-3223(03)00708-x. [DOI] [PubMed] [Google Scholar]
- Walther A, Nili H, Ejaz N, Alink A, Kriegeskorte N, Diedrichsen J. Reliability of dissimilarity measures for multi-voxel pattern analysis. Neuroimage. 2016;137:188–200. doi: 10.1016/j.neuroimage.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Weiner MW, Friedl KE, Pacifico A, Chapman JC, Jaffee MS, Little DM, Manley GT, McKee A, Petersen RC, Pitman RK, Yaffe K, Zetterberg H, Obana R, Bain LJ, Carrillo MC. Military risk factors for Alzheimer's disease. Alzheimers Dement. 2013;9(4):445–451. doi: 10.1016/j.jalz.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR, Jagust W, Liu E, Morris JC, Petersen RC, Saykin AJ, Schmidt ME, Shaw L, Siuciak JA, Soares H, Toga AW, Trojanowski JQ. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2012;8(1 Suppl):S1–68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, Veitch DP, Hayes J, Neylan T, Grafman J, Aisen PS, Petersen RC, Jack C, Jagust W, Trojanowski JQ, Shaw LM, Saykin AJ, Green RC, Harvey D, Toga AW, Friedl KE, Pacifico A, Sheline Y, Yaffe K, Mohlenoff B. Effects of traumatic brain injury and posttraumatic stress disorder on Alzheimer's disease in veterans, using the Alzheimer's Disease Neuroimaging Initiative. Alzheimers Dement. 2014;10(3 Suppl):S226–235. doi: 10.1016/j.jalz.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Res Rev. 2004;3(4):369–382. doi: 10.1016/j.arr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Bigler ED, Hunter JV, Fearing MA, Scheibel RS, Newsome MR, Johnson JL, Bachevalier J, Li X, Levin HS. Hippocampus, amygdala, and basal ganglia morphometrics in children after moderate-to-severe traumatic brain injury. Dev Med Child Neurol. 2007;49(4):294–299. doi: 10.1111/j.1469-8749.2007.00294.x. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Hunter JV, Newsome MR, Scheibel RS, Bigler ED, Johnson JL, Fearing MA, Cleavinger HB, Li X, Swank PR, Pedroza C, Roberson GS, Bachevalier J, Levin HS. Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. J Neurotrauma. 2005;22(3):333–344. doi: 10.1089/neu.2005.22.333. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Merkley TL, Bigler ED, Max JE, Schmidt AT, Ayoub KW, McCauley SR, Hunter JV, Hanten G, Li X, Chu ZD, Levin HS. Longitudinal changes in cortical thickness in children after traumatic brain injury and their relation to behavioral regulation and emotional control. Int J Dev Neurosci. 2012;30(3):267–276. doi: 10.1016/j.ijdevneu.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolz R, Julkunen V, Koikkalainen J, Niskanen E, Zhang DP, Rueckert D, Soininen H, Lotjonen J. Multi-method analysis of MRI images in early diagnostics of Alzheimer's disease. PLoS ONE. 2011;6(10):e25446. doi: 10.1371/journal.pone.0025446. [DOI] [PMC free article] [PubMed] [Google Scholar]