Abstract
The orbits and eyes of modern humans are situated directly below the frontal lobes and anterior to the temporal lobes. Contiguity between these orbital and cerebral elements could generate spatial constraints, and potentially lead to deformation of the eye and reduced visual acuity during development. In this shape analysis we evaluate whether and to what extent covariation exists between ocular morphology and the size and spatial position of the frontal and temporal areas in adult modern humans. Magnetic resonance imaging (MRI) was used to investigate patterns of variation among the brain and eyes, while computed tomography (CT) was used to compare cranial morphology in this anatomical region among modern humans, extinct hominids and chimpanzees. Seventeen landmarks and semi‐landmarks that capture the outline of the eye, frontal lobe, anterior fossa/orbital roof and the position of the temporal tips were sampled using lateral scout views in two dimensions, after projection of the average grayscale values of each hemisphere, with midsagittal and parasagittal elements overlapped onto the same plane. MRI results demonstrated that eye position in adult humans varies most with regard to its horizontal distance from the temporal lobes and, secondly, in its vertical distance from the frontal lobes. Size was mainly found to covary with the distance between the eye and temporal lobes. Proximity to these cerebral lobes may generate spatial constraints, as some ocular deformation was observed. Considering the CT analysis, modern humans vary most with regard to the orientation of the orbits, while interspecific variation is mainly associated with separation between the orbits and endocranial elements. These findings suggest that size and position of the frontal and temporal lobes can affect eye and orbit morphology, though potential effects on eye shape require further study. In particular, possible effects of these spatial and allometric relationships on the eye and vision should be examined using ontogenetic samples, vision parameters such as refractive error in diopters, and three‐dimensional approaches that include measures of extraocular soft tissues within the orbit.
Keywords: eyeball, frontal lobes, functional craniology, geometric morphometrics, orbits, temporal lobes
Introduction
The unique craniofacial configuration of modern humans with a globular vault and reduced facial block (Lieberman et al. 2002) places the orbits directly below and anterior to the frontal and temporal lobes, respectively. This anatomical proximity involves structural and functional interactions between facial and neurocranial elements during developmental and evolutionary morphogenesis (Moss & Young, 1960; Bruner, 2007). In other words, spatial and volumetric reorganization of the frontal and temporal lobes may induce changes in the orbital region, given that integration between the brain and face is mediated by the lateral cranial base (Lieberman et al. 2000; Bastir & Rosas, 2006; Bruner & Ripani, 2008; Neaux et al. 2013a), and because changes in facial morphology are associated with basicranial variation. Furthermore, the anterior cranial base and the orbits are structurally aligned with the mandibular ramus, and rotate as an entire anatomical block during growth and development (Lieberman et al. 2000; McCarthy & Lieberman, 2001). According to Bastir & Rosas (2016), facial rotation is associated with an elongation of lateral cranial base structures and a change in their overall orientation, while facial reduction and retraction are related to the position of the sphenoid wings relative to that of the sphenoid body.
Compared with Neanderthals and Middle Pleistocene humans, modern humans have smaller, narrower and vertically shorter faces that are flatter and more retracted (Bastir & Rosas, 2016). It remains to be established whether modern human facial morphology is derived, or rather, is a primitive condition (Arsuaga et al. 1999; Bermúdez de Castro et al. 2010; Freidline et al. 2013; Lacruz et al. 2015; Stringer, 2016). Nonetheless, this unique morphological configuration, characterized by reduced facial block and expanded braincase, is highly specific to our species.
The frontal lobes of modern humans underwent an enlargement, which is proportional to the general increase in brain size according to the scaling rules of apes (Semendeferi et al. 1997; Semendeferi & Damasio, 2000). However, at least in modern humans and Neanderthals, there was a change in frontal proportions, in which the frontal lobes became wider relative to more archaic hominids (Bruner & Holloway, 2010). Such lateral redistribution of the cortical mass can be a morphogenetic consequence of vertical constraints imposed by the superposition of the brain and orbits (Bruner, 2004).
Considering the temporal lobes, these are larger than expected in humans, both in terms of absolute and relative volume (Rilling & Seligman, 2002). The middle cranial fossae that house the temporal lobes are also wider (Lieberman et al. 2002) and more axially elongated (Bastir et al. 2008) in modern humans compared with other human species. This antero‐posterior elongation has displaced the temporal poles into a more anterior position relative to the optic canal (Bastir et al. 2008), which taken together, may have influenced the rotation and positioning of facial structures (Lieberman et al. 2000, 2002).
As a developmentally connected part of the facial skeleton (Waitzman et al. 1992), the orbits follow the same evolutionary trends as the face. Though by contrast, the eye is dimensionally (Scammon & Armstrong, 1925) and genetically (Mak et al. 2006) associated with the brain. Nonetheless, there is a strong correlation between the size of the orbital aperture and the transverse diameter of the eye among primates. However, this relationship differs between suborders, as there is a greater disparity between the two measures in haplorhines, where larger‐sized anthropoids exhibit larger eye diameters relative to orbital aperture size (Kirk, 2006). Orbital aperture size is also related to activity pattern in primates, in which nocturnal species generally have larger orbital apertures, despite similar eye sizes between diurnal and nocturnal primates (Kirk, 2006). Instead, orbital aperture size might be more associated with variation in eye shape, i.e. size of the cornea relative to size of the eye (Kirk, 2006; Ross & Kirk, 2007). For instance, nocturnal primates have larger corneal diameters relative to the transverse diameter of the eye and its focal length, while diurnal primates, and especially anthropoids, have smaller corneas relative to eye size (Kirk, 2004; Ross & Kirk, 2007), indicating that the relationship between eye and orbit size depends on both primate phylogeny and activity pattern.
In terms of volume, according to Schultz (1940), size of the eye does not directly determine size of the orbit, as both have different but somewhat complimentary patterns of morphogenesis during ontogeny. Additionally, both the eye and orbit have an opposite allometric relationship to body size, in which larger‐bodied primates have proportionately small eyes in relatively large orbits, while smaller primates have eyes that occupy a larger proportion of the orbital cavity by comparison. In spite of this general allometric pattern, humans have a larger eye to orbit ratio than what would be expected of a primate of our body size. For example, the eye occupies about 32% of orbital volume in adult male humans, but only about 21% of orbital volume in adult male chimpanzees; and this dichotomy exists in light of the fact that chimp eye volume can occasionally reach values similar to that of humans (Schultz, 1940).
Thus, the structural relationship between the eye and adjacent anatomical elements must be different in humans and chimpanzees, which is particularly evident from looking at the spatial relationship among the orbit, ocular tissues, facial, neurocranial and cerebral structures in the sagittal profile of these two species (Fig. 1). Compared with modern humans, chimps exhibit more separation between ocular and neural tissues, with eyes that occupy a smaller proportion of orbital volume, and which reside in a far more anterior and superior position relative to the brain, and to the frontal lobes in particular. Moreover, in chimps and gorillas the orbital roof is primarily made up of the browridge, and its antero‐posterior development is negatively correlated with the neuro‐orbital angle, or the angle between the orbital axis and the anterior cranial vault (Ravosa, 1991). By contrast, in modern humans this feature has largely disappeared (Moss & Young, 1960), and the orbital roof consists of only a thin sliver of bone separating highly adjacent ocular and cerebral tissues. In fact, the thinnest portion of the anterior cranial fossa – averaging between 0.66 and 1.13 mm in adult humans – resides in this area of the orbital roof directly above the eyes (Lang, 2012). This extremely thin portion of the anterior cranial base, which occasionally shows patches of rarefaction and entire gaps in the bone, must now provide support for both intraorbital soft tissues and the wider and more anteriorly located frontal lobes of modern humans. By comparison, archaic humans such as H. ergaster/erectus maintained a more ape‐like configuration, with greater separation between facial structures and the braincase. However, because the orbits have become situated directly beneath the anterior cranial fossae in modern humans and Neanderthals, we might expect a more direct interaction between the neural and ocular tissues in these two species (Bruner et al. 2014).
Figure 1.
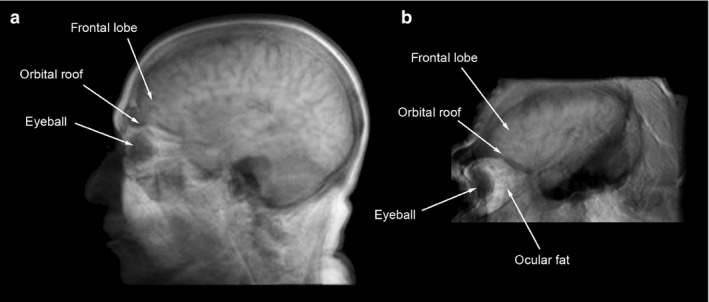
Comparison between modern human (a) and chimpanzee (b), regarding the anatomical relationship between the eyeball and the frontal and temporal lobes, as seen in the lateral scout view of the sagittal and parasagittal scans (see Materials and methods).
Masters (2012) hypothesized that the location of the prefrontal cortex directly above, and the reduced and retracted facial anatomy directly below the orbits in modern humans could result in spatial conflicts among cerebral, ocular and craniofacial structures. In this way, the coalescence of neuro and viscerocranial components during recent hominid evolution may act to constrain development of the orbit and internal ocular tissues during ontogeny, and potentially lead to deformation of the eye, and consequent visual defects such as juvenile‐onset myopia. Supero‐inferior pressure resulting from structural constraints imposed by the frontal lobes above, and a more anterior projection of the temporal lobes behind the orbits (Bastir et al. 2008), would be expected to cause ocular distortion, and specifically axial elongation of the eye, as it shifts more toward the narrowing concave rim of the anterior orbital margins in recent human evolution (Bruner et al. 2014). The disparate impact of these changes would also be expected to vary with population‐specific craniofacial variation, and particularly among certain Far East populations, where the orbits have increased in height, but have decreased in width and overall volume since the Holocene in this region (Brown & Maeda, 2004; Wu et al. 2007).
Masters et al. (2015) analyzed the relationship among eye, orbit, and frontal and occipital lobe volumes, and found that ocular and orbital volumes are slightly more associated with frontal lobe volumes than with those of the visual cortex of the occipital lobes. This indicates that ocular and orbital sizes might be more reflective of the structural constraints imposed by the frontal lobe than of the functional demands of the visual system. The study also showed that the correlation between ocular and orbital volume is low, which corroborates the results of previous research demonstrating independent ontogenetic growth trajectories in these structures (Schultz, 1940; Todd et al. 1940; Weale, 1982; Waitzman et al. 1992; Hoyte, 1997), and that orbital size is largely independent of eye size in humans, regardless of how large the myopic eye grows within it (Chau et al. 2004; Masters, 2012).
Ocular growth can induce morphological changes on immature osseous aspects of the orbit during prenatal and early postnatal ontogeny (Wagner et al. 2000). However, recent research shows that following the first year of postnatal life, and as the various anatomical components comprising the orbit begin to ossify, no significant change is observable in size or shape of the orbital aperture (Barbeito‐Andrés et al. 2016). In fact, during later stages of postnatal development, when the eye continues to grow rapidly but after the orbit has already ossified, the effects of added ocular growth on orbital size are negligible (Washburn & Detwiler, 1943; Hoyte, 1997). This is further indicated by research showing that a 15% reduction in size of the orbital aperture occurs in children enucleated prior to the age of 5 years, but that enucleation produces no appreciable change in this feature at 9 years of age and older (Taylor, 1939).
As a result of this disconnect between ocular and orbital size following the early stages of postnatal life, a disproportionate enlargement of the eyeball – rather than increasing orbital size – may result in its compression against adjacent soft‐tissues circumscribed by the orbital walls. Additionally, because of the shape of the orbit and constraints imposed by cerebral, neurocranial and viscerocranial structures around it, added ocular growth would be expected to result in distortion toward the same eye form common among myopes (Masters et al. 2015), which is generally an overly large and axially elongated eye with a steeper cornea and, subsequently, a higher axial length/corneal radius of curvature ratio relative to emmetropes (Grosvenor & Goss, 1998; Lam et al. 1999, 2008; Atchison et al. 2004; Llorente et al. 2004; Stone & Flitcroft, 2004; Dirani et al. 2006; Ip et al. 2007; Foo et al. 2016).
In the current study, we use geometric morphometrics analysis to investigate the relationship among these cerebral, neurocranial and visual components of the skull, examining variation in orbital and ocular anatomy relative to the size and spatial position of the anterior frontal and temporal areas. This research consists of two parts; firstly we assess the spatial relationship between the eye and brain in a sample of magnetic resonance imaging (MRI) taken from adult modern humans and, secondly, we examine the relationship between the orbit and braincase using computed tomography (CT) of adult modern humans and fossil hominid specimens. The principal aim of this research is to evaluate whether spatial variation of the frontal and temporal lobes may constrain the orbital area and, in the case of modern humans, if these patterns of cerebral and craniofacial variation are associated with size and anatomical position of the eye.
Materials and methods
MRI sample
The adult modern human MRI sample comprises 63 individuals (36 female, 27 male), with ages ranging from 19 to 80 years (mean age 45 ± 15 years). The voxel size for each image is isometric and measures 1.0 × 1.0 × 1.0 mm. These MR images were obtained as part of the International Consortium for Brain Mapping (ICBM) (Mazziotta et al. 2001), and were provided by the Laboratory of Neuro Imaging (LONI) at the University of Southern California.
Using this MRI sample, relationships among orbital, ocular and fronto‐temporal profiles were examined in two‐dimensions (2D) as seen in the sagittal plane. Sagittal spatial organization is relevant to evaluate interactions between vertical (frontal lobes/orbits) and longitudinal (temporal lobes/orbits) features. Lateral scout views were created by overlapping the midsagittal and parasagittal elements through projection of the average intensity of the whole‐stack grayscale values onto the same 2D plane. Two images representing each cerebral hemisphere were generated for each subject and were used to sample a set of 17 landmarks and semi‐landmarks (Fig. 2a), which capture the outline of the eye, frontal lobe, anterior fossa (AF)/orbital roof and the position of the temporal tips (anterior‐most point of the temporal lobes, sensu Bastir et al. 2008).
Figure 2.
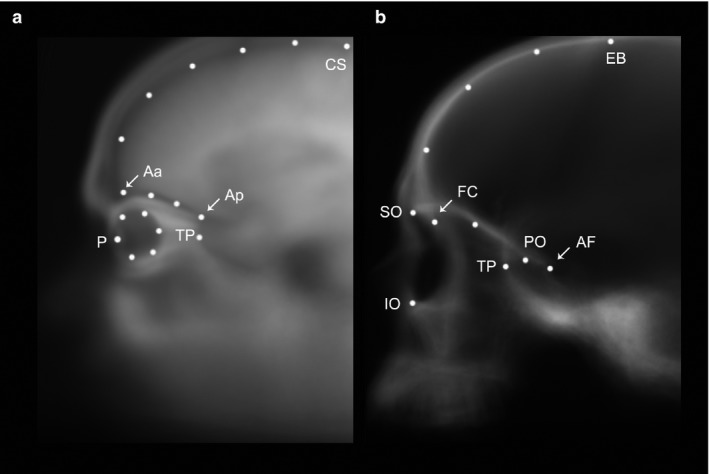
Landmark set chosen to sample fronto‐orbital morphology on the magnetic resonance imaging (MRI) sample (a), and on the computed tomography (CT) sample (b). On the MRI sample landmarks are: central sulcus (CS); anterior point of the anterior fossa (Aa); posterior point of the anterior fossa (Ap); pupil (P); temporal pole (TP); and 12 equidistant semi‐landmarks sampling the curvature of the frontal profile, anterior fossa and eye. The landmarks on the CT sample are: endobregma (EB); foramen caecum (FC); posterior end of the anterior fossa (AF); temporal pole (TP); superior (SO) and inferior (IO) orbit aperture; posterior orbital (PO) point on the aperture of the optic nerve canal; and 3 and 1 equidistant semi‐landmarks sampling the curvature of the frontal profile and orbital roof, respectively.
The landmark configuration was then analyzed using geometric morphometrics (Bookstein, 1991; Zelditch et al. 2004), carried out by first superimposing this landmark configuration to the same location, scale and orientation by Procrustes registration, which minimizes the distance between corresponding landmarks. The resulting shape coordinates were then analyzed using principal component analysis (PCA) in order to examine patterns of shape variation. Independent PCA on right and left sides were strongly correlated (R = 0.88, 0.85, 0.90 for PC1, PC2 and PC3, respectively), so the two sides were averaged and the final PCA was computed on the individual mean shape.
To investigate the correlation between shape and size, total shape and each significant PC were regressed against cortical volume of the frontal and temporal lobes, the ratio of frontal and temporal lobe volumes (F/T) and centroid size (CS). Results were considered significant at the 5% level. These cerebral lobe volumes had been previously generated (Masters et al. 2015) with LONI Brain Parser 56 ROI (Tu et al. 2008), which uses a pipeline workflow with automatic segmentation based on standard parcellation schemes (Dinov et al. 2009).
CT sample
The CT sample consists of 30 scans of adult modern humans of both sexes (15 females and 15 males) and distinct geographical origins (resolution between 0.214 and 0.700 mm), three adult chimpanzee specimens (voxel size 0.488 × 0.488 × 0.625 mm), and fossil specimens, which include Bodo [estimated age 600 thousand years (ka); Middle Awash, Ethiopia; voxel size 0.49023 × 0.49023 × 1.0 mm], Broken Hill 1 (estimated age 300–125 ka; Kabwe, Zambia; isometric voxel 0.7 × 0.7 × 0.7 mm) and Gibraltar 1 (estimated age 70–45 ka; Forbes Quarry, Gibraltar; isometric voxel 1.0 × 1.0 × 1.0 mm). Bodo and Broken Hill 1 are generally attributed to H. heidelbergensis/rhodesiensis, and Gibraltar 1 to H. neanderthalensis. With the exception of Bodo, which was acquired from the University of Vienna, the remaining specimens were obtained from the NESPOS database (Neanderthal Museum, Mettmann, Germany).
The CT sample was analyzed with the same procedure used for the MRI sample, which involved using lateral scout views in 2D, after projection of the average grayscale values of each hemisphere. The landmark set was comparable to that of the MRI sample, and comprised 11 landmarks capturing the lateral profile of the orbits, the frontal pole of the endocranial cavity and the anterior‐most point of the temporal tips (Fig. 2b). Left and right sides for each individual were sampled separately (with the exception of Bodo, which retained only one complete orbit), and the two hemispheres were averaged together for the final analysis.
A first PCA was conducted on the modern human sample to assess within‐species variation, and a second PCA was computed, which included the chimpanzee and fossil human specimens, in order to evaluate principal phylogenetic differences among them. Because the frontal bone of Gibraltar 1 is broken it could not be included in this broader analysis. However, given that its orbits are complete it was possible to carry out a thin‐plate spline comparison using only landmarks on the orbital region. This analysis allows for visualization of major shape changes through a pairwise comparison in cases of reduced sample size. The average shape of each species was also contrasted with the average of modern humans using pairwise comparisons, and Gibraltar 1 was further examined in a pairwise comparison with the average shape of H. heidelbergensis, a phylogenetically closer species. For both the MRI and CT samples, 2D images were generated with imagej 1.48v (Schneider et al. 2012), the landmarks were digitized on tpsDig2 (Rohlf, 2013), and the geometric morphometrics analysis was performed on morphoj (Klingenberg, 2011) and past 2.17c (Hammer et al. 2001).
Results
MRI shape analysis
According to the PCA (Fig. 3a–f), the first four components explain 77% of the total variance, while the subsequent PCs are below the threshold for random variation. PC1 (Fig. 3c) accounts for 30% of the variance and is associated with a retraction/projection of the eye relative to the temporal pole. PC2 (19% of variation; Fig. 3d) describes vertical separation/proximity of the eye and the AF. In PC1 and PC2, increased ocular protrusion is apparently associated with a rounder eyeball, while eyes that are closer to the temporal pole (PC1) or to the AF (PC2) are somewhat shorter horizontally and more vertically elongated. PC3 (18%; Fig. 3e) describes the dimensions and spatial relationship between the eye and AF, in which an antero‐posteriorly elongated AF is associated with larger eyes, that are also closer to the orbital roof. PC4 (10%; Fig. 3f) illustrates dolichocephaly‐brachycephaly neurocranial proportions, with the former morphotype associated with a larger eyeball, and increased distance between the eye and temporal pole. PC1 and PC4, despite describing distinct shape changes, mainly denote the horizontal distance between the eye and temporal lobes, while PC2 and PC3 describe similar changes related to the vertical distance between the eye and the AF/orbital roof. A discriminant analysis reveals that no significant differences exist between males and females with regard to shape variation.
Figure 3.
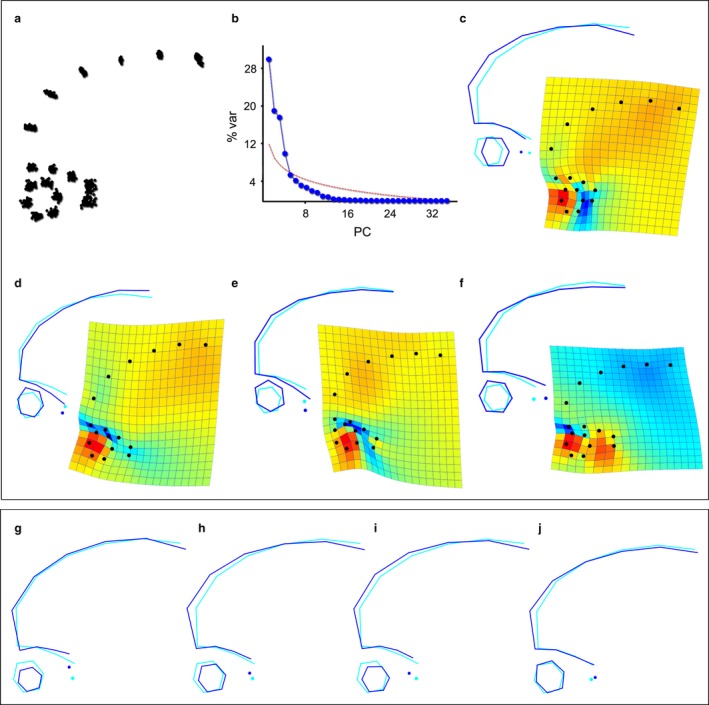
Shape variation within modern humans according to the magnetic resonance imaging (MRI) sample (a–f) and regression with size variables (g–j). After Procrustes superimposition, the distance between correspondent landmarks is the difference in shape (a), and the principal component analysis (PCA) (b–f) shows the main vectors of shape changes. The first four principal components (PCs) are above the standard threshold of statistical significance (b, red line), while the subsequent PCs account for < 6% of variance. PC1 (c: 30%) is associated with the spatial relationship between the eye and the temporal pole; PC2 (d: 19%) and PC3 (e: 18%) describe the distance between the eye and the fossa; and PC4 (f: 10%) deals with the antero‐posterior dimensions of the entire structure. Increasing CS (g) is associated with greater separation between the AF and eye, and a decrease in eye size. Increasing cortical volume of the frontal (h) and temporal (i) lobes is associated with a bulging frontal and a slight decrease in eye size, separation from the anterior fossa (AF), but closer proximity to the temporal pole. Lastly, an increase in the F/T ratio (j) is primarily associated with a bulging of the frontal profile.
Cortical volume of the frontal and temporal lobes are correlated (Pearson's R = 0.78, P < 0.001), and both lobe volumes are positively correlated with CS (frontal: R = 0.76; P < 0.001; temporal: R = 0.66; P < 0.001), though the relationship with the frontal lobe is stronger, as this region is more represented in the landmark set. Additionally, males are larger than females across all metrics considered in this analysis (t‐test, P < 0.001). Table 1 shows results from the regression of shape variables with CS and cortical volumes, and indicates that the three size measures are significantly correlated with whole shape changes, but that they explain a rather low percentage of the total variation (CS: 8%; frontal volume: 8%; temporal volume: 7%). Considering the correlation between size and shape, it can be seen that CS is associated with a bulging frontal profile, and a smaller, vertically shorter eyeball that is more separated from the anterior part of the AF (Fig. 3g). Regarding each of the principal components individually, CS is correlated with PC2 (R 2 = 0.14) and PC3 (R 2 = 0.11), denoting further separation of the eye from the AF in larger individuals. An increase in cortical volume of the frontal and temporal lobes is associated with a bulging of the frontal profile, as well as smaller eyes that reside closer to the temporal poles (Fig. 3h,i). These shape changes are reflected in the correlation of both lobes with PC1 (frontal: R 2 = 0.19; temporal: R 2 = 0.15) and in the correlation of frontal volume with PC2 (R 2 = 0.08). The ratio frontal/temporal (Fig. 3j) explains only 3% of whole shape variation, and is mostly associated with a dolichocephalic morphology in individuals with much larger frontals relative to their temporal lobe volumes. The ratio of F/T is not significantly correlated with any PC.
Table 1.
Correlation of the whole shape and significant PCs (1–4) with Centroid size, frontal and temporal volumes, and the ratio of frontal to temporal volume
| Centroid size | Frontal volume | Temporal volume | Ratio F/T | |||||
|---|---|---|---|---|---|---|---|---|
| % predicted | P‐value | % predicted | P‐value | % predicted | P‐value | % predicted | P‐value | |
| Whole shape | 7.83 | < 0.0001 | 8.19 | 0.0002 | 6.52 | 0.0005 | 3.31 | 0.052 |
| PC1 | 5.07 | 0.080 | 19.2 | 0.0006 | 14.94 | 0.002 | 1.73 | 0.306 |
| PC2 | 13.95 | 0.003 | 8.16 | 0.025 | 2.41 | 0.221 | 4.48 | 0.094 |
| PC3 | 10.54 | 0.010 | 0.73 | 0.511 | 5.77 | 0.058 | 4.58 | 0.093 |
| PC4 | 5.25 | 0.078 | 0.15 | 0.766 | 0.55 | 0.560 | 3.55 | 0.140 |
P‐values in italics indicate statistical significance (P<0.05).
CT shape analysis
When considering modern humans only, the first four PCs are above the threshold of random variation, and together they explain 75% of the total variance (Fig. 4). PC1 (34% of the total variance; Fig. 4c) distinctively characterizes the morphospace, describing the orientation/inclination of the orbits relative to the frontal profile; PC2 (17%; Fig. 4d) regards the antero‐posterior size of the orbit, which is shorter in individuals with a bulging superior portion of the frontal profile; PC3 (14%; Fig. 4e) deals with alignment between the orbital margins and the bulging of the anterior portion of the frontal profile; and PC4 (10%; Fig. 4f) with supero‐inferior (vertical) proportions of the orbital aperture.
Figure 4.
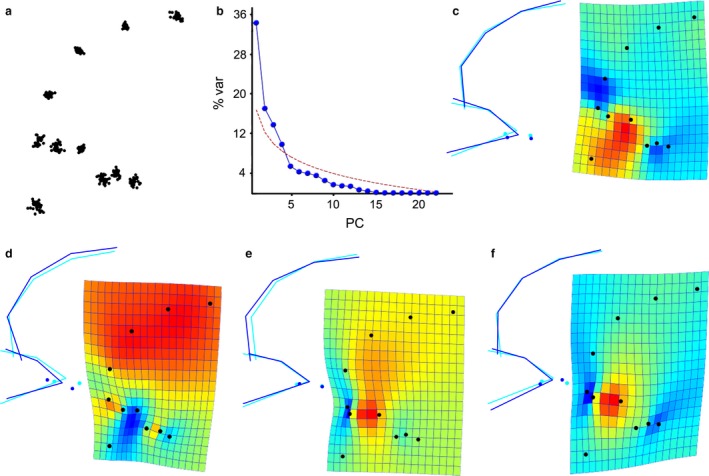
Shape variation within modern humans according to the landmark configuration applied to the computed tomography (CT) sample (a). The majority of variation is explained by the first four vectors (b). Principal component (PC)1 (c: 34%) is associated with orientation of the orbit relative to the frontal profile; PC2 (d: 17%) describes anteroposterior size of the orbit and bulging of the frontal profile; PC3 (e: 14%) is associated with alignment of the superior orbital margin with the frontal profile; and PC4 (f: 10%) deals with alignment of the orbit with the frontal, and the vertical and horizontal proportions of the orbit.
After including chimpanzees and the two H. heidelbergensis specimens in the sample, PC1 (70%), which clearly separates the three species based on length and height of the orbits, is the only vector representing significant shape changes (Fig. 5). Most notably, chimpanzees have far more protruding orbits that are located anterior and superior to the temporal tips. By contrast, the temporal poles of modern humans are closer to the orbits, and are situated in a more superior position relative to the optic canal, while the orbits themselves are antero‐posteriorly shorter and are tucked up under the frontal region. The two H. heidelbergensis specimens exhibit a morphology that falls halfway between that of modern humans and the chimpanzee. Shape variation described by PC2 (10%) is similar to that of PC1 in modern humans, in that it is mainly associated with orientation of the orbits. It primarily describes variation within modern humans, with chimps falling close to the mean modern human shape. However, the fossils (and especially Broken Hill 1) can be seen to display more inferiorly oriented orbits by comparison.
Figure 5.
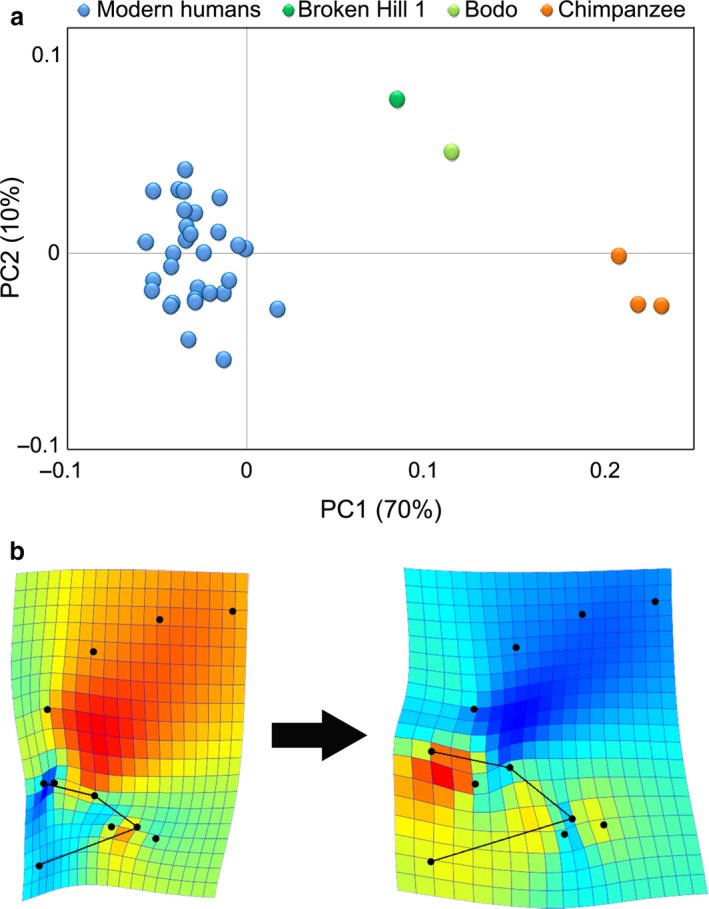
Shape variation within the whole computed tomography (CT) sample. When introducing the fossil human and chimpanzee specimens, two principal components (PCs) explain the largest percentage of variation (a). PC1 is the main vector of variation, describing the dimensions of the orbit and its alignment with the frontal profile (b).
Pairwise comparisons of average orbital shape between these species demonstrate differences similar to the variation described by PC1 (Fig. 6). For instance, compared with chimpanzees and H. heidelbergensis, modern humans have anteroposteriorly shorter orbits, with superior margins closer to the foramen caecum, and a temporal pole that projects more forward relative to the posterior orbit. As expected, these shape changes are more overt between modern humans and chimpanzees. However, the same general shape pattern is also observable when comparing modern humans with Gibraltar 1, albeit to a somewhat lesser extent. In turn, Gibraltar 1 differs from the average of Bodo and Broken Hill 1 by having a more anteriorly situated temporal pole and a foramen caecum in a different position relative to the orbital roof, suggesting that Neanderthal orbital morphology may be intermediate between that of modern humans and H. heidelbergensis.
Figure 6.
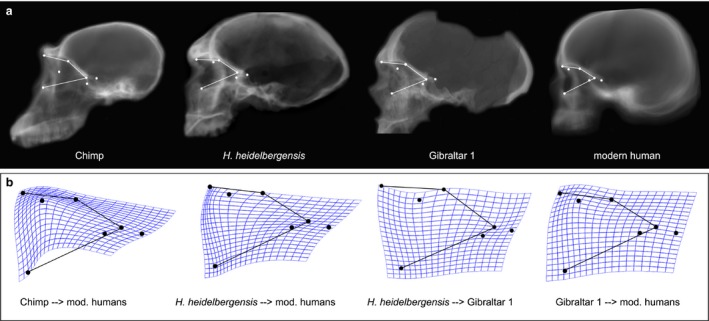
Between‐species comparisons of average orbital shape. Upper row (a), from left to right: average orbital contour of chimpanzees, H. heidelbergensis, Gibraltar 1 representing H. neanderthalensis, and modern humans. Lower row (b): thin‐plate spline deformation grids of the species average deformed into modern humans (the first three) and of modern humans’ average deformed into Gibraltar 1.
Discussion
The orbits and eyes of modern humans are located directly below the frontal lobes and anterior to the temporal lobes, which together with facial reduction and retraction, has been suggested as a factor contributing to improper ocular development and the potential generation or amplification of vision problems, as a result of competition among structural and functional features in this confined region of the human skull (Masters, 2012). In the present study, we describe variation in the orbit and eye relative to the spatial position of the frontal and temporal lobes among adult modern humans as seen in the sagittal scout view, using both MRI and CT data. The MRI sample allows the inclusion of the eye in this morphological analysis, while the CT analysis allows for the inclusion of human fossils and chimpanzees, which can aid in inferring evolutionary trends with regard to human fronto‐orbital spatial organization.
Brain‐eye spatial relationship
The main pattern of morphological variation in adult humans is associated with the antero‐posterior (horizontal) position of the eye relative to the temporal lobes. Individuals with a greater distance between the eyes and temporal tips tend to have apparently rounder eyes, which are more anteriorly projected beyond the frontal profile (protrusion). Conversely, in individuals with more posteriorly located eyes, the space between the eye and temporal poles is reduced, and the frontal outline is more curved. In this case, a possible constraint is associated with spatial reduction between the eye and middle cranial fossa, which may actually involve an antero‐posterior compression of the eye, as it appears taller and shorter when positioned nearer to the temporal tips.
The successive component of variation is due to the supero‐inferior (vertical) position of the eye relatively to the frontal lobes. This vertical change is associated with the second and third PCs, which are rather similar in terms of variance explained, involving changes in eye shape and size, respectively. Also in this case, the distance between the eye and the frontal lobes matches minor changes in ocular form. Thus, this analysis has succeeded in separating the two main morphological factors, namely the distance between the eye and the middle fossa, and the distance between the eye and the anterior fossa, and suggests that both patterns of variation are associated with minor changes in the geometry of the eye. The fact that the distance from the temporal lobes represents the main morphological axis of variation may be the result of less stringent constraints in this direction, given that the orbital margins represent the only opening of the orbital cavity. In fact, in terms of structure and development, horizontal changes in the position of the eye are mainly restricted by the posterior limit with the temporal lobes, while vertical changes are limited by the frontal lobes above, and the middle face below.
Frontal and temporal lobe volumes are correlated, which is in agreement with Allen et al. (2002), who found a significant high correlation between the volumes of these districts (left: 0.8; right: 0.7). Indeed, our results show that the regression of both lobe volumes against shape gives the same outcome, explaining about 7–8% of the variation (15–19% when considering only the first PC). With increased ‘fronto‐temporal’ size, the eye is positioned more posteriorly, nearer to the temporal lobe, which could involve some spatial conflicts. In this case, the position of the eye, but not its general shape, seems to vary with size of the fronto‐temporal lobes. These results only partially support Masters’ (2012) hypothesis, in that size and position of the frontal and temporal lobes may restrain size and position of the eye in modern humans, eventually leading to certain visual defects.
Myopic eyes tend to be larger and more axially elongated than emmetropic eyes (Atchison et al. 2004; Stone & Flitcroft, 2004; Goldschmidt & Fledelius, 2011), and it is well‐established that loss of vision occurs as a result of these changes to ocular size and shape in association with the development of myopic refractive error in humans (Holladay et al. 1991; Zhao et al. 2000; He et al. 2004; Remón et al. 2006). In the current study, we found that the distance between the eye and these cerebral lobes is associated with changes in eye form, but the size of the lobes is only associated with ocular proximity/distance, and does not involve any deformation of the eye outline. Hence, although brain size has an effect on eye position, it is not sufficient in and of itself to induce ocular deformation. We must also assume that the distance between the eye and brain depends upon additional factors other than brain size, which can induce antero‐posterior flattening of the eyeball when it approaches the braincase. Even so, it must be taken into account that myopic eyes are neither exclusively nor always elongated (Chau et al. 2004; Stone & Flitcroft, 2004; Guo et al. 2017) and, even when they are, this axial elongation may not always be detectable, and particularly in individuals with lower levels of refractive error (Cheng et al. 1992; Palmowski‐Wolfe et al. 2009).
Eye form is likely more associated with cranial architecture than with brain size. For example, our data suggest that antero‐posterior elongation and outward projection of the eyeball may be associated with flatter frontal curvature, that is, with a more dolichocephalic cranial morphology. The normal range of ocular protrusion can vary with multiple factors, such as the depth of the orbital floor (Migliori & Gladstone, 1984), or the proportion of extraocular fat within the orbit (Peyster et al. 1986). Moreover, ocular protrusion is inversely correlated with orbital capacity, i.e. volume of the orbit minus volume of the eye (Detorakis et al. 2010), meaning that relative to eye size, reduced space within smaller orbits contributes to greater ocular protrusion. Considering that variation in frontal and temporal cortical volume explains < 20% of the variation in eye size and position, one might expect that variation in size, shape and relative position of the orbital contents are also important to consider.
There are further factors that could have influenced our results, such as intra‐individual variation and age for example. Various studies have shown that ocular dimensions conform to a regular diurnal rhythm synchronized to the external light/dark cycle (Read et al. 2008; Chakraborty et al. 2011; Nickla, 2013; Stone et al. 2013) and, as such, images might display distinct peaks of axial eye length depending upon the time of the day the images were recorded. Also, our sample includes a broad range of age groups, introducing senescence as a further factor (i.e. orbicularis muscle laxity, changes in fat position, atrophy, etc.) that may perhaps influence certain morphological variables (Berger & Kahn, 2012). Although our results showed no significant correlation between shape changes and age, further analyses on specific age ranges may add to subsequent studies. In addition, it is recommended that future research examining cerebral/craniofacial anatomy and the form and function of the eye also include individuals with high myopia, use Standard Error of Refraction as a measure of reduced visual acuity, and investigate spatial and volumetric relationships using a three‐dimensional approach that also includes measures of extraocular soft tissues within the orbit.
Braincase‐orbit spatial relationship
The orbital and fronto‐temporal morphology of modern humans as captured in the CT sample mainly varies with regard to the orientation of the orbits. Subsequent shape changes concern the position of the orbit relative to the frontal profile, orbital axial proportions, and changes in size of the orbital aperture. Although we attempted to sample corresponding regions in both the MRI and CT samples, the landmark sets are inherently different, and thus describe different shape changes. More specifically, the MRI sample primarily describes changes in the relationship among soft tissue components of the upper skull, while results from the CT analysis mostly describe changes in craniofacial‐braincase morphology. For instance, the frontal profile is more posteriorly extended in the MRI sample than in the CT sample, as its posterior limit is marked by the central sulcus in the former, and by endobregma in the latter, which are two landmarks that have previously been shown to deviate from each other by about 57.9 ± 6.8 mm (Bruner et al. 2015). Similarly, the roof of the orbit differs in the two samples, with the antero‐posterior extension of the orbital roof being captured only on the CT sample. Hence, variation in modern humans is mostly determined by the position of the eye in the MR images, and by the orientation of the orbits in the CT scans. Nonetheless, some comparable changes are observable. For instance, it is possible that shape changes describing the spatial relationship between the eye and the orbital roof in the MRI analysis also depict variation in orbital orientation as observed in the CT analysis. Moreover, variation in the space between the eye and frontal lobe may be related to height of the orbit, although it must be taken into account that this is a secondary variation in the CT sample.
Differences in the orientation of the orbits might be associated with dolichocephalic/brachycephalic proportions. Analyzing integration between the face and lateral basicranium in the sagittal view, Bastir & Rosas (2006) separated two main patterns related to height of the face. More specifically, they discriminated between vertically longer (taller) and antero‐posteriorly shorter faces with shallower cranial bases (middle cranial fossae), from vertically shorter but antero‐posteriorly stretched faces associated with deeper cranial bases. This study revealed that in individuals with taller faces, the orbital roof also appeared antero‐posteriorly shorter, and the authors attributed this retraction of the orbits to an upward rotation of the anterior cranial base (Bastir & Rosas, 2006). Orientation of the orbits is strongly associated with orientation of the anterior cranial base and, together, these form an angle with the posterior maxillary plane that is close to 90° (McCarthy & Lieberman, 2001). In turn, the mandibular ramus is vertically aligned with the middle cranial fossae (Bastir et al. 2004; Bastir & Rosas, 2005), and integration among each of these structures – which in our study is indicated by somewhat coordinated variation among the orbital landmarks, the temporal pole and the posterior limit of the AF– causes the face to rotate as an entire block relative to the posterior cranial base, which acts to influence overall craniofacial shape (McCarthy & Lieberman, 2001; Neaux et al. 2013b).
Within adult modern humans, size of the parietal bones is a major source of variability, and larger parietal bones are associated with a forward rotation of the cranial base and facial block (Bruner et al. 2017), which influences the functional axis of the head. This is in agreement with our main morphological vector of variation concerning orientation of the orbits. If these two studies are referring to the same process, they suggest that when the parietal bones are larger the facial block is more ventrally flexed, and the orbits undergo a corresponding adjustment. On the other hand, the frontal profile was found to be rather invariable in its shape, and variation observed in its lower limit might be due to variability in the morphology of crista galli, influencing the location of the foramen caecum (Moss, 1963).
Regarding the inter‐specific analysis, differences between modern humans and the other species considered did not follow the main pattern of intra‐specific variation seen in the former, but rather, it largely centered on separation between the orbits and braincase. For instance, when compared with more archaic humans and chimpanzees, modern humans display a specific cranial architecture, in which the orbits are positioned directly below the frontal lobes (Bruner & Holloway, 2010) and in close proximity to the temporal lobes, which are positioned directly behind the orbits (Bastir et al. 2008). On the other extreme, chimpanzees exhibit larger and more anteriorly projected orbits, which remain highly separated from the frontal and temporal areas.
The fossil hominids in this analysis display a morphology that is intermediate between modern humans and chimpanzees, as their faces and orbits are larger and more detached from the braincase when compared with modern humans, but less so relative to the chimps in this study. Moreover, the temporal poles of these fossil humans are situated in a lower position than that of the orbits, which is more characteristic of chimpanzees; however, the position of their foramen caecum was found to be more like that of modern humans by comparison. Interestingly, although the main spatial relationship separating these species involves both the frontal and temporal areas, it is the proximity with the latter that could generate spatial conflicts in modern humans. Indeed, in our species the temporal tip is more anteriorly projected than in chimpanzees or fossil humans, and this close proximity with the middle cranial fossa could constrain the orbit in terms of antero‐posterior development. On the other hand, the anterior portion of the temporal lobe in modern humans is flexed against the anterior wall of the middle cranial fossa, twisting toward the midline (Bruner et al. 2017), which could be a direct consequence of spatial constraints between the temporal pole and orbits as evidenced in this study.
Although we could not include Neanderthals in this analysis due to a lack of specimens with cranial anatomy complete enough to sample all required landmarks, it was possible to compare the orbital morphology of Gibraltar 1 with that of modern humans and H. heidelbergensis. This assessment revealed that with a more anteriorly located temporal pole and less separation between the orbits and frontal areas, this specimen appears to have an orbital morphology between that of H. heidelbergensis and modern humans. Naturally, with only one Neanderthal specimen, broader generalizations cannot be made in terms of species‐wide variation. Nonetheless, Gibraltar 1 is a good representative of the Neanderthals, a species with a limited degree of variation (when compared with other hominid taxa), and with characteristic facial traits (Schwartz & Tattersal, 2003). More generally, a scarcity of complete human fossils available for this type of analysis is a limitation of the study, as orbits and frontal bones are often broken, missing or deformed in fossil specimens. However, Bodo and Broken Hill 1 retained intact frontal bones and at least one complete orbit, and thus were the only specimens complete enough to be included. Notwithstanding this inherent limitation, the specimens representing each species grouped together, and were well‐separated in shape space from specimens of the other species considered. Furthermore, Bodo and Broken Hill 1 adequately represent the morphotype associated with archaic human species preceding the differentiation towards modern humans and Neanderthals (Stringer, 2012). Therefore, considering that they share a similar morphology according to the geometrical model used in this study, we can assume that inter‐specific differences are sufficiently marked to be generally detected in this analysis. However, future surveys using larger fossil samples could be used to investigate the degree of variation of extinct species, and their specific patterns of variability.
According to Bastir & Rosas (2016), the flatter faces of modern humans are associated with retraction of the midline anterior cranial fossae and sphenoid body, and relative projection of the sphenoid wings. This pattern seems to be corroborated by our results showing anterior projection of the temporal pole and antero‐posterior retraction of the orbits in modern humans. As in the modern human sample, change in the frontal profile of the different species was negligible, confirming the unvarying outline of the internal frontal table during human evolution (Bookstein et al. 1999). However, the frontal squama is more curved in modern humans when compared with non‐modern human species, which is likely a spatial consequence of reduction in the facial block, positioned below the braincase (Bruner et al. 2013). Also, a surface analysis of archaic and modern frontal lobes suggests that the degree of frontal bulging is proportional to the proximity between the frontal lobes and orbits, with extinct human species showing an intermediate morphotype between modern humans and living apes (Beaudet & Bruner, 2017). While variation explained by the second PC in the current study was not significant, it is noteworthy that chimps fall within the same range of variation as modern humans, which could indicate a similar mechanism for orientation of the orbits between the two species (Neaux et al. 2013b, 2015). Patterns of covariation between the cranial base and face are undoubtedly complex, and a complete understanding of the spatial relationship between these structures requires a three‐dimensional approach that includes both hard and soft tissue morphology, as this could detect patterns of integration that are indistinguishable in a two‐dimensional study.
The dimensionality of the geometrical model represents a limitation of this study. However, both frontal and temporal lobes interact with the orbital space through longitudinal variations and sagittal changes, which is why only morphological variation in this anatomical plane was considered. Nonetheless, we cannot exclude that factors along the coronal plane can also elicit some structural effects. For instance, Moriyama et al. (2011) found that myopia is associated with a temporally distorted eyeball, with asymmetric posterior elongation of the temporal portion of the eye. Also, in a recent study Takada et al. (2015), associated pathogenic exophthalmos with lateral expansion of the ethmoidal sinus and consequent inflation of the medial orbital walls, which acts to constrict the contents of the orbit and shift the eye anteriorly. Beyond the sagittal and axial relationships revealed here, a three‐dimensional approach incorporating coronal dimensions could add relevant information to aid in evaluating other potential constraints associated with the spatial relationship among these cranial districts.
Although it is impossible to know how the eyes were positioned within the orbits of fossil specimens, comparing modern humans and chimpanzees can provide some clues. For instance, in both species the eye assumes a rather anterior position within the orbit (Fig. 1), though in modern humans it is closer to the frontal lobe, but is located below the browridge in chimpanzees. Moreover, chimpanzees can be seen to have a much larger amount of extraocular fat within the orbit, which might contribute to a more forward projection of the eye, or it may simply exist to fill the extraocular space within what is proportionately a much larger orbit in chimps. According to Denion et al. (2015a,b), modern humans have more protruding eyes and more rearward lateral orbital margins relative to other hominoids, which are hypothesized to help increase lateral vision in our species. By comparison, Neanderthals and H. heidelbergensis have large and projecting browridges, and their eyes may also have been positioned below these structures, more separated from the neural tissues than what is characteristic of modern humans. Still, having a larger brain than chimpanzees, their eyes could also have been larger (Scammon & Armstrong, 1925), which would be expected to change their relative spatial position among circumscribing hard and soft tissues in this anatomical region of the skull. Furthermore, because of observed variation in ocular protrusion and relative position of the lateral orbital margins, analyzing the morphology of the lateral orbital walls in fossil crania could potentially provide insight regarding the lateral angle of vision, and relative protrusion of the eye in fossil specimens.
It is worth noting that certain structural constraints might not necessarily be the target of negative selection in terms of evolutionary processes. Many characters that involve sub‐optimal conditions, or even negative effects, can be positively selected if associated with more beneficial traits (antagonistic pleiotropy), or if their effects do not influence general reproductive success (such as in the case of detrimental conditions that occur during later life stages). Therefore, the localization of possible spatial conflicts in terms of morphogenesis is crucial to understand in the context of the rules behind evolutionary schemes and limitations, and potentially in considering eventual clinical consequences, though they should not be interpreted individually in terms of evolutionary fitness.
Conclusion
According to the MRI analysis, variation within adult modern humans is primarily related to the horizontal position of the eye relative to the temporal lobes and, secondly, to the vertical position of the eye relative to the base of the frontal lobes. Both cases involve an apparent deformation of the eye, and possible constraints associated with proximity to the temporal and frontal lobes, respectively. The size of the frontal and temporal lobes seems to have the same effect on the eye, which in larger individuals, appears smaller and closer to the temporal lobe, suggestive of a possible posterior constraint. On the one hand, these results support Masters’ (2012) hypothesis, as a slight deformation of the eye, coupled with possible spatial constraints with the temporal and frontal lobes were identified. On the other hand, it was not possible to verify a clear association between cortical size and eye shape.
According to the CT analysis, shape variation within adult modern humans is primarily determined by the orientation of the orbits. Though by contrast, an interspecific comparison with other human species and chimpanzees shows that the main change is a shift of the whole orbit from a position anterior to the braincase (apes), to one inferior to the braincase (modern humans), with fossil humans representing an intermediate position between the two. In our species this change involves more direct contact between the temporal and orbital spaces. Taken together, these results suggest that the specific hypothesis of competition among the eye, extraocular soft tissues, the orbit, brain and broader craniofacial anatomy should be further examined using a 3D morphometric approach, which can extend the analysis to include the entire orbital and frontal surfaces. Moreover, an ontogenetic study of patterns of variation among these anatomical components during different stages of growth, and particularly prior to and following age 9 years (Taylor, 1939), could elucidate more about the development of vision problems, and to what extent they may arise in association with spatial constraints among adjacent hard and soft tissue features in this confined region of the modern human skull.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
Ana Sofia Pereira‐Pedro is funded by Fundación Atapuerca, Spain. Michael Masters was supported by the National Institute of General Medical Sciences of the National Institutes of Health, award number 8 P20 GM103474‐12. Emiliano Bruner is funded by the Spanish Government (Proyecto Atapuerca – CGL2015‐65387‐C3‐3‐P).
References
- Allen JS, Damasio H, Grabowski TJ (2002) Normal neuroanatomical variation in the human brain: an MRI‐volumetric study. Am J Phys Anthropol 118, 341–358. [DOI] [PubMed] [Google Scholar]
- Arsuaga J‐L, Martínez I, Lorenzo C, et al. (1999) The human cranial remains from Gran Dolina Lower Pleistocene site (Sierra de Atapuerca, Spain). J Hum Evol 37, 431–457. [DOI] [PubMed] [Google Scholar]
- Atchison DA, Jones CE, Schmid KL, et al. (2004) Eye shape in emmetropia and myopia. Invest Ophthalmol Vis Sci 45, 3380. [DOI] [PubMed] [Google Scholar]
- Barbeito‐Andrés J, Anzelmo M, Ventrice F, et al. (2016) Morphological integration of the orbital region in a human ontogenetic sample. Anat Rec 299, 70–80. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A (2005) Hierarchical nature of morphological integration and modularity in the human posterior face. Am J Phys Anthropol 128, 26–34. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A (2006) Correlated variation between the lateral basicranium and the face: a geometric morphometric study in different human groups. Arch Oral Biol 51, 814–824. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A (2016) Cranial base topology and basic trends in the facial evolution of Homo. J Hum Evol 91, 26–35. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A, Kuroe K (2004) Petrosal orientation and mandibular ramus breadth: evidence for an integrated petroso‐mandibular developmental unit. Am J Phys Anthropol 123, 340–350. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A, Lieberman DE, et al. (2008) Middle cranial fossa anatomy and the origin of modern humans. Anat Rec 291, 130–140. [DOI] [PubMed] [Google Scholar]
- Beaudet A, Bruner E (2017) A frontal lobe surface analysis in three African human fossils: OH 9, Buia, and Bodo. C R Palevol 16, 499–507. [Google Scholar]
- Berger AJ, Kahn D (2012) Growth and development of the orbit. Oral Maxillofac Surg Clin North Am 24, 545–555. [DOI] [PubMed] [Google Scholar]
- Bermúdez de Castro JM, Martinón‐Torres M, Prado L, et al. (2010) New immature hominin fossil from European Lower Pleistocene shows the earliest evidence of a modern human dental development pattern. PNAS 107, 11 739–11 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein FL (1991) Morphometric Tools for Landmark Data: Geometry and Biology. Cambridge: Cambridge University Press. [Google Scholar]
- Bookstein F, Schäfer K, Prossinger H, et al. (1999) Comparing frontal cranial profiles in archaic and modern Homo by morphometric analysis. Anat Rec 257, 217–224. [DOI] [PubMed] [Google Scholar]
- Brown P, Maeda T (2004) Post‐Pleistocene diachronic change in East Asian facial skeletons: the size, shape and volume of the orbits. Anthropol Sci 112, 29–40. [Google Scholar]
- Bruner E (2004) Geometric morphometrics and paleoneurology: brain shape evolution in the genus Homo. J Hum Evol 47, 279–303. [DOI] [PubMed] [Google Scholar]
- Bruner E (2007) Cranial shape and size variation in human evolution: structural and functional perspectives. Childs Nerv Syst 23, 1357–1365. [DOI] [PubMed] [Google Scholar]
- Bruner E, Holloway RL (2010) A bivariate approach to the widening of the frontal lobes in the genus Homo. J Hum Evol 58, 138–146. [DOI] [PubMed] [Google Scholar]
- Bruner E, Ripani M (2008) A quantitative and descriptive approach to morphological variation of the endocranial base in modern humans. Am J Phys Anthropol 137, 30–40. [DOI] [PubMed] [Google Scholar]
- Bruner E, Athreya S, de la Cuétara JM, et al. (2013) Geometric variation of the frontal squama in the genus homo: frontal bulging and the origin of modern human morphology. Am J Phys Anthropol 150, 313–323. [DOI] [PubMed] [Google Scholar]
- Bruner E, De la Cuétara JM, Masters M, et al. (2014) Functional craniology and brain evolution: from paleontology to biomedicine. Front Neuroanat 8, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner E, Amano H, de la Cuétara JM, et al. (2015) The brain and the braincase: a spatial analysis on the midsagittal profile in adult humans. J Anat 227, 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner E, Pereira‐Pedro AS, Bastir M (2017) Patterns of morphological integration between parietal and temporal áreas in the human skull. J Morphol 278(10), 1312–1320. [DOI] [PubMed] [Google Scholar]
- Chakraborty R, Read SA, Collins MJ (2011) Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci 52, 5121–5129. [DOI] [PubMed] [Google Scholar]
- Chau A, Fung K, Pak K, et al. (2004) Is eye size related to orbit size in human subjects? Ophthalmic Physiol Opt 24, 35–40. [DOI] [PubMed] [Google Scholar]
- Cheng HM, Singh OS, Kwong KK, et al. (1992) Shape of the myopic eye as seen with high‐resolution magnetic resonance imaging. Optom Vis Sci 69, 698–701. [DOI] [PubMed] [Google Scholar]
- Denion E, Hitier M, Guyader V, et al. (2015a) Unique human orbital morphology compared with that of apes. Sci Rep 5, 11 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denion E, Hitier M, Levieil E, et al. (2015b) Human rather than ape‐like orbital morphology allows much greater lateral visual field expansion with eye abduction. Sci Rep 5, 12 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detorakis ET, Drakonaki E, Papadaki E, et al. (2010) Effective orbital volume and eyeball position: an MRI study. Orbit 29, 244–249. [DOI] [PubMed] [Google Scholar]
- Dinov ID, Van Horn JD, Lozev KM, et al. (2009) Efficient, distributed and interactive neuroimaging data analysis using the LONI Pipeline. Front Neuroinform 3, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirani M, Chamberlain M, Shekar SN, et al. (2006) Heritability of refractive error and ocular biometrics: the genes in myopia (GEM) twin study. Invest Ophthalmol Vis Sci 47, 4756–4761. [DOI] [PubMed] [Google Scholar]
- Foo VHX, Verkicharla PK, Ikram MK, et al. (2016) Axial length/corneal radius of curvature ratio and myopia in 3‐year‐old children. Transl Vis Sci Technol 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidline SE, Gunz P, Harvati K, et al. (2013) Evaluating developmental shape changes in Homo antecessor subadult facial morphology. J Hum Evol 65, 404–423. [DOI] [PubMed] [Google Scholar]
- Goldschmidt E, Fledelius HC (2011) Clinical features in high myopia. A Danish cohort study of high myopia cases followed from age 14 to age 60. Acta Ophthalmol 89, 97–98. [DOI] [PubMed] [Google Scholar]
- Grosvenor T, Goss DA (1998) Role of the cornea in emmetropia and myopia. Optom Vis Sci 75, 132–145. [DOI] [PubMed] [Google Scholar]
- Guo X, Xiao O, Chen Y, et al. (2017) Three‐dimensional eye shape, myopic maculopathy, and visual acuity: the Zhongshan Ophthalmic Center – Brien Holden Vision Institute high myopia cohort study. Ophthalmology 124, 679–687. [DOI] [PubMed] [Google Scholar]
- Hammer Ø, Ryan P, Harper D (2001) PAST: Paleontological Statistics software package for education and data analysis. Palaeontol Electronica 4, 9. [Google Scholar]
- He M, Zeng J, Liu Y, et al. (2004) Refractive error and visual impairment in urban children in southern China. Invest Ophthalmol Vis Sci 45, 793–799. [DOI] [PubMed] [Google Scholar]
- Holladay JT, Lynn M, Waring G III, et al. (1991) The relationship of visual acuity, refractive error, and pupil size after radial keratotomy. Arch Ophthalmol 109, 70–76. [DOI] [PubMed] [Google Scholar]
- Hoyte D (1997) Growth of the orbit In: Fundamentals of Craniofacial Growth. (eds Dixon AD, Hoyte D, Rönning O.), pp. 225–255. New York: CRC Press. [Google Scholar]
- Ip JM, Huynh SC, Kifley A, et al. (2007) Variation of the contribution from axial length and other oculometric parameters to refraction by age and ethnicity. Invest Ophthalmol Vis Sci 48, 4846–4853. [DOI] [PubMed] [Google Scholar]
- Kirk EC (2004) Comparative morphology of the eye in primates. Anat Rec 281A, 1095–1103. [DOI] [PubMed] [Google Scholar]
- Kirk EC (2006) Effects of activity pattern on eye size and orbital aperture size in primates. J Hum Evol 51, 159–170. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP (2011) MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour 11, 353–357. [DOI] [PubMed] [Google Scholar]
- Lacruz RS, Bromage TG, O'Higgins P, et al. (2015) Ontogeny of the maxilla in Neanderthals and their ancestors. Nat Commun 6, 8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CS, Edwards M, Millodot M, et al. (1999) A 2‐year longitudinal study of myopia progression and optical component changes among Hong Kong schoolchildren. Optom Vis Sci 76, 370–380. [DOI] [PubMed] [Google Scholar]
- Lam DSC, Fan DSP, Lam RF, et al. (2008) The effect of parental history of myopia on children's eye size and growth: results of a longitudinal study. Invest Ophthalmol Vis Sci 49, 873–876. [DOI] [PubMed] [Google Scholar]
- Lang J (2012) Clinical Anatomy of the Head: Neurocranium, Orbit, Craniocervical Regions. New York: Springer Science & Business Media. [Google Scholar]
- Lieberman DE, Pearson OM, Mowbray KM (2000) Basicranial influence on overall cranial shape. J Hum Evol 38, 291–315. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, McBratney BM, Krovitz G (2002) The evolution and development of cranial form in Homo sapiens. PNAS 99, 1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente L, Barbero S, Cano D, et al. (2004) Myopic versus hyperopic eyes: axial length, corneal shape and optical aberrations. J Vis 4, 288–298. [DOI] [PubMed] [Google Scholar]
- Mak W, Kwan MWM, Cheng TS, et al. (2006) Myopia as a latent phenotype of a pleiotropic gene positively selected for facilitating neurocognitive development, and the effects of environmental factors in its expression. Med Hypotheses 66, 1209–1215. [DOI] [PubMed] [Google Scholar]
- Masters MP (2012) Relative size of the eye and orbit: an evolutionary and craniofacial constraint model for examining the etiology and disparate incidence of juvenile‐onset myopia in humans. Med Hypotheses 78, 649–656. [DOI] [PubMed] [Google Scholar]
- Masters MP, Bruner E, Queer S, et al. (2015) Analysis of the volumetric relationship among human ocular, orbital and fronto‐occipital cortical morphology. J Anat 227, 460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, et al. (2001) A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos Trans R Soc Lond B Biol Sci 356, 1293–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy RC, Lieberman DE (2001) Posterior maxillary (PM) plane and anterior cranial architecture in primates. Anat Rec 264, 247–260. [DOI] [PubMed] [Google Scholar]
- Migliori ME, Gladstone GJ (1984) Determination of the normal range of exophthalmometric values for black and white adults. Am J Ophthalmol 98, 438–442. [DOI] [PubMed] [Google Scholar]
- Moriyama M, Ohno‐Matsui K, Hayashi K, et al. (2011) Topographic analyses of shape of eyes with pathologic myopia by high‐resolution three‐dimensional magnetic resonance imaging. Ophthalmology 118, 1626–1637. [DOI] [PubMed] [Google Scholar]
- Moss ML (1963) Morphological variations of the crista galli and medial orbital margin. Am J Phys Anthropol 21, 159–164. [DOI] [PubMed] [Google Scholar]
- Moss ML, Young RW (1960) A functional approach to craniology. Am J Phys Anthropol 18, 281–292. [DOI] [PubMed] [Google Scholar]
- Neaux D, Guy F, Gilissen E, et al. (2013a) Covariation between midline cranial base, lateral basicranium, and face in modern humans and chimpanzees: a 3D geometric morphometric analysis: basicranium and face integration. Anat Rec 296, 568–579. [DOI] [PubMed] [Google Scholar]
- Neaux D, Guy F, Gilissen E, et al. (2013b) Facial orientation and facial shape in extant great apes: a geometric morphometric analysis of covariation. PLoS One 8, e57026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neaux D, Gilissen E, Coudyzer W, et al. (2015) Implications of the relationship between basicranial flexion and facial orientation for the evolution of hominid craniofacial structures. Int J Primatol 36, 1120–1131. [Google Scholar]
- Nickla DL (2013) Ocular diurnal rhythms and eye growth regulation: where we are 50 years after Lauber. Exp Eye Res 114, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmowski‐Wolfe AM, Kober C, Berg I, et al. (2009) Globe restriction in a severely myopic patient visualized through oculodynamic magnetic resonance imaging (od‐MRI). J AAPOS 13, 322–324. [DOI] [PubMed] [Google Scholar]
- Peyster R, Ginsberg F, Silber J, et al. (1986) Exophthalmos caused by excessive fat: CT volumetric analysis and differential diagnosis. Am J Roentgenol 146, 459–464. [DOI] [PubMed] [Google Scholar]
- Ravosa MJ (1991) Interspecific perspective on mechanical and nonmechanical models of primate circumorbital morphology. Am J Phys Anthropol 86, 369–396. [DOI] [PubMed] [Google Scholar]
- Read SA, Collins MJ, Iskander DR (2008) Diurnal variation of axial length, intraocular pressure, and anterior eye biometrics. Invest Ophthalmol Vis Sci 49, 2911–2918. [DOI] [PubMed] [Google Scholar]
- Remón L, Tornel M, Furlan WD (2006) Visual acuity in simple myopic astigmatism: influence of cylinder axis. Optom Vis Sci 83, 311–315. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Seligman RA (2002) A quantitative morphometric comparative analysis of the primate temporal lobe. J Hum Evol 42, 505–533. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ (2013) tpsDig version 2.17, Department of Ecology and Evolution, State University of New York at Stony Brook. [Google Scholar]
- Ross CF, Kirk EC (2007) Evolution of eye size and shape in primates. J Hum Evol 52, 294–313. [DOI] [PubMed] [Google Scholar]
- Scammon RE, Armstrong EL (1925) On the growth of the human eyeball and optic nerve. J Comp Neurol 38, 165–219. [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz AH (1940) The size of the orbit and of the eye in primates. Am J Phys Anthropol 26, 389–408. [Google Scholar]
- Schwartz JH, Tattersal I (2003) The Human Fossil Record, Volume Two: Craniodental Morphology of Genus Homo (Africa and Asia). New York: John Wiley & Sons. [Google Scholar]
- Semendeferi K, Damasio H (2000) The brain and its main anatomical subdivisions in living hominoids using magnetic resonance imaging. J Hum Evol 38, 317–332. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Damasio H, Frank R, et al. (1997) The evolution of the frontal lobes: a volumetric analysis based on three‐dimensional reconstructions of magnetic resonance scans of human and ape brains. J Hum Evol 32, 375–388. [DOI] [PubMed] [Google Scholar]
- Stone RA, Flitcroft DI (2004) Ocular shape and myopia. Ann Acad Med Singapore 33, 7–15. [PubMed] [Google Scholar]
- Stone RA, Pardue MT, Iuvone PM, et al. (2013) Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res 114, 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer C (2012) The status of Homo heidelbergensis (Schoetensack 1908). Evol Anthropol 21, 101–107. [DOI] [PubMed] [Google Scholar]
- Stringer C (2016) The origin and evolution of Homo sapiens . Philos Trans R Soc Lond B Biol Sci 371, 20150237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K, Sakamoto Y, Shimizu Y, et al. (2015) A hypothesis for the pathologic mechanism of idiopathic exophthalmos based on computed tomographic evaluations. J Craniofac Surg 26, 1639–1642. [DOI] [PubMed] [Google Scholar]
- Taylor W (1939) The effect of enucleation of one eye in childhood upon the subsequent development of the face. Trans Ophthalmol Soc UK 59, 361–371. [Google Scholar]
- Todd TW, Beecher H, Williams GH, et al. (1940) The weight and growth of the human eyeball. Hum Biol 12, 1–20. [Google Scholar]
- Tu Z, Narr KL, Dollár P, et al. (2008) Brain anatomical structure segmentation by hybrid discriminative/generative models. IEEE Trans Med Imaging 27, 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A, Schneider C, Lagogiannis G, et al. (2000) Pulsatile expansion therapy for orbital enlargement. Int J Oral Maxillofac Surg 29, 91–95. [PubMed] [Google Scholar]
- Waitzman AA, Posnick JC, Armstrong DC, et al. (1992) Craniofacial skeletal measurements based on computed tomography: Part II. Normal values and growth trends. Cleft Palate Craniofac J 29, 118–128. [DOI] [PubMed] [Google Scholar]
- Washburn SL, Detwiler SB (1943) An experiment bearing on the problems of physical anthropology. Am J Phys Anthropol 1, 171–190. [Google Scholar]
- Weale RA (1982) A Biography of the Eye: Development, Growth, Age. London: H. K. Lewis. [Google Scholar]
- Wu X, Liu W, Zhang Q, et al. (2007) Craniofacial morphological microevolution of Holocene populations in northern China. Chin Sci Bull 52, 1661–1668. [Google Scholar]
- Zelditch ML, Swiderski DL, Sheets HD, et al. (2004) Geometric Morphometrics for Biologists: A Primer. New York; London: Elsevier Academic Press. [Google Scholar]
- Zhao J, Pan X, Sui R, et al. (2000) Refractive error study in children: results from Shunyi District, China. Am J Ophthalmol 129, 427–435. [DOI] [PubMed] [Google Scholar]


