Abstract
The palmaris brevis (PB) is a small cutaneous hand muscle that has been described as the most mysterious muscle from a functional and developmental perspective [Kaplan (1984) Kaplan's Functional and Surgical Anatomy of the Hand]. Functionally, the PB is considered to deepen the hollow of the palm and to protect the neurovasculature of the ulnar canal. Although the function of the PB has been inferred from cadaveric observations, the electromyographic (EMG) activity of this muscle has not been explored systematically during specific movements of the hand. The purpose of this study was to record PB intramuscular EMG activity during dynamic grasping tasks, and to quantify the change in PB muscle length (ML) and thickness (MT) incurred during maximal contraction using ultrasound imaging. Intramuscular EMG was recorded from the PB in the dominant hands of 12 healthy participants (11 males, one female; age: 27 ± 4 years) during maximal abduction, flexion and opposition of the 5th digit, and two grasping tasks. Abduction of the 5th digit yielded the greatest EMG activity in most individuals (seven out of 11), and produced significantly less PB EMG activity when compared with grasping a cylindrical‐shaped object (P = 0.003) but not a spherical‐shaped object (P = 0.130). During maximal abduction of the 5th digit, PB ML decreased in both the left (28 ± 11%; P = 0.002) and right (32 ± 5%; P = 0.002) hands. Similarly, a concomitant increase in PB MT was observed in the left (68 ± 30%; P = 0.002) and right (85 ± 44%; P = 0.002) hands during the same contraction. These EMG results indicate that the PB is voluntarily activated during prehensile and non‐prehensile movements of the hand with significant changes in muscle architecture. The study supports the preservation of the PB in surgical procedures based on its proposed protective role as a muscular barrier to the neurovasculature within the ulnar canal.
Keywords: electromyography, functional anatomy, muscle architecture, palmaris brevis, ultrasound
Introduction
The palmaris brevis (PB) is a small muscle of variant morphology originating from the palmar aponeurosis to insert in the skin and fascia of the medial palm (Przystasz, 1977). The PB is uniquely innervated by the only motor component of the superficial branch of the ulnar nerve. Clinically, the innervation of the PB facilitates diagnosis of the site of ulnar nerve lesion at the wrist based on whether function to the PB is affected or remains intact (PB sign; Pleet & Massey, 1978). Interestingly, Andreas Vesalius overlooked the PB in his classical dissections of the human body perhaps due to its subcutaneous location (Tubbs et al. 2007). Unlike the relatively frequent absence of the palmaris longus (PL; ~14%; Moore et al. 2014), the PB is rarely absent (~ 3%) in humans (Przystasz, 1977). The PL is well developed in mammalian species that use the forelimb for weight‐bearing and ambulation, and may explain its regression in humans (Stecco et al. 2009); however, the PB may still provide a functional role based on its position in the palm.
Several researchers have postulated various functions of the PB, ranging from deepening the palm to aiding in palmar grip; protecting the neurovasculature of the ulnar canal (Shrewsbury et al. 1972; Przystasz, 1977); and preventing the displacement of the hypothenar fat pad during grasping (Kirk, 1924). Cadaveric studies have investigated the gross anatomy of the PB, including descriptions of its muscle width, length of attachments at points of origin and insertion (Shrewsbury et al. 1972; Chiou‐Tan et al. 1998), and its variant morphology (Przystasz, 1977; Nayak & Krishnamurthy, 2007), yet morphological measures in vivo of PB muscle length (ML) and thickness (MT) during rest and contraction at the ulnar canal have not been assessed. Investigating PB muscle architecture during dynamic contractions using ultrasound imaging provides insight as to whether the PB acts as a protective muscular barrier or simply tenses with no significant change in ML or MT.
Surveying several texts and clinical electromyographic (EMG) investigations of the PB reveals a disparity in the hand movement necessary to evoke its muscle activity. Specific movements of the 5th digit (abduction, flexion, opposition; Serratrice et al. 1995; Chiou‐Tan et al. 1998; Iyer, 1998; Standring, 2008; Perotto et al. 2011) or applying mechanical pressure superficial to the pisiform bone (Serratrice et al. 1995; Liguori et al. 2003; Perotto et al. 2011) have been described as actions that evoke PB contraction. Furthermore, some PB descriptions from clinical case reports state that the PB is not under voluntary control (Serratrice et al. 1995; Iyer, 1998; Eswaradass et al. 2014), which may suggest a smooth muscle composition, under a conditioned (Montagu, 1952) or reflexive control (Boynton‐Lee, 1888), like those found in other panniculus carnosus derivatives such as the dartos or corrugator cutis ani muscles (Patil, 2013). Although PB EMG has been investigated in clinical examinations, a systematic investigation of PB EMG activity during simple movements of the 5th digit and functional grasping tasks has yet to be explored. Thus, the purpose of this study was to investigate the EMG activity of the PB as well as muscle architecture changes during specific hand movements to provide further insight into its function in the palm.
Materials and methods
Participants
Twelve healthy participants (11 men and one woman; age: 27 ± 4 years; height: 182 ± 7 cm; weight: 86 ± 11 kg) volunteered to participate in this study. PB EMG recordings could not be obtained from one participant and he was removed from the EMG portion of the study. The local research ethics board approved the study procedures, and informed written consent was obtained from each participant prior to testing. This study protocol required participants to attend two separate experimental sessions: (1) PB EMG session followed by (2) a PB ultrasound investigation. The ultrasound investigation was performed in both the left and right hands, whereas the EMG investigation was restricted to the dominant hand (left handed: 1, right handed: 11) to minimize the discomfort associated with indwelling EMG insertion into the glabrous skin of the hand. Furthermore, PB muscle morphology is typically more developed in the right hand (Przystasz, 1977), which could potentially yield better EMG recordings than in the left hand.
EMG experimental setup
The medial palmar skin was swabbed with 70% ethanol prior to the EMG procedures. Custom‐made indwelling fine wire, hooked‐tipped electrode pairs (50 μm; California Fine Wire Company, Grover Beach, California, USA) were inserted into the PB via a small‐diameter hypodermic needle (27G × 1/2; Becton Dickinson PrecisionGlideTM Needle, REF 305109; Basmajian & Stecko, 1962) using an approach angle parallel to the palm. Approximately 5 mm of insulation was removed from the fine wires, thereby exposing an adequate recording surface to create a global indwelling EMG interference pattern. Chiou‐Tan et al. (1998) identified the PB from the abductor digiti minimi based on single motor unit rise times using a clinical needle examination. Because we could not determine MU rise times due to the use of global EMG recordings, ultrasound imaging was used to visualize the location of the PB relative to the skin prior to needle insertion. A common ground electrode was placed on the skin at the metacarpophalangeal (MCP) joint of the thumb. Indwelling electrodes are advantageous over surface electrodes for PB recordings, because cross‐talk from the hypothenar muscles may interfere with the EMG signal recorded at the skin surface. Furthermore, the indwelling fine wires allow participants to grasp objects while performing functional movements, which cannot be achieved when using clinical concentric needle electrodes.
The global EMG recorded from the indwelling fine wires was pre‐amplified (1000×; NeuroLog System NL844 Pre‐amplifier), band‐pass filtered (10 Hz–10 kHz; 60 Hz notch filter) and sampled at 2500 Hz before being converted to a digital signal using a 16‐bit analog‐to‐digital converter (Micro 1401 mkII board; Cambridge Electronic Design, CED). All EMG data analyses were performed offline using Spike2 software (v.7.0; CED, Cambridge, UK).
Prehensile and non‐prehensile tasks
Participants were instructed to perform a series of movements of the 5th digit and grasping tasks while PB EMG activity was recorded from the indwelling fine wires. The non‐prehensile tasks involved specific movements of the 5th digit: abduction, flexion at MCP joint only, and opposition to the thumb (Fig. 1). Abduction and flexion of the 5th digit were performed against a rigid surface to provide resistance to the movement. The functional tasks required participants to make two prehensile movements: grasping the shaft of a carpenter's hammer and tennis ball using a power grip and spherical grip, respectively (Napier, 1956; Fig. 1). Participants were instructed to make maximal contractions during all movements, and each task was held isometric for a minimum of 3 s.
Figure 1.
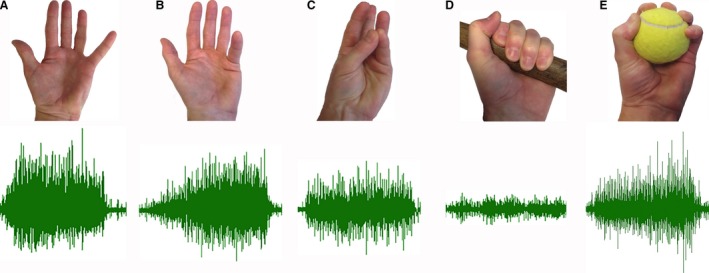
Unprocessed electromyogram (EMG) recorded from the palmaris brevis (PB) during maximal effort movements of the 5th digit and grasping tasks. (A) Abduction of the 5th digit; (B) 5th digit flexion [metacarpophalangeal (MCP) joint only]; (C) opposition; (D) power grip; (E) spherical grip.
EMG normalization
From the unprocessed EMG signal, the average root mean square (EMGRMS) was calculated over a constant time interval of 3 s for all non‐prehensile and prehensile tasks. To compare relative EMGRMS recorded in each task among participants, the PB EMGRMS recorded during each task was normalized to 100% of the 3 s PB EMGRMS evoked during maximal abduction of the 5th digit. Normalization of EMG signals to maximal peak levels is a reliable and valid method to compare relative values of EMG activity among participants (Halaki & Ginn, 2012).
Ultrasound imaging
Visualization of the PB prior to hypodermic needle insertion and dynamic morphological changes in PB architecture, ML and MT, were imaged using a Vivid‐7 ultrasound system (GE Healthcare, Mississauga, ON, Canada; linear array probe: GE model M12L, 4.9 mm, 5–13 MHz). A single investigator with experience in musculoskeletal ultrasound acquired PB images from the palms using the following ultrasound settings: probe frequency = 11.4 MHz, frame rate: 19.0, power = −2 dB, dynamic range = 9, depth = 4.0 cm. To ensure adequate standoff distance for imaging superficial palmar structures, a liberal application of ultrasound gel (Aquasonic 100 Ultrasound transmission gel, Parker Laboratories) was applied and the ultrasound probe frequency was increased to its optimal setting (11.4 MHz). Multiple focus points were set on the ultrasound image within a 2 cm depth from the surface as the PB was typically located within this depth (Fig. 4). Each hand was supported and fully supinated during the imaging. The ultrasound probe was rotated until the PB muscle fibers could be viewed in‐plane and were visible from origin to insertion in the longitudinal plane. Static ultrasound images of the PB at rest were acquired from each participant at the point of maximal PB MT. PB images during maximal contraction were acquired at the same position of maximal PB MT. PB muscle contraction was imaged longitudinally during maximal abduction of the 5th digit (Fig. 4). To prevent the ultrasound probe from moving, participants were instructed to gradually abduct the 5th digit until maximum abduction was achieved.
Figure 4.
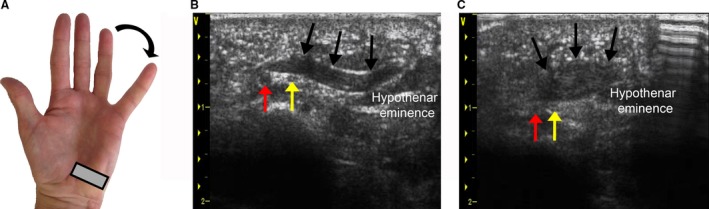
Visualizing dynamic changes in palmaris brevis (PB) muscle architecture during abduction of the 5th digit using ultrasound imaging. (A) Ultrasound probe (gray rectangle) aligned longitudinally with the PB muscle. (B) PB appearance at rest. (C) PB appearance during contraction. Ulnar artery (red arrow), ulnar nerve (yellow arrow), superficial border of the PB (black arrow).
Ultrasound images were exported from the ultrasound unit and analyzed using osirix imaging software (version. 8.0.2, Geneva, Switzerland). PB muscle borders were determined by visual inspection using the echogenicity of both epimysium surrounding the muscle, and the perimysium producing linear reflections surrounding and within the muscle along the longitudinal axis (Figs 2 and 4; Pillen, 2010). MT and ML measurements were performed using the length tool in OsiriX. Measurement lines were drawn perpendicular to the superficial and deep borders, and along the long axis of the muscle to determine MT and ML measures, respectively.
Figure 2.
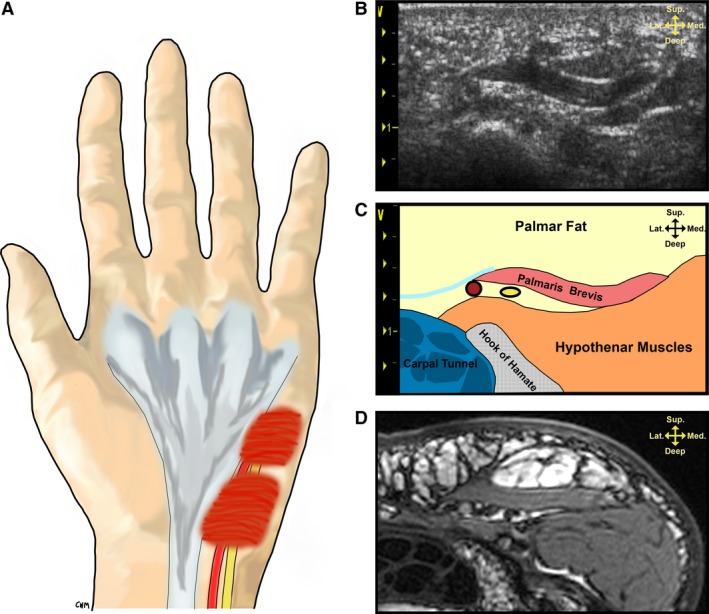
Palmaris brevis (PB) gross morphology and its ultrasound appearance at the level of the hook of the hamate. (A) Illustration demonstrating the spatial relationship of the PB to the ulnar artery and nerve. (B) Ultrasound appearance of the PB at rest. (C) Schematic depiction of palmar structures located in the ultrasound image from (B). (D) Axial T2‐weighted magnetic resonance image of the PB at similar location to the ultrasound in (B). Note the following structures in (C): palmar aponeuroses (turquoise), ulnar artery (red), ulnar nerve (yellow).
Histological analysis
Palmaris brevis specimens were harvested from the hands of four (three fresh frozen, one formalin‐embalmed) cadavers [four left hands, four right hands; mean age at death: 78 years (range: 44–88 years)]. Cadaveric specimens were obtained with permission from the body bequeathal program at the University of Western Ontario, London, ON, Canada, and approved for research use by the Committee for Cadaver Use in Research (REF#: 21092016). Tissue samples were immediately immersed in a 10% formalin solution for a minimum of 24 h prior to paraffin embedding. Specimens were sectioned 5 μm thick using a Microm HM‐325 Microtome. Tissues were mounted on slides and warmed at 60 °C for 30 min. Longitudinal‐ and transverse‐orientated PB tissue samples were stained with hematoxylin‐eosin and hematoxylin only, respectively. Histology slides were imaged using a Zeiss AxioCam MRc microscope camera.
Statistical analysis
Data were analyzed using spss statistical software (Version 24, SPSS, Chicago, IL, USA). A Shapiro–Wilk test determined that the normalized EMG during the spherical grip task was not normally distributed. Therefore, a non‐parametric test (Friedman) was used to determine whether a significant main effect was present in the % PB EMGRMS/ABD recorded during the hand positions. Pairwise comparisons were performed as a post hoc analysis (Wilcoxon signed‐ranks test) of a significant main effect during the five hand movements (5th digit: abduction, flexion, opposition; and power and spherical grips). For the ultrasound measures, a Shapiro–Wilk test determined that the variable, MT (right hand, contracted state), was not normally distributed. Therefore, a non‐parametric t‐test (Wilcoxon signed‐rank test) was used to determine whether a statistically significant change occurred in mean PB ML and MT, at rest and during contraction of both the left and right hands. Effect sizes (r) from Wilcoxon signed‐rank tests were calculated manually using microsoft excel software (version 14.5.8). A Bonferroni correction was applied to both the EMG and ultrasound data to account for multiple statistical comparisons. All data are presented as means ± SD.
Results
EMG
Of the two contraction types used in clinical examination, the PB EMGRMS evoked during abduction of the 5th digit was selected as a method of normalization as this contraction task evoked the greatest PB muscle activity in seven of the 11 (~60%) participants (Fig. 3). In the remainder of subjects, flexion of the 5th digit evoked the greatest PBEMG activity and was only 5% less compared with the % PBEMG evoked during 5th digit abduction. An analysis of main effects revealed a significant difference in the % PB EMG recorded during prehensile and non‐prehensile hand movements [χ2 (4) = 23.799, P = 0.0001]. Post hoc analysis with Wilcoxon signed‐rank tests was conducted with a Bonferroni correction applied, resulting in a significance level set at P = 0.005.
Figure 3.
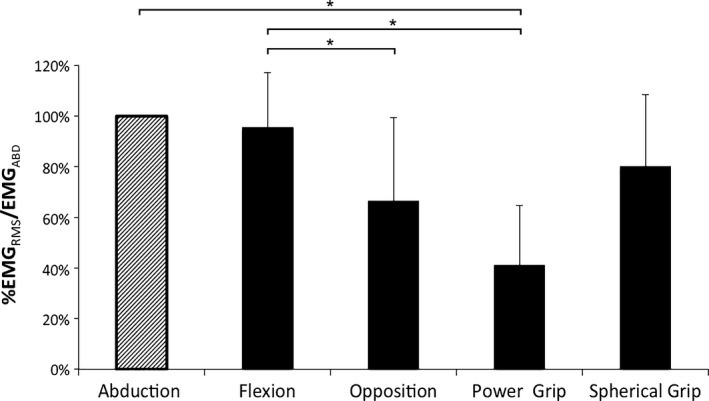
Palmaris brevis (PB) muscle activity during maximal effort movements of the 5th digit and functional grasping tasks. The PB muscle activity recorded from each task is displayed as a percentage of PB EMGRMS normalized to the PB muscle activity recorded during maximal abduction of the 5th digit (EMGABD). Non‐prehensile tasks (5th digit): abduction, flexion, opposition; prehensile tasks: power grip (carpenter's hammer), spherical grip (tennis ball). EMGRMS, root mean square electromyography; all data presented as means ± SD; * denotes P < 0.005. Statistical trends were observed in the comparisons between both 5th digit abduction and opposition (P = 0.016), and the spherical and power grips (P = 0.011).
There were no significant differences in the mean % PB EMGRMS/ABD between abduction and flexion of the 5th digit (P = 0.44, r = 0.16). The % PB EMGRMS/ABD recorded during opposition was significantly reduced by 29% when compared with 5th digit flexion (P = 0.004, r = 0.61). The opposition task produced 34% less % PB EMGRMS/ABD compared with 5th digit abduction, but did not reach statistical significance (P = 0.016, r = 0.51). Similarly, the power grip task produced 39% less % PB EMGRMS/ABD compared with the spherical grip, but did not reach statistical significance (P = 0.011, r = 0.54). The % PB EMGRMS/ABD recorded during the power grip was significantly reduced by 59% and 54% compared with abduction (P = 0.003, r = 0.63) and flexion of the 5th digit (P = 0.003, r = 0.62), respectively (Fig. 3). The % PB EMGRMS/ABD during the spherical grip was 20% and 15% less than abduction (P = 0.13, r = 0.32) and flexion of the 5th digit (P = 0.17, r = 0.29), respectively; however, these comparisons were not statistically significant (P > 0.005). Similarly, the % PB EMGRMS/ABD recorded during the opposition task was 14% lower compared with the spherical grip, but did not reach statistical significance (P = 0.25, r = 0.25).
Ultrasound imaging
Pairwise comparisons using Wilcoxon signed‐rank tests were conducted with a Bonferroni correction applied, resulting in a significance level set at P = 0.006. During abduction of the 5th digit, the mean length of the PB decreased by 28 ± 11% (range: 8–40%, P = 0.002, r = 0.62) and 32 ± 5% (range: 18–59%, P = 0.002, r = 0.62) in the left and right hands, respectively (Table 1). PB muscle thickness increased by 68 ± 30% (range: 23–130%, P = 0.002, r = 0.62) and 85 ± 44% (range: 39–162%, P = 0.002, r = 0.63) in the left and right hands, respectively (Table 1). There were no significant differences between resting and contracted states between the left and right hands (P > 0.006). The ulnar artery and nerve were located deep to the PB in all ultrasound images, and these structures were identifiable in both images acquired at rest (Fig. 2) and during dynamic contraction [Fig. 4; see supplementary material for an ultrasound video of the PB undergoing dynamic contraction upon maximal abduction of the 5th digit (Video S1)].
Table 1.
Ultrasound‐derived measures of PB muscle architecture
| (n = 12) | Left hand | P‐value | Right hand | P‐value | ||
|---|---|---|---|---|---|---|
| Rest | Contraction | Rest | Contraction | |||
| Length (cm) | 2.0 ± 0.3 (1.5–2.5) | 1.4 ± 0.2a(1.2–1.8) | 0.002 | 2.0 ± 0.3 (1.1–2.5) | 1.3 ± 0.3a(0.9–1.7) | 0.002 |
| Thickness (mm) | 1.9 ± 0.6 (1.3–2.9) | 3.1 ± 1.0a(1.9–4.7) | 1.6 ± 0.5 (1.1–3.0) | 3.0 ± 1.7a(1.7–7.7) | ||
Contraction: maximal abduction of 5th digit. Values are means ± SD (range).
Denotes significant from resting condition using Bonferroni correction factor (P < 0.006).
Histology
Histological investigation of the PB tissue revealed typical features of skeletal muscle tissue, striations and peripherally located nuclei, when viewed in longitudinal and cross‐sectional orientations, respectively (Fig. 5).
Figure 5.
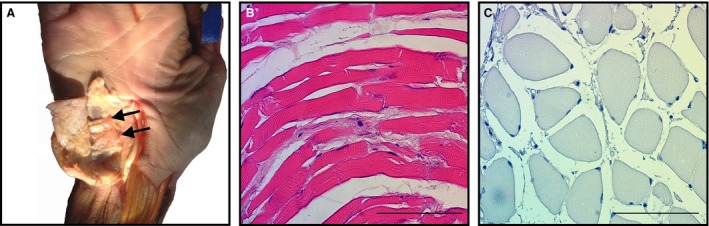
Histological appearance of the palmaris brevis (PB). (A) PB harvested from a fresh frozen cadaveric specimen (black arrows). (B) PB muscle fibers oriented longitudinally (H–E). (C) PB muscle fibers oriented in cross‐section (hematoxylin stain). Note the presence of muscle fiber striations, and peripherally located nuclei typical of skeletal muscle. Scale bar: 100 μm.
Discussion
This study examined the PB EMG and muscle architecture during specific movements of the 5th digit and during functional grasping tasks. A few studies have examined single motor unit PB EMG in vivo in healthy participants (Chiou‐Tan et al. 1998), during clinical examination (Serratrice et al. 1995; Liguori et al. 2003; Tarsy et al. 2004; Eswaradass et al. 2014), and its muscle architecture from cadavers (Shrewsbury et al. 1972; Przystasz, 1977; Chiou‐Tan et al. 1998); however, we investigated PB global EMG from a functional perspective and imaged the PB muscle during dynamic contractions using ultrasound. The results indicate that PB EMG activity is under voluntary control and is highly dependent on movements of the 5th digit, and that the PB muscle is capable of significant changes in muscle architecture during voluntary muscle contraction. In addition, histological analyses indicated that the PB is composed of striated skeletal muscle fibers.
For resting PB MT and ML, the ultrasound‐determined values (Table 1) are in agreement with measurements from embalmed (MT: 1–3 mm; Przystasz, 1977) and fresh (mean ML: 2.1 cm; Kim et al. 2017) cadaveric specimens. Although a reliability study assessing the inter‐ and intra‐rater reliability of the ultrasound‐derived PB measurements was not performed, the results were comparable to those data obtained from cadavers (Przystasz, 1977; Kim et al. 2017). An inter‐ and intra‐rater reliability study of the PB validated against magnetic resonance images from the same participants might be useful for future studies. From a functional perspective, a resting MT of 1–3 mm may be insufficient to protect the neurovasculature of the ulnar canal. Passive movements in which the palm is simply resting on a surface will likely not produce PB muscle contraction, thereby providing minimal protection during prolonged palmar compression. This could explain reports of PB spasm syndrome in occupations requiring long hours using a computer mouse and keyboard (Liguori et al. 2003). The PB may not provide the necessary relief from the prolonged overlying pressure during conditions in which the palm is passively resting on a surface such as a classic‐style handlebar typical of a road bicycle, in which the overlying pressure on the hypothenar eminence experienced by endurance cyclists depends on the handlebar grip position adapted by the rider (Slane et al. 2011).
When the PB contracts, it visibly draws the skin of the hypothenar eminence radially producing a dimpling effect on the ulnar margin of the hand. Ultrasound imaging allows for improved visualization of dynamic PB contraction and its relationship to the ulnar neurovasculature beyond dimpling on the skin surface, or static cadaveric and magnetic resonance imaging investigations. In all subjects, maximal abduction of the 5th digit produced significant changes in PB muscle architecture resulting in a relatively thick muscular barrier between the neurovasculature of the ulnar canal and the palmar hypothenar fat (Fig. 4). The unique quantification through ultrasound showed significant changes in PB MT of 68% and 85%, in the left and right hands, respectively, indicating that the muscle is capable of forming a relatively thick muscular barrier to the neurovasculature of the ulnar canal but only during hypothenar muscle contraction. Kirk (1924) proposed that the PB is essential for anchoring the mobile hypothenar fat pad during grasping movements. Considering this potential muscular barrier in conjunction with the hypothenar fat pad found adjacent to the PB suggests a protective function to the ulnar nerve and ulnar artery. Although we imaged the PB during abduction of the 5th digit, we expect similar changes in PB muscle architecture during 5th digit flexion at the MCP joint, and during the spherical grip based on the % PB EMGRMS/ABD evoked during these tasks.
The power grip produced the least amount of PB muscle activity when grasping the carpenter's hammer. During the power grip, finger flexion is achieved mainly by the forearm flexors (flexor digitorum superficialis, flexor digitorum profundus) while relying on the thenar and hypothenar intrinsic hand muscles for support and stabilization (Napier, 1956). The hypothenar eminence acts as a muscular cushion to the hammer during this functional task (Napier, 1956). The PB EMG activity was reduced significantly during the power grip as grasping the carpenter’s hammer does not require the 5th digit to deepen the palm to achieve a ‘cupping’ action typical of a spherical grip. Thus, the PB may not provide a protective benefit to the ulnar canal neurovasculature during this functional task based on the reduced PB EMG recorded.
The spherical grip is considered a powerful grip yet with more precision and reliance on the intrinsic hand muscles for object manipulation compared with grasping the carpenter's hammer (Napier, 1956). During the spherical grip, the hand is positioned by producing a ‘cupping’ action in which the thumb and the 5th digit are in a position of support. To achieve this position, the 5th digit moves by contracting all hypothenar muscles in a combination of flexion, abduction and opposition. This coordinated hypothenar muscle contraction would explain the large % PB EMGRMS/ABD activity (80%) as the 5th digit supports the ball and resists the movement of the thumb. The % PB EMGRMS/ABD recorded during the spherical grip supports the postulate by Shrewsbury et al. (1972) of the protective benefit of the PB during functional tasks associated with repetitive intermittent trauma or contact associated with prehensile maneuvers. Therefore, the PB may provide a protective benefit when the hand repeatedly grasps a spherical‐shaped object such as catching a baseball or grasping various‐sized and shaped elements during climbing tasks.
PB function in palmar grip
Textbooks typically describe the function of the PB as deepening the palm to aid in palmar grip (Standring, 2008; Moore et al. 2014); however, the extent that the PB deepens the palm seems insignificant to the depth created by the muscles of thenar and hypothenar eminences. Shrewsbury et al. (1972) disagreed with the interpretation that the PB improves palmar grip as the muscle is found in the forelimbs of quadriped mammals such as the cat, mouse and opossum species, which are not capable of grasping objects. This idea seems probable considering the PB can be excised for surgical reconstruction of palmar thumb defects (Ueda & Inoue, 1994) or is divided during standard open carpal tunnel release surgery (Rodner & Katarincic, 2006; Malhotra et al. 2007); however, a systematic description of the functional limitations in grip ability and susceptibility to compression‐related deficits due to the absence of a PB has yet to be explored. Despite the relatively unknown functional limitations imposed by PB absence, some clinicians have proposed preserving this muscle during surgical procedures based on its proposed protective functions of ulnar neurovasculature of the palm (Shrewsbury et al. 1972) and use in diagnosing the location of an ulnar neuropathy at the wrist (Pleet & Massey, 1978; Saadeh, 1989). Compared with an open approach to carpal tunnel release surgery, an improved recovery time of grip and pinch strength using the endoscopic approach has been attributed by some to the preservation of both the PB and palmar fascia (Malhotra et al. 2007). The current study results provide support for the preservation of the PB during surgical procedures, especially in individuals whose palms are frequently subjected to repetitive trauma or compression whether through sport or occupational demands.
Involuntary PB activation
Although we could evoke PB EMG and PB contraction during specific movements of the hand, the PB contractions were not in isolation but occurred in conjunction with hypothenar muscle contractions. The idea that the PB is not under voluntary control has likely been precipitated by historical reports of automatic reflex contraction of the PB initially referred to as the palm reflex (Boynton‐Lee, 1888; Montagu, 1952). Boynton‐Lee (1888) reported involuntary reflexive contraction of the PB by pinching of the skin above the pisiform bone or by firm mechanical compression of the same region. Montagu (1952) reported that the PB muscle could be involuntarily activated by compressing the ulnar nerve at the wrist or it could be conditioned to contract without any tactile stimulation. It is unknown whether the PB response is a physiological reflex by definition, acting in a spinal loop, or whether compression of the pisiform bone produces an involuntary discharge, or spasm, by indirectly compressing the superficial branch of the ulnar nerve. However, based on the evidence provided by the histological examination, the PB is under voluntary control as it contains skeletal muscle fibers and thus is capable of voluntary contraction.
Conclusion
Although the PB is a small rudimentary muscle of variant morphology, it is capable of significant changes in muscle architecture overlying the neurovasculature of the ulnar canal. The PB EMG and ultrasound imaging findings support cadaveric observations that the PB can function as a potential protective muscular barrier, but only when actively engaging the 5th digit either independently or during functional movements. Although involuntary contraction of the PB may be possible through potential reflexive or indirect mechanical compression of the ulnar nerve, the PB muscle is a dynamic structure that can be voluntarily contracted in conjunction with muscles of the hypothenar eminence. This study further supports suggestions that the PB should be spared during surgical interventions based on its proposed protective function to the ulnar artery and nerve in the palm.
Author contributions
Both authors were involved in study conceptualization and design. CWM recruited the participants, collected the data, and performed the primary analyses. CWM and CLR interpreted the data, and prepared the figures and manuscript for publication.
Supporting information
Video S1 Palmaris brevis muscle contraction
Acknowledgements
This study was supported by the Natural Sciences and Engineering Research Council of Canada (Grant #180790). The authors wish to thank all the participants for volunteering their time to participate in this study, and the donors who generously donated their bodies to science, research and education. The authors would like to thank Dr Kevin Shoemaker and Arlene Fleischhauer for providing and facilitating access to the ultrasound imaging equipment used in this study. The authors would like to also thank Linda Jackson from the Department of Oral Pathology for providing lab space and equipment for histological investigations. Finally, the authors would like to thank Tyler Beveridge and Dr Brian Allman for providing supplies, assistance and guidance in histological staining procedures.
References
- Basmajian JV, Stecko G (1962) A new bipolar electrode for electromyography. J Appl Physiol 17, 849–849. [Google Scholar]
- Boynton‐Lee HB (1888) Palm reflex (?). Lancet 2, 1267. [Google Scholar]
- Chiou‐Tan FY, Reno SB, Magee KN, et al. (1998) Electromyographic localization of the palmaris brevis muscle. Am J Phys Med Rehabil 77, 243–246. [DOI] [PubMed] [Google Scholar]
- Eswaradass PV, Kalidoss R, Ramasamy B, et al. (2014) Familial palmaris brevis spasm syndrome. Ann Indian Acad Neurol 17, 141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaki M, Ginn K (2012) Normalization of EMG signals: to normalize or not to normalize and what to normalize to? In: Computational Intelligence in Electromyography Analysis – a Perspective on Current Applications and Future Challenges. (ed. Naik G.). Rijeka, Croatia: InTech. [Google Scholar]
- Iyer VG (1998) Palmaris brevis sign in ulnar neuropathy 1998. Muscle Nerve 21, 675–677. [DOI] [PubMed] [Google Scholar]
- Kaplan EB (1984) Kaplan's functional and surgical anatomy of the hand. Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Kim DH, Bae JH, Kim HJ (2017) Anatomical insights of the palmaris brevis muscle for clinical procedures of the hand. Clin Anat 30, 397–403. [DOI] [PubMed] [Google Scholar]
- Kirk T (1924) Some Points in the Mechanism of the Human Hand. J Anat 58, 228–230. [PMC free article] [PubMed] [Google Scholar]
- Liguori R, Donadio V, Di Stasi V, et al. (2003) Palmaris brevis spasm: an occupational syndrome. Neurology 60, 1705–1707. [DOI] [PubMed] [Google Scholar]
- Malhotra R, Kiran EK, Dua A, et al. (2007) Endoscopic versus open carpal tunnel release: a short‐term comparative study. Indian J Orthop 41, 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagu MF (1952) Conditioning of the palmaris brevis muscle. Science 115, 153. [DOI] [PubMed] [Google Scholar]
- Moore KL, Dalley AF, Agur AMR (2014) Clinically Oriented Anatomy. Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Napier JR (1956) The prehensile movements of the human hand. J Bone Joint Surg Br 38‐B, 902–913. [DOI] [PubMed] [Google Scholar]
- Nayak SR, Krishnamurthy A (2007) An unusually large palmaris brevis muscle and its clinical significance. Clin Anat 20, 978–979. [DOI] [PubMed] [Google Scholar]
- Patil S (2013) Clinico‐anatomical considerations of palmaris brevis muscle. Int J Adv Res 1, 341–344. [Google Scholar]
- Perotto A, Delagi E, Iazzetti J, et al. (2011) Anatomical Guide for the Electromyographer: the Limbs and Trunk. Springfield, IL: Charles C Thomas Publisher. [Google Scholar]
- Pillen S (2010) Skeletal muscle ultrasound. Eur J Transl Myol 20, 145–156. [Google Scholar]
- Pleet AB, Massey EW (1978) Palmaris brevis sign in neuropathy of the deep palmar branch of the ulnar nerve. Ann Neurol 3, 468–469. [DOI] [PubMed] [Google Scholar]
- Przystasz T (1977) Morphology of the palmaris brevis muscle in man. Folia Morphol (Warsz) 36, 77–82. [PubMed] [Google Scholar]
- Rodner CM, Katarincic J (2006) Open carpal tunnel release. Tech Orthop 21, 3–11. [Google Scholar]
- Saadeh PB (1989) Examination of the palmaris brevis. Muscle Nerve 12, 425–426. [PubMed] [Google Scholar]
- Serratrice G, Azulay JP, Serratrice J, et al. (1995) Palmaris brevis spasm syndrome. J Neurol Neurosurg Psychiatry 59, 182–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrewsbury MM, Johnson RK, Ousterhout DK (1972) The palmaris brevis – a reconsideration of its anatomy and possible function. J Bone Joint Surg Am 54, 344–348. [PubMed] [Google Scholar]
- Slane J, Timmerman M, Ploeg HL, et al. (2011) The influence of glove and hand position on pressure over the ulnar nerve during cycling. Clin Biomech (Bristol, Avon) 26, 642–648. [DOI] [PubMed] [Google Scholar]
- Standring S (2008) Gray's Anatomy: the Anatomical Basis of Clinical Practice. Edinburgh: Churchill Livingstone. [Google Scholar]
- Stecco C, Lancerotto L, Porzionato A, et al. (2009) The palmaris longus muscle and its relations with the antebrachial fascia and the palmar aponeurosis. Clin Anat 22, 221–229. [DOI] [PubMed] [Google Scholar]
- Tarsy D, Apetaurova D, Rutkove SB (2004) Palmaris brevis spasm: an occupational syndrome. Neurology 62, 838. [DOI] [PubMed] [Google Scholar]
- Tubbs RS, Louis RG Jr, Loukas M, et al. (2007) The first description of the palmaris brevis muscle. J Hand Surg Eur 32, 382–383. [DOI] [PubMed] [Google Scholar]
- Ueda K, Inoue T (1994) The new palmaris brevis musculocutaneous flap. Ann Plast Surg 32, 529–534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 Palmaris brevis muscle contraction


