Abstract
Squamates present a unique challenge to our understanding of dental evolution in amniotes because they are the only extant tooth‐bearing group for which a ligamentous tooth attachment is considered to be absent. This has led to the assumption that mammals and crocodilians have convergently evolved a ligamentous tooth attachment, composed of root cementum, periodontal ligament, and alveolar bone, whereas squamates are thought to possess a single bone of attachment tissue that fuses teeth to the jaws. The identity and homology of tooth attachment tissues between squamates, crocodilians, and mammals have thus been a focal point of debate for decades. We provide a novel interpretation of the mineralized attachment tissues in two focal taxa in this debate, mosasaurids and snakes, and compare dental tissue histology with that of the extant crocodilian Caiman sclerops. We identify a periodontal ligament in these squamates that usually exists temporarily as a soft connective tissue anchoring each tooth to the alveolar bone. We also identify two instances where complete calcification of the periodontal ligament does not occur: in a durophagous mosasaur, and in the hinged teeth of fossil and modern snakes. We propose that the periodontal ligament rapidly calcifies in the majority of mosasaurids and snakes, ankylosing the tooth to the jaw. This gives the appearance of a single, bone‐like tissue fusing the tooth to the jaw in ankylosed teeth, but is simply the end stage of dental tissue ontogeny in most snakes and mosasaurids.
Keywords: ankylosis, cementum, gomphosis, Mosasauridae, periodontal ligament, snake, tooth attachment, tooth implantation
Introduction
Mammals and crocodilians are unique among extant amniotes in possessing a ligamentous form of tooth attachment in which a periodontal ligament (PDL) anchors into root cementum coating each tooth and into the alveolar bone forming each tooth socket. This form of tooth attachment, called a gomphosis, provides a flexible connection between the teeth and the jaws, which can dissipate the compressive forces of dental occlusion and high bite forces (Miller, 1968; Nanci, 2013). Despite having diverged from a common ancestor over 300 million years ago (Reisz, 1997), the presence of nearly identical tooth attachment modes in mammals and crocodilians is implied to be a remarkable case of convergence (Kvam, 1960; Peyer, 1968; Berkovitz & Sloan, 1979; McIntosh et al. 2002), because these tooth attachment tissues are apparently absent in most fossil reptiles (Peyer, 1968; Osborn, 1984; Rieppel, 2001) and in the other major extant tooth‐bearing amniote group, Squamata (Zaher & Rieppel, 1999).
Squamates are a diverse group of diapsid reptiles with teeth that are fused to the jaws by a spongy bone tissue, traditionally called ‘bone of attachment’ (Tomes, 1882; Peyer, 1968). Tomes’ original definition of ‘bone of attachment’ replaced Owen's (1840) ‘dental cement’ (a term Owen applied to cementum in mammals and non‐mammalian vertebrates) and described a peculiar bone tissue that was intimately associated with the attachment of each tooth in snakes specifically (Tomes, 1882). This definition has since been expanded to include osseous tooth attachments in other non‐mammalian, non‐crocodilian amniotes (Peyer, 1968; Osborn, 1984; Gaengler, 2000). In the vast majority of extinct and living squamates there appears to be very little variation in tooth attachment mode, and squamate ‘bone of attachment’ is typically treated as non‐equivalent to the mammalian and crocodilian attachment tissue complex or as a homologue to either cementum or alveolar bone (Tomes, 1882; Peyer, 1968; Gaengler, 2000). Over the past two decades, this view has come under scrutiny, spurring extensive debates regarding the histology and development of squamate tooth attachment tissues and their potential homologues in mammals and archosaurs (Zaher & Rieppel, 1999; Caldwell et al. 2003; Rieppel & Kearney, 2005; Budney et al. 2006; Caldwell, 2007; Luan et al. 2009). The extinct mosasaurids as well as modern and extinct snakes have continued to feature prominently in this debate, because of their controversial affinities with each other and within Squamata (Lee, 1997; Zaher & Rieppel, 1999; Caldwell, 2007), and because both possess modes of tooth implantation that are more reminiscent of thecodonty in mammals and archosaurs than of pleurodonty or acrodonty in other lizards.
Despite renewed interest in mosasaurid and snake tooth attachment and development, many still consider squamate tooth attachment tissues to be plesiomorphic, autapomorphic, or simply not homologous to those of crocodilians or mammals (Luan et al. 2009; Liu et al. 2016). This interpretation is a long‐standing one that centres around the absence of a soft PDL in squamates (Tomes, 1882; Peyer, 1968). This hypothesis predicts that the PDL must have arisen at least twice in the evolutionary history of Amniota: once in the lineage leading to all crocodilians and dinosaurs, and again in the lineage including all mammals (Fig. 1). Interestingly, this evolutionary hypothesis is at odds with the reports of cementum, PDL, and alveolar bone in several extinct amniote clades (Fig. 1), ligamentous hinged teeth of several snakes and legless lizards (Savitsky, 1981; Patchell & Shine, 1986), the presence of alveolar bone and attachment points for a PDL (Sharpey's fibers) in a fossil snake with hinged teeth (Budney et al. 2006), and traces of soft tissue attachment within the cementum and alveolar bone of mosasaurids (Caldwell et al. 2003; Caldwell, 2007; Luan et al. 2009). What remains to be determined, however, is if, when, and where the PDL is present in the development of the supporting tissues of the tooth in squamates, because fully developed teeth are usually completely fused to the jaws, with no space for a PDL. This information is necessary to better understand the evolution and development of the periodontium in squamates and amniotes more generally.
Figure 1.
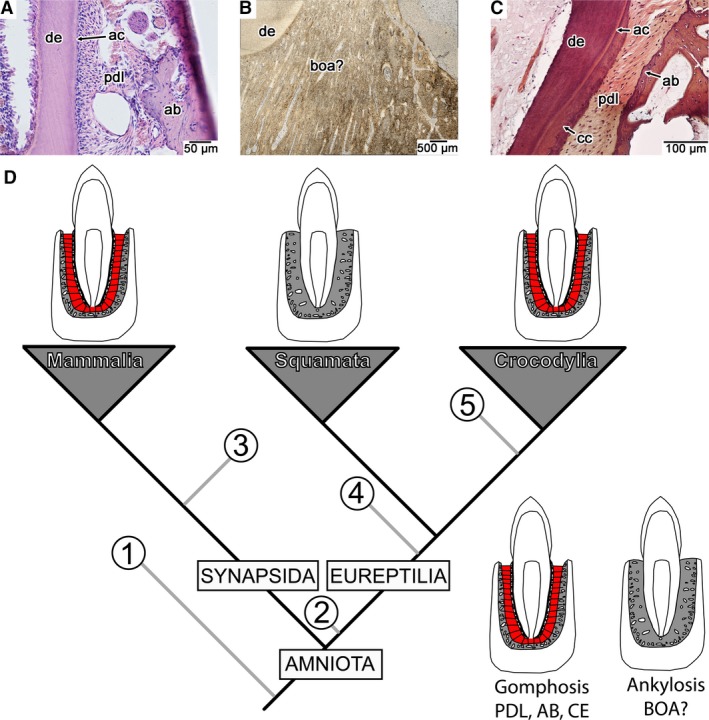
Dental attachment tissues and their distributions across extant, tooth‐bearing amniotes. (A) Transverse section of the attachment tissues in a modern Pika (AMF histology slide collection, cabinet 28 tray 19). (B) Coronal section of the attachment tissues in the extinct mosasaurid Platecarpus (UALVP 57044). (C) Coronal section of the attachment tissues in the crocodilian Caiman sclerops (CMNAR 25747‐4). (D) Phylogenetic relationships of the extant tooth‐bearing amniote clades and reported presence of cementum, PDL, and alveolar bone in extinct clades (grey branches). Among extinct clades, cementum, PDL, and alveolar bone have been reported in (1) diadectids (LeBlanc & Reisz, 2013), (2) mesosaurs (Pretto et al. 2014), (3) early synapsids (LeBlanc et al. 2016a), (4) ichthyosaurs and pliosaurids (Maxwell et al. 2011; Sassoon et al. 2015), (5) non‐avian dinosaurs and toothed birds (Dumont et al. 2016; Fong et al. 2016; García & Zurriaguz, 2016; LeBlanc et al. 2016b). ab, alveolar bone; ac, acellular cementum; boa?, tissue interpreted as ‘bone of attachment’ by previous authors; cc, cellular cementum; ce, cementum (acellular and cellular); de, dentine; pdl, periodontal ligament.
Here we re‐assess the periodontal tissues in the mosasaurids Platecarpus and Globidens using plain‐ and cross‐polarized light microscopy with an emphasis on the nature, development, and maintenance of the squamate PDL. We then compare attachment tissue histology and development to those of several species of extant snakes, which possess similar modes of tooth attachment to mosasaurids. Finally, we compare mosasaurid and snake dental tissue histology to that of the extant crocodilian Caiman sclerops to identify fundamental shifts in the evolution of the reptilian tooth attachment system between archosaurs and squamates.
Materials and methods
Material examined in this study was borrowed from the Canadian Museum of Nature, Ottawa, Canada (NMC), Smithsonian Museum, United States National Museum, Washington, DC, USA (USNM), University of Alberta Laboratory of Vertebrate Paleontology, Edmonton, Canada (UALVP), University of Alberta Museum of Zoology (UAMZ), and the University of Alberta, Department of Biological Sciences Advanced Microscopy Facility (AMF) histology slide collection. Three partial dentaries and two isolated teeth with intact roots of the mosasaurid Platecarpus (NMC 40957, NMC 40967, UALVP 57044, UALVP 57045, UALVP 57046) and a tooth root of the durophagous mosasaur Globidens (UALVP 51746) were sectioned. Thin sections of fossil material were made by first embedding specimens in either EpoThin epoxy or Castolite AC polyester resins and placed under vacuum. The encased specimens were then cut using a Buehler Isomet 1000 low‐speed wafer blade saw, ground down using a Hillquist grinding machine, and further ground using progressively finer grits of silicon carbide powder on a glass plate.
Dentaries of the snake species Cylindrophis rufus (CMNAR 35067), Ungaliophis continentalis (CMNAR 30958), Lichanurua trivirgata (CMNAR 27262), and Epicrates cenchria (CMNAR 35170), Acrochordus javanicus (CMNAR 25026), and Kolophis sp. (CMNAR 17921) were removed for sectioning, as well as a dentary of the crocodilian Caiman sclerops (CMNAR 25747‐4) and a pika (AMF). Thin sections of modern samples were made from ethanol‐preserved museum specimens (CMNAR, UAMZ) by L. Budney at the Advanced Microscopy Facility (AMF) in the University of Alberta Biological Sciences Department and Histo Best Inc., Edmonton, Alberta, originally as part of a Master's dissertation (Budney, 2004). The thin sectioning and staining methods employed by Budney (2004) are summarized here.
Sections were taken from preserved museum specimens. These specimens were preserved in 70% ethanol (some of which were initially preserved in formaldehyde). A 5‐mm portion of the posterior end of the right dentary was sampled for each specimen. Samples were decalcified in RDO, embedded in paraffin wax (AMF) or embedded in beeswax (Histo Best Inc.) and sectioned at 5 μm using table top microtomes. At least 10 slides were made of each specimen with approximately 15 sections per slide. Sections were then stained with Gabe's (1976) modified Gomori's trichome (stains preferentially for collagen), haematoxylin and eosin, or Masson's trichrome.
Thin sections were imaged using Nikon nis elements‐d imaging software, and a Nikon DS‐Fi3 camera mounted to a Nikon Eclipse E600 POL polarizing microscope. Images were taken of teeth at various stages of development in the holotype of the mosasaurid Eremiasaurus heterodontus (UALVP 51744), a skeletonized specimen of the snake genus Python (UALVP 57047), an isolated tooth of the mosasaurid Platecarpus (UALVP 53595), and the left dentary and an isolated tooth of the mosasaurid Globidens (UALVP 51746). Scanning electron microscope (SEM) images were also taken of the skull of the hinged‐tooth snake Xenopeltis unicolor (USNM 287277).
Results
Mosasaur tooth attachment
Each tooth in Platecarpus is ankylosed to the jaw by an extensive layer of highly vascularized, bone‐like tissue (Fig. 2). This tissue has been termed a pedicel of ‘bone of attachment’ (Rieppel & Kearney, 2005), or a thick layer of osteocementum (Caldwell et al. 2003). Transverse sections through ankylosed teeth of Platecarpus reveal that the dentine portion of the tooth root is coated in a thin, avascular band of acellular cementum (Caldwell et al. 2003), a root tissue also found in mammals and crocodilians (Miller, 1968; Nanci, 2013). The surrounding attaching tissue contains abundant vascular spaces that extend parallel to the apico‐occlusal axis of the tooth. Each vascular space is surrounded by concentric lamellae of calcified tissue, akin to the lamellar bone surrounding osteons, but the lamellae in this attachment tissue lack osteocyte or cementocyte lacunae (Fig. 2C). Caldwell et al. (2003) referred to these structures as cementeons and considered these to be the defining characteristic of osteocementum. For reasons highlighted later, we continue to refer to this tissue as osteocementum. Upon closer inspection, the calcified matrix forming the bulk of the osteocementum is unusual. What appear to be cell lacunae within the osteocementum are actually large, non‐mineralized collagen fiber bundles that are surrounded by concentric layers of acellular, calcified matrix (Fig. 2B,C). When viewed under cross‐polarized light, the osteocementum surrounding the dentine root is composed of thick, mineralized collagen fiber bundles that are all obliquely oriented towards the tip of the crown (Fig. 2D). These structures are more appropriately termed Sharpey's fibers and bear a strong similarity to transverse sections through the collagen fiber bundles in the crocodilian PDL (Berkovitz & Sloan, 1979).
Figure 2.
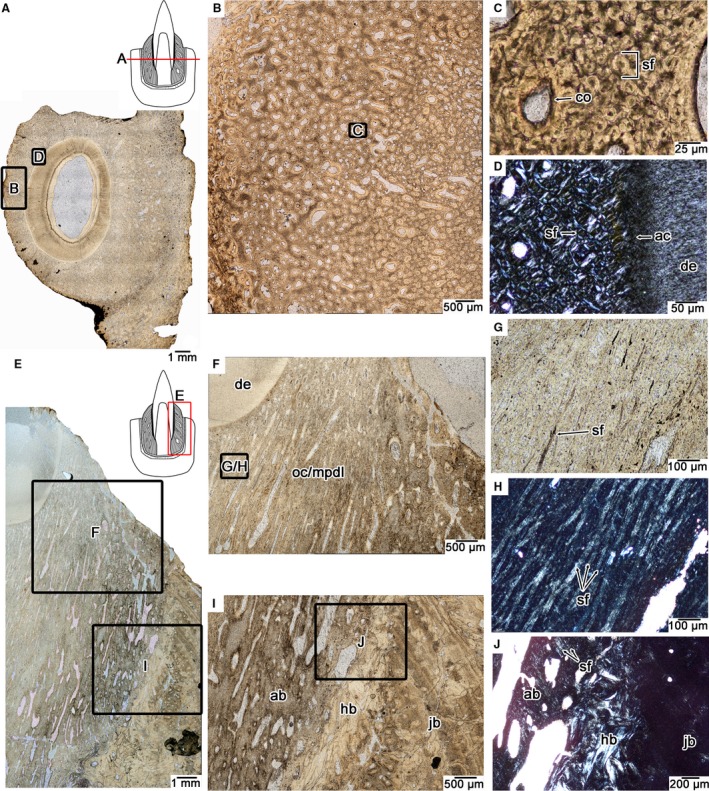
Tooth attachment tissue histology in the mosasaurid Platecarpus. (A) Wholeview of a transverse section through a single tooth root (UALVP 57045). (B) Closeup image of the tissue previously referred to as ‘bone of attachment’ (Rieppel & Kearney, 2005) or osteocementum (Caldwell et al. 2003) that fuses the tooth to the surrounding jawbone. (C) Closeup of (B) showing the fibrous microtexture of the attachment tissue. Most of the matrix is composed of Sharpey's fibers that extend parallel to the long axis of the tooth and are therefore circular in cross‐section. (D) Closeup of the attachment tissue surrounding the tooth root under cross‐polarized light, highlighting its fibrous microtexture. (E) Wholeview image of a Platecarpus dentary tooth in coronal section (UALVP 57044). (F) Closeup of (E) tooth attachment tissues in coronal view. (G) closeup of attachment tissue in coronal section under plain and cross‐polarized light (H) showing the network of Sharpey's fibers constituting the attachment tissue. (G and H) are taken from the same location. (I) Interface between the mineralized periodontal ligament, alveolar bone, remodelled attachment tissues, and jawbone. (J) Closeup image of alveolar bone and underlying remodelled attachment tissues. Note the presence of Sharpey's fibers, which mark the anchoring points for the periodontal ligament into the alveolar bone. ab, alveolar bone; ac, acellular cementum; co, cementeon; de, dentine; hb, Haversian bone; jb, bone of the jaw; mpdl, mineralized periodontal ligament; oc, osteocementum; sf, Sharpey's fibers.
Coronal sections through the teeth of Platecarpus confirm that the bulk of this tissue is actually a network of large Sharpey's fiber bundles. The only portions of these fiber bundles that are visible under plain‐polarized light are the dark, non‐mineralized cores, which only appear sporadically in coronal view (Fig. 2E–G). These bundles are best seen under cross‐polarized light (Fig. 2H), where their calcified outer shells are also visible and show that these bundles extend through the entire length of the osteocementum up to the acellular cementum coating the dentine of the tooth root. Each bundle and its calcified shell measure approximately 20 μm in total diameter, with the internal non‐mineralized core being approximately 5–8 μm across. These fiber bundles extend parallel to each other in coronal section, forming a highly organized network that is reminiscent of the PDL fibers that suspend the teeth in crocodilians and mammals. We interpret this tissue, which forms the bulk of the root in a mosasaur tooth (Caldwell, 2007), as osteocementum that has completely calcified the PDL in fully erupted teeth. Along the fringes of the alveoli, where the osteocementum and calcified PDL meets the jawbone, is a thin layer of woven bone that is also perforated by Sharpey's fibers (Fig. 2E,I,J). This layer is the alveolar bone and it is separated from the more external bone layers by a reversal line, indicative of resorption and redeposition following the formation of each tooth generation (Caldwell et al. 2003; Budney et al. 2006; Caldwell, 2007). External to the alveolar bone are successive layers of heavily remodelled bone that represent the previous generations of dental attachment tissues. This layer separates the functional alveolar bone layer from the jawbone proper, which is formed by fibrolamellar and parallel‐fibered bone (Fig. 2I,J).
Sections through isolated Platecarpus teeth that must have fallen out postmortem reveal the same Sharpey's fiber bundles along the peripheries of the intact roots, indicating that these teeth were held in their sockets by a non‐calcified PDL prior to the death of the animal (Fig. 3). Subsequent calcification of the PDL must have occurred along the peripheries of the tooth roots, preserving the orientations of each fiber bundle along the entire length of the ligament and gradually infilling the periodontal space with a calcified matrix. Based on previous work (Caldwell et al. 2003; Caldwell, 2007), thin section images of incompletely ankylosed teeth in the mosasaurine Clidastes (Rieppel & Kearney, 2005), and first‐hand examination of the teeth of other mosasaurids at successive stages of development (Fig. 3D), show that the mineralization front within the PDL proceeded from the surface of an initially thin layer of osteocementum along the root towards the alveolar bone as the tooth erupted into the oral cavity. Complete ankylosis was achieved when the mineralization front met the surrounding alveolar bone in fully erupted teeth (Fig. 3C,D,H). Given the observed centrifugal mineralization of the root tissue and the presence of cementeons (Figs 2C and 3F–H), we still designate the tissue surrounding the tooth root in a mosasaurid as osteocementum, although the cellular component is much smaller than previously recognized. One key difference is that we also acknowledge that this tissue contains the majority of the PDL within its calcified matrix and thus constitutes both osteocementum and the mineralized PDL (Figs 2 and 3).
Figure 3.
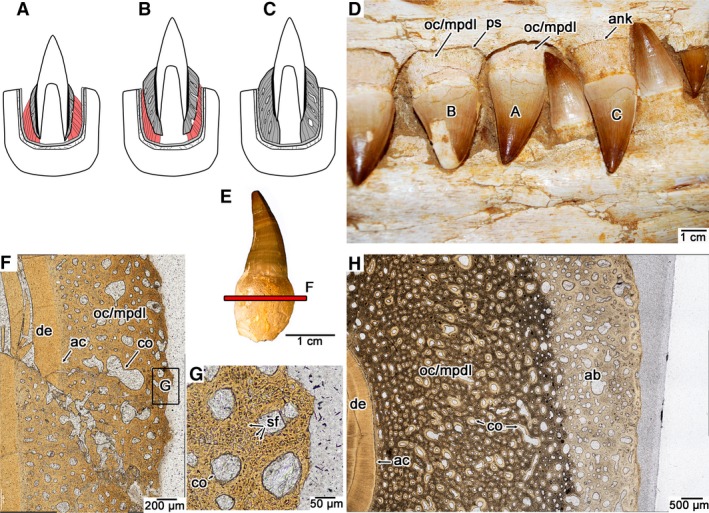
Ontogeny of tooth attachment in mosasaurids. (A) Early stage gomphosis in an erupting tooth, where cementum (darker grey) and alveolar bone (lighter grey) are incipiently developed, but must be attached by PDL (red). (B) Later stage gomphosis in an erupted tooth, where cementum (darker grey) is continuing to mineralize centrifugally, calcifying the PDL (red). (C) Ankylosis in an erupted tooth, where cementum (darker grey) meets the alveolar bone (lighter grey), resulting in a completely calcified PDL. (D) Image of a set of maxillary teeth from the holotype of the mosasaurid Eremiasaurus (UALVP 51744) at the three stages of dental ontogeny (specimen was not used for thin sectioning). (E) Isolated tooth of Platecarpus with an intact root (UALVP 53595). These types of teeth were not shed and must have fallen out of the jaws postmortem, due to decay of the PDL. (F) Closeup image of a transverse section through an isolated tooth root of Platecarpus (UALVP 57046) showing the early development of the cementum (which is mainly composed of Sharpey's fibers of the PDL, and thus is also termed the mineralized PDL here). (G) Closeup image of the external root surface in the cross‐section figured in (F), showing Sharpey's fibers entombed in the osteocementum. (H) Closeup image of an ankylosed tooth of Platecarpus (UALVP 57045) showing the completed centrifugal calcification of the PDL. ab, alveolar bone; ac, acellular cementum; ank, ankylosis; co, cementeon; de, dentine; mpdl, mineralized periodontal ligament; oc, osteocementum; ps, space for the periodontal ligament; sf, Sharpey's fibers.
We also sectioned an isolated tooth root of the durophagous mosasaur Globidens from the Maastrichtian (Late Cretaceous) of Morocco (Fig. 4). This tooth root was associated with a disarticulated skull, including both dentaries and numerous isolated teeth with intact roots. UALVP 51746 is tentatively assigned to Globidens phosphaticus, given the similarities in dental and cranial morphology to other material described from Angola and Morocco (Bardet et al. 2005; Polcyn et al. 2010). Stomach contents, tooth morphology, and skull element shape all indicate that Globidens was a specialist on hard‐shelled molluscs (Gilmore, 1912; Bardet et al. 2005; Martin, 2007; Martin & Fox, 2007; Polcyn et al. 2010) and makes for interesting comparisons of tooth attachment with the more generalist‐type dentition of Platecarpus. The dentaries of UALVP 51746 are nearly devoid of teeth, and in the case of the left dentary (Fig. 4A), all of the teeth have fallen out postmortem, which is consistent with other described jaws of G. phosphaticus (Polcyn et al. 2010). The frequency of postmortem tooth loss in this species led Polcyn et al. (2010) to hypothesize that the teeth of G. phosphaticus were held in place by soft tissue in life.
Figure 4.
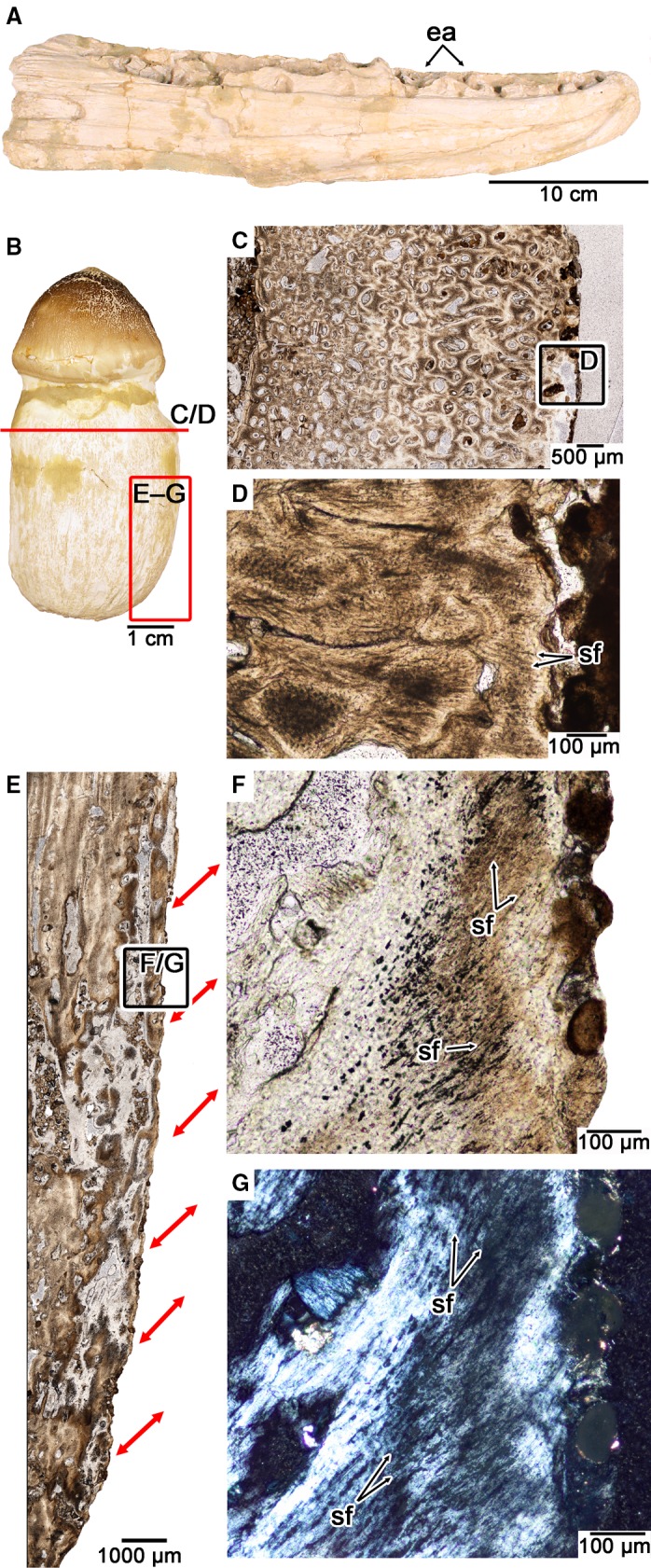
Tooth attachment tissue histology in the durophagous mosasaurid Globidens phosphaticus. (A) Medial view of the left dentary of UALVP 51746, referred to G. phosphaticus. (B) Isolated tooth of UALVP 51746 (this tooth was not used for thin sectioning). (C) Closeup of transverse section through the osteocementum of an isolated tooth root of UALVP 51746. (D) Closeup of the root surface in (C) showing radiating Sharpey's fibers. (E) Closeup of coronal section through the osteocementum of the same tooth root sectioned in (C). Red arrows indicate inclinations of PDL fiber bundles as indicated by Sharpey's fibers along the root surface. (F) Closeup of root surface in (E) showing the arrangement of Sharpey's fibers under plain polarized light. (G) Same image as (G) under cross‐polarized light. ea, empty alveoli; sf, Sharpey's fibers.
The thin sections of a tooth root of UALVP 51746 show that the root is composed entirely of osteocementum, but unlike the condition in Platecarpus, the external layers of this tissue possess radiating Sharpey's fibers that extend out to the surface of the tooth root in transverse section (Fig. 4B–D). In coronal section, the Sharpey's fibers form thick parallel bundles, similar to Platecarpus. However, these fiber bundles in G. phosphaticus extend crownwards and outwards in coronal section and terminate at the external surface of the root (Fig. 4E–G). Their orientation and position clearly indicate that these fibers are of extrinsic origin and are the mineralized portion of a highly organized PDL. The result is sling‐like arrangements of the fiber bundles of a PDL that extended into the root cementum of the teeth in G. phosphaticus (Fig. 4E).
The histology of the osteocementum in G. phosphaticus and the frequency of postmortem tooth loss (Fig. 4; Polcyn et al. 2010) provide the first evidence of a non‐mineralized, ligamentous tooth attachment in a mosasaurid. Histologically, the osteocementum in G. phosphaticus differs from that of Platecarpus in the orientations of the collagen fiber bundles that anchored the tooth to the socket. The other key difference is the extent of centrifugal mineralization of the osteocementum. Unlike in Platecarpus, the root cementum and alveolar bone in G. phosphaticus probably never met to ankylose a tooth to the jaws. The end result is a non‐calcified portion of the PDL that suspended the tooth in place. After death, the non‐mineralized portion of the PDL probably decayed, making the teeth more prone to falling out of the jaws, which is a preservational feature seen in other amniotes with a PDL attachment (LeBlanc & Reisz, 2013; LeBlanc et al. 2016b).
Snake tooth attachment
Our snake sample consisted of a broad taxonomic sampling of Alethinophidia: Cylindrophis (Anilioidea), Xenopeltis (Xenopeltidae), Ungaliophis, Lichanurua, and Epicrates (Booidae), Acrochordus (Caenophidia), and Kolophis (Elapidae; taxonomic assignments sensu Reeder et al. 2015). Teeth from all of the aforementioned genera were examined as histological sections, except for Xenopeltis, which was examined as a dried specimen (USNM 287277) under SEM and compared with histological descriptions of Xenopeltis and other hinge‐tooth snake tooth attachment by Savitsky (1981).
Zaher & Rieppel (1999) identified snake tooth implantation and attachment as a modified form of pleurodonty, where the teeth are ankylosed to the jaws by ‘bone of attachment’. Indeed, most snake teeth are ankylosed to the jaws by a unique bone‐like tissue that Tomes originally termed ‘bone of attachment’, but some exceptions occur in snakes with hinged teeth (Savitsky, 1981; Patchell & Shine, 1986), including Xenopeltis. In snakes with ankylosed teeth, the attachment tissue surrounding the bases of the teeth is a vascular, bone‐like tissue, similar to that in mosasaurids (Fig. 5). Most snake teeth are extremely small relative to those of even the smallest mosasaurid. This size discrepancy and the limited amount of attachment tissue anchoring snake teeth to the jaws makes it much more difficult to characterize without thin section data.
Figure 5.
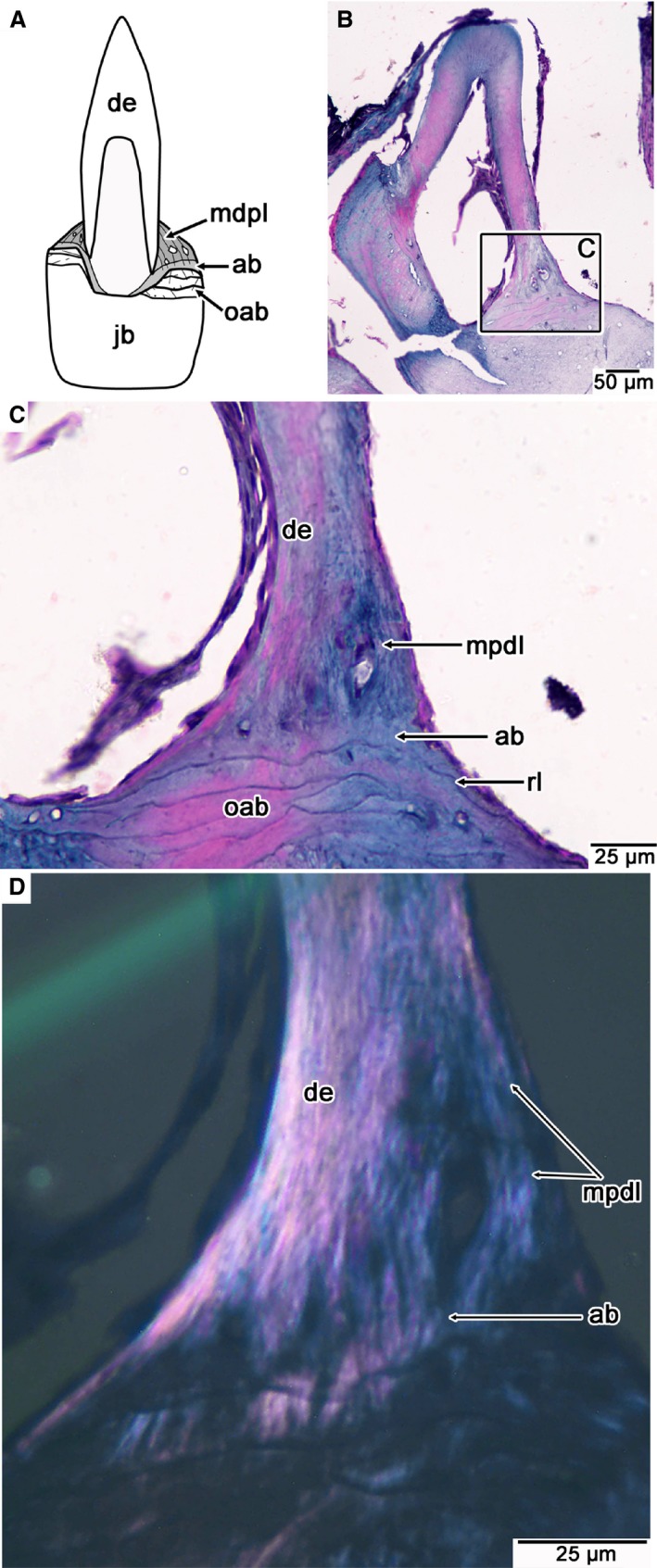
Tooth attachment tissue histology in the modern snake Ungaliophis. (A) Illustration of the general arrangements of attachment tissues in snakes with ankylosed teeth. Lingual is to the right. (B) Wholeview image of a coronal section through a dentary tooth of Ungaliophis. Lingual is to the right. (C) Closeup of attachment tissues along the lingual surface of the tooth root figured in (B). (D) Closeup of (C) under cross‐polarized light, highlighting the fibrous texture of the alveolar bone and MPDL. ab, alveolar bone; de, dentine; mpdl, mineralized periodontal ligament; oab, old generations of alveolar bone; rl, reversal line.
In thin section, the attachment tissue is restricted to a small portion of the base of the tooth that is usually thicker on the lingual side (Fig. 5A,B). This tissue is easily distinguished from the underlying bone of the jaw at higher magnifications by a reversal line, which marks bone resorption, followed by deposition of the new generation of attachment tissue (Fig. 5C). Several species actually show evidence of two zones within the attachment tissue: a calcified, fibrous tissue adjacent to the dentine root of the tooth, and a basal tissue that lacks parallel fibers (Figs 5C,D and 6A–D). The bone layer adjacent to the dentine contains vascular spaces and a high density of partially mineralized collagen fibers (Sharpey's fibers) that extend obliquely to the long axis of each tooth, similar to those in the calcified PDL of mosasaurids. Under cross‐polarized light, the fibrous tissue is organized into discrete, parallel fiber bundles that make the tissue readily distinguishable from the underlying jawbone (Figs 5C,D and 6A–H). The basal layer contains sparse vascular spaces but probably provides the anchorage point for the ligament fibers. These characteristics lead us to interpret the fibrous tissue as the calcified PDL, and the basal layer as alveolar bone. Alveolar bone has also been previously identified forming the tooth sockets in the early snake Dinilysia (Budney et al. 2006).
Figure 6.
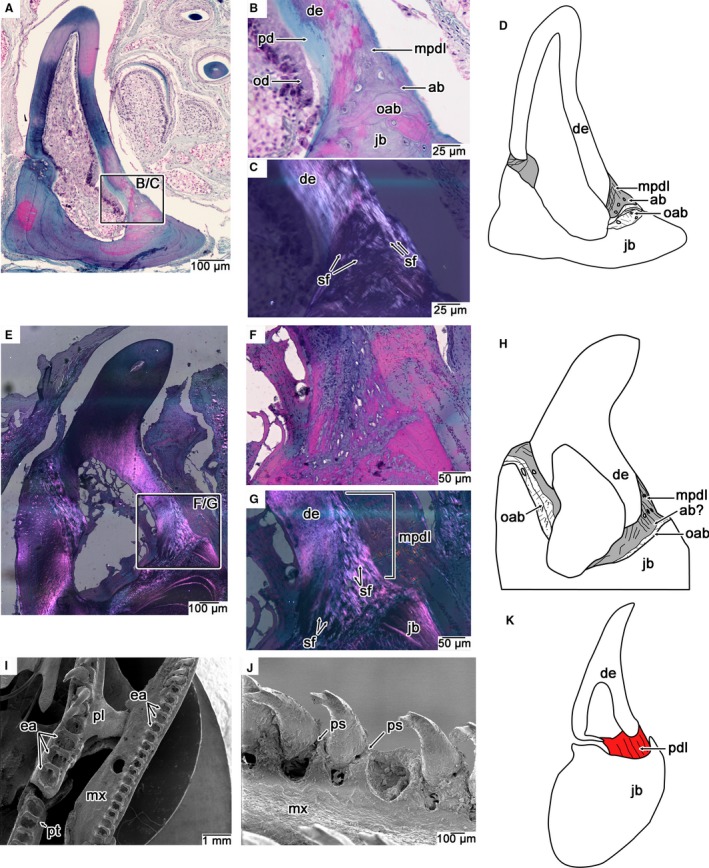
Comparisons of dental attachment in modern snakes with ankylosed and hinged teeth. (A) Wholeview image of a coronal section through a nearly completely ankylosed dentary tooth of Kolophis. Lingual is to the right. (B,C) Closeup images of the lingual attachment surface in (A) under plain (B) and cross‐polarized (C) light. Note the fibrous microtexture of the attachment tissue under cross‐polarized light. (D) Illustration of tooth attachment tissues in Kolophis, highlighting the position of the mineralized attachment tissues (grey). (E) Wholeview image of a coronal section through an ankylosed tooth of Cylindrophis under cross‐polarized light. Note the textural difference between the attachment tissue and jawbone under cross‐polarized light. (F,G) Closeup images of the lingual attachment surface in (F) cross‐polarized and (G) plain‐polarized light. Attachment tissues and jawbone are more easily distinguished by their fibrilar organization under cross‐polarized light. (H) Illustration of tooth attachment tissues in Cylindrophis, highlighting position of the mineralized attachment tissues (grey). (I) SEM of the left tooth‐bearing marginal and palatal bones of the hinged‐tooth snake Xenopeltis. Note the frequency of postmortem tooth loss due to decay of the ligamentous hinge. (J) Closeup of several hinged maxillary teeth of Xenopeltis showing positions of periodontal space where ligamentous hinge was situated in life. (K) Illustration of a histological section through a hinged maxillary tooth of Scaphiodontophis (lingual is to the right) taken from Savitsky (1981) showing position of the ligamentous hinge (red), here referred to as the PDL. ab, alveolar bone; de, dentine; mpdl, mineralized periodontal ligament; mx, maxilla; ea, empty alveoli; jb, jawbone; oab, old generations of alveolar bone; od, odontoblasts; pd, predentine; pdl, periodontal ligament; pl, palatine; ps, space for periodontal ligament; pt, pterygoid; sf, Sharpey's fibers.
SEM examination of the hinged‐tooth snake Xenopeltis provides evidence for a soft tissue attachment of the teeth to the sockets. Similar to the mosasaurid G. phosphaticus, the teeth of skeletonized specimens of Xenopeltis are typically nearly all missing due to decay of the ligament connection (Fig. 6I). Postmortem tooth loss has also been used to argue for hinged teeth in the early snake Dinilysia (Budney et al. 2006). Some of the teeth of Xenopeltis were still preserved in life position and show a gap between the tooth base and the alveolar margins that would have been spanned by the ligament in life (Fig. 6J). Moreover, histological studies of hinged snake teeth have revealed that this space, which is occupied by calcified fibrous tissue in snakes with ankylosed teeth, is occupied by a non‐calcified ligament that attaches to a basal layer of bone, thus forming an elastic hinge on one side of each tooth (Savitsky, 1981; Patchell & Shine, 1986; Budney et al. 2006; Fig. 6K).
In several examples, multiple layers of attachment tissue are found underneath the functional layer and are buried deep within the jaw (Figs 5C and 6). A reversal line separates each layer and they accumulate more along the lingual surfaces of the jaws and in between adjacent tooth positions (Figs 5, 6, 7, 8). Interdental ridges are typically considered stand‐alone wedges of bone that separate adjacent tooth positions (Zaher & Rieppel, 1999; Luan et al. 2009); however, our histological sections reveal an alternative origin of this tissue. Thin sections of the interdental ridges and socket margins reveal a clear reversal line separating each ridge from the underlying jawbone (Figs 7 and 8). The tissue making up each interdental ridge consists of layer‐caked arrangements of attachment tissues (Figs 7 and 8). Close histological inspection of the interdental ridges reveals remnants of alveolar bone (see also Caldwell et al. 2003), mineralized PDL (Figs 7 and 8A–D), and even remnants of dentine (Fig. 8F). These accumulations of attachment tissues are likely the remnants of dentine, alveolar bone, and mineralized PDL that were left behind following a replacement event. Interdental ridges, contra Zaher & Rieppel (1999), are therefore neither dental attachment tissue nor jawbone in snakes, but simply remnants of previous generations of tooth bases and tooth attachment tissues, which are found in other polyphyodont amniotes (Caldwell et al. 2003; LeBlanc & Reisz, 2013), including fossil snakes (Budney et al. 2006).
Figure 7.
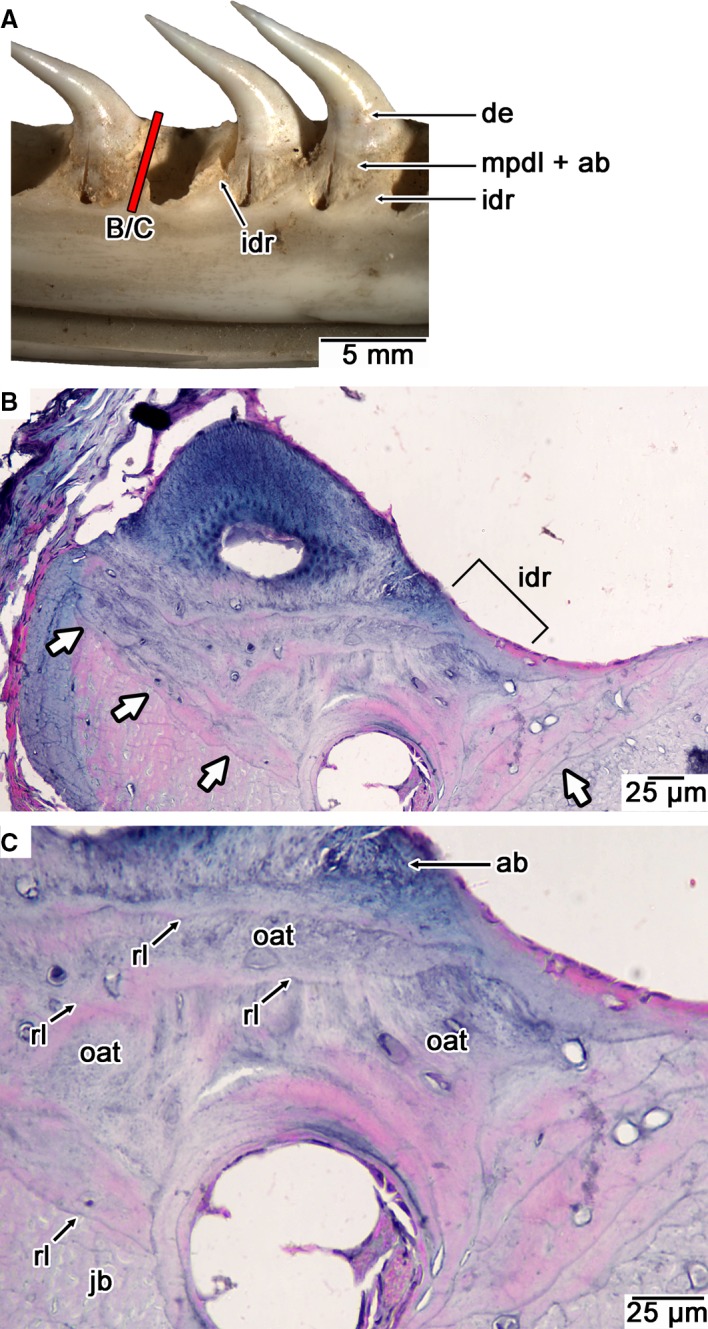
Histology of the interdental ridges in a modern snake. (A) Image of the interdental ridges in a modern Python (UALVP 57047). (B) Coronal section through an interdental ridge in Ungaliophis showing a reversal line between interdental ridge tissue and bone of the jaw (white arrows; lingual is to the right). (C) Closeup of interdental ridge in (B) showing layers of previous generations of tooth attachment tissue (alveolar bone and MPDL) separated by reversal lines. ab, alveolar bone; de, dentine; idr, interdental ridge; jb, bone of the jaw; mpdl, mineralized periodontal ligament; oat, old generations of tooth attachment tissue (alveolar bone and MPDL); rl, reversal line.
Figure 8.
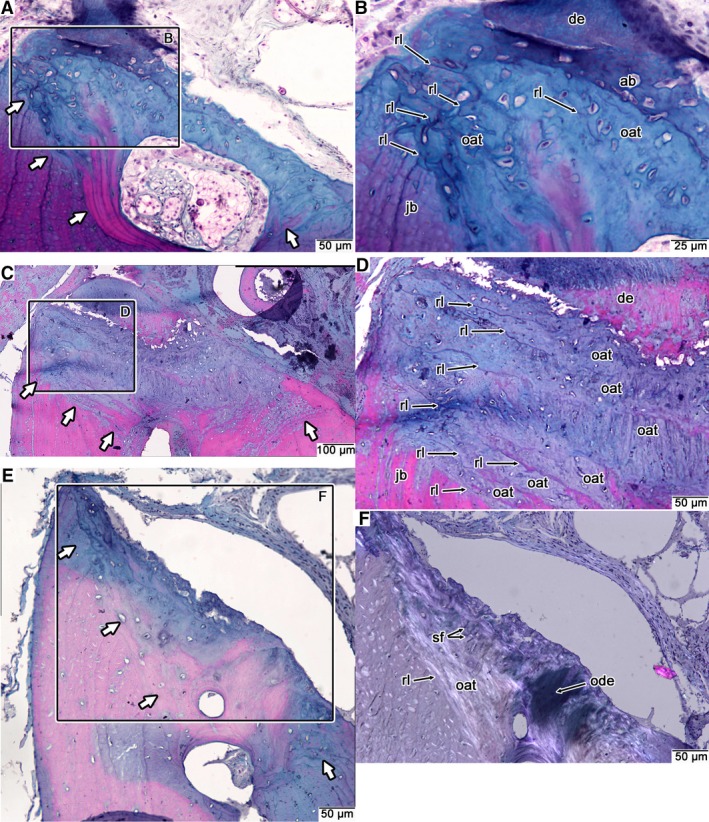
Comparative histology of snake interdental ridges. (A) Coronal section through an interdental ridge of Kolophis. (B) Closeup of interdental ridge in (A) showing the accumulations of old generations of attachment tissue that form the interdental ridge. (C) Coronal section through an interdental ridge in Cylindrophis. (D) Closeup of interdental ridge in (C) showing the numerous layers of previous generations of attachment tissue forming the interdental ridge. (E) Coronal section through an interdental ridge in Pituophis. (F) Closeup of interdental ridge in (E) under cross‐polarized light. Note the presence of a remnant of dentine and Sharpey's fibers from previous generations of attachment tissue that are barely visible under plain‐polarized light. White arrows indicate the deepest reversal line separating interdental ridge tissue from the bone of the jaw. ab, alveolar bone; de, dentine; jb, bone of the jaw; oat, old generations of tooth attachment tissue (alveolar bone and MPDL); ode, remnant of dentine; rl, reversal line; sf, Sharpey's fibers.
Unfortunately, we were unable to reconstruct an ontogenetic series of the attachment tissues in any snake because the teeth were either fully ankylosed to the jaws, or in the process of being replaced. The absence of intermediate stages of attachment tissue development suggests that ankylosis may be more rapid in snakes relative to the larger teeth of mosasaurids. In general, the teeth of most lizards fuse to the jaws extremely rapidly and the probability of capturing a tooth at an intermediate stage of attachment tissue mineralization is extremely low (Zahradnicek et al. 2014). Most snakes, with the exclusion of hinged teeth, appear to be no exception (Figs 5 and 6). In one instance, we observed a tooth of Kolophis that must have very recently attached to the jaws (Fig. 5B), but the attachment tissue had already completely calcified. This tissue had completely calcified prior to the calcification of the predentine along the base of the tooth. This suggests that the alveolar bone and mineralized PDL of snakes forms and calcifies completely at an extremely rapid rate and is not a product of the odontoblast layer, a process that has been suggested for the attachment tissues of other squamates (Buchtová et al. 2013). Without intermediate stages of tissue mineralization, we are unable to determine whether it is the alveolar bone that continues to calcify centripetally, as occurred in some fossil synapsids (LeBlanc et al. 2016a), or the cementum continues to calcify centrifugally, as it did in mosasaurids. We therefore simply refer to the calcified tissue attaching snake teeth to the alveolar bone as the mineralized PDL.
Crocodilian tooth attachment
Crocodilian tooth attachment histology and development have been studied extensively due to their similarity to those of mammals (Kvam, 1960; Miller, 1968; Peyer, 1968; Berkovitz & Sloan, 1979; McIntosh et al. 2002). The presence of polyphyodonty in crocodilians also makes them well suited for studying the development and ontogeny of the dental gomphosis, because a single specimen will have teeth at different stages of maturity (Fig. 9). Nearly erupted and recently erupted teeth are attached to the jaws by a highly organized network of PDL fiber bundles. Unlike in mosasaurids (except G. phosphaticus) or snakes, the PDL fibers are oriented such that their alveolar bone insertions are coronal to their insertions into the cementum (Fig. 9B–I). The result is a sling‐like organization of the PDL around the tooth root.
Figure 9.

Ontogeny of tooth attachment tissues in dentary teeth of the crocodilian Caiman sclerops. (A) Wholeview image of early stage erupting tooth (section angle missed the tooth crown) in coronal section. (B) Closeup of labial root surface in (A) showing early development of cementum. Only acellular cementum is visible along the labial surface of the tooth in this section. The PDL already attaches the tooth to the jaw, but alveolar bone has not ossified. (C) Closeup of lingual root surface in (A) showing early formation of cellular cementum and calcification of PDL fiber bundles along the root surface. (D) Wholeview image of a slightly later stage of attachment tissue formation in a dentary tooth (note the presence of an early stage of replacement tooth development). (E) Closeup of labial root surface showing thin layers of cellular cementum, well developed PDL, and early formation of alveolar bone. (F) Same image as (E) under cross‐polarized light, showing mineralized ends of PDL fiber bundles (Sharpey's fibers) in the cellular cementum. (G) Wholeview image of an older, partially resorbed functional tooth. (H) Closeup of labial root surface showing extensive development of cellular cementum, and alveolar bone. (I) Cross‐polarized light image of the mature periodontium, showing Sharpey's fibers (calcified PDL fiber bundles) within the cellular cementum, and the highly organized collagen fiber network of the PDL. ab, alveolar bone; ac, acellular cementum; cc, cellular cementum; ct, cementocyte; de, dentine; dl, dental lamina; ft, functional tooth; jb, bone of the jaw; ot, osteocyte; pdl, periodontal ligament; rl, reversal line; rt, replacement tooth; sf, Sharpey's fibers.
At early stages of dental ontogeny, the PDL attaches directly to the bone of the jaw and to very thin layers of acellular cementum and the first layers of cellular cementum along the tooth root (Fig. 9A,B). In place of the bulk of the cellular cementum is a mass of highly cellular, non‐calcified connective tissue that is composed of PDL fiber bundles, fibroblasts, and large, rounded cells that may be cementoblasts or cementoprogenitor cells (Fig. 9B). At progressively later ontogenetic stages, the fibrous, cellular mass surrounding the tooth calcifies centrifugally (Fig. 9D–F). Mineralization appears to occur in two ways: (i) cementoblasts form regular layers of cementum along the advancing mineralization front, eventually becoming entombed in the matrix, and differentiating into cementocytes; and (ii) calcification of the PDL fiber bundles, forming a jagged mineralization front that proceeds from the cementum surface outwards into the main body of the PDL (Fig. 9A–F).
Cementum formation in crocodilians is therefore a unidirectional, centrifugal calcification of connective tissue coating the tooth root, and entombs the ends of the PDL fiber bundles. The end result in mature, fully erupted teeth is a thick stratified mass of cellular cementum that contains numerous entombed cementocytes, and the mineralized ends of the ligament fiber bundles (Sharpey's fibers). At this stage, alveolar bone also ossifies along the fringes of the jawbone, forming a reversal line separating the bone of the jaw from the alveolar bone, which ossifies centripetally (Fig. 9G–I). Similar to the cellular cementum, the alveolar bone consists of entombed cells (osteocytes) and Sharpey's fibers from the PDL. Contrary to previous interpretations (Miller, 1968; McIntosh et al. 2002), the presence of a new layer of alveolar bone in the teeth of C. sclerops provides clear evidence of repeated development of alveolar bone with each newly formed tooth, instead of the alveolar bone remaining in place with subsequent tooth replacement (Berkovitz & Sloan, 1979).
Discussion
Mosasaurs and snakes have a PDL that normally mineralizes during dental ontogeny
Extensive debates concerning the nature and homology of mosasaurid and snake tooth attachment tissues have gradually improved our understanding of the evolution of the reptilian periodontium. Mosasaur and snake teeth were initially thought to attach to the jaws by ‘bone of attachment’ (Tomes, 1882; Zaher & Rieppel, 1999; Rieppel & Kearney, 2005), a tissue that was of unknown homology to crocodilian and mammalian attachment tissues (Peyer, 1968; Ten Cate, 1997; Budney et al. 2006). More recently, Caldwell et al. (2003), Budney et al. (2006), and Caldwell (2007) demonstrated the presence of acellular and cellular cementum coating the tooth roots in mosasaurs and snakes, which were in turn anchored to alveolar bone, thus explicitly hypothesizing a homology between squamate tooth attachment and that of crocodilians and mammals.
Luan et al. (2009) acknowledged the presence of root cementum in mosasaurs but suggested that mosasaur teeth fundamentally differed from those of crocodilians and mammals in being fused to an interdental ridge of bone via a mineralized PDL. Despite finding evidence for a mineralized PDL, Luan et al. (2009) and Liu et al. (2016) argued that mosasaurid tooth attachment could not be homologized with the three‐tissue attachment system (cementum, PDL, and alveolar bone) in crocodilians and mammals. In their view, there was no conclusive evidence that mosasaurid teeth were ever attached to a tooth socket formed by alveolar bone via a true PDL.
We disagree with this interpretation based on our new observations and interpretations: what was previously referred to as cellular cementum (Luan et al. 2009) or osteocementum (Caldwell et al. 2003) in mosasaurs preserves the main body of the PDL, which spans the entire width of the tooth socket and anchors into thin layers of alveolar bone (Figs 2 and 3). These collagen fiber bundles in mosasaurid attachment tissue are identical in appearance to the mineralized collagen fiber bundles in osteoderms (Scheyer & Sander, 2004), which in the latter case represent ossified collagen fiber bundles of the dermis (Vickaryous & Sire, 2009). Similarly then, in mosasaurs, the PDL probably persisted only temporarily as a mass of collagen fiber bundles (a PDL) supporting each erupting tooth before it completely calcified in ankylosed teeth (Fig. 3). Calcification of the PDL occurred centrifugally from the tooth root surface as the osteocementum continued to entomb the soft tissues of the PDL (Fig. 3). This interpretation is supported by the presence of a ligament fiber network preserved inside the osteocementum, which must have already been present before being encased in calcified tissue. Moreover, the teeth of the durophagous mosasaur G. phosphaticus provide strong evidence for a true, non‐mineralized PDL attachment of the teeth to the jaws in a mosasaurid (Fig. 4), a feature we hypothesize simply to be the result of impeding the complete calcification of the PDL. Moreover, the sling‐like arrangement of the PDL fibers that anchored the teeth of G. phosphaticus is reminiscent of the PDL fiber arrangement in extant crocodilians (Fig. 9) and may have provided compressive resistance for these shell‐crushing teeth.
A similar condition must occur in the teeth of fossil (Budney et al. 2006) and modern snakes (Savitsky, 1981; Patchell & Shine, 1986; Figs 5 and 6). Despite modest interest in the histology of hinged snake teeth, very little work has been done to characterize the microstructure and development of the calcified attachment tissues in extant snakes. Hermyt et al. (2017) described the attaching tissue in the egg teeth of the extant grass snake Natrix natrix as a poorly calcified, fibrous tissue, but beyond this work, the dearth of detailed examinations of snake tooth attachment histology has thus far perpetuated Tomes’ (1882) identification of this tissue as ‘bone of attachment’. The microstructure of these tissues can only be characterized in thin section and using cross‐polarized light, which has seldom been reported in squamates. Cross‐polarized light reveals the orientations of collagen fiber bundles in the PDL and the organization of the collagen fibers in calcified tissues (Figs 5 and 6). Such comparisons highlight the organized network of fiber bundles that span the mineralized attachment tissues in mosasaurs and snakes, which is inconsistent with any supposedly diagnostic features of ‘bone of attachment’ (Kearney et al. 2006; Luan et al. 2009). We hypothesize that, like mosasaurs, snake teeth are temporarily anchored to the jaws by a PDL that rapidly calcifies in erupted teeth. This would explain the microstructure of the attachment tissue we have identified as the mineralized PDL, which extends into the underlying alveolar bone (Fig. 4).
Mosasaur, snake, and crocodilian tooth attachment tissues are homologous
Zaher & Rieppel (1999) and Luan et al. (2009) distinguished squamate tooth attachment and implantation from true thecodonty in crocodilians and mammals based on the presence of ‘bone of attachment’ or a mineralized PDL, which is attached to an interdental ridge of bone that separates adjacent teeth. By comparison, true thecodont dentitions are set in discrete sockets of alveolar bone and attached by a soft PDL. The problem with this distinction is three‐fold.
First, as we show here and previously (Budney et al. 2006; Caldwell, 2007; LeBlanc & Reisz, 2013), the interdental ridges of bone in squamates, and amniotes more generally, form passively and are simply accumulations of previous generations of dentine, alveolar bone, and any other mineralized dental tissues following a replacement event (Figs 2, 7 and 8). Whereas these accumulations may alter the geometry of tooth attachment through ontogeny, these ridges are not tooth attachment tissues that form from the dental follicle with each successive tooth (the defining characteristic of tooth attachment tissues (Ten Cate & Mills, 1972; Ten Cate, 1997) and they therefore have no bearing on the complexity or evolution of tooth attachment, only tooth implantation (see the third objection below).
Secondly, we propose that mosasaurs, snakes, and crocodilians have the same tooth attachment tissues (Fig. 10), but are variably mineralized. Cementum, alveolar bone, and even a PDL have also been identified in numerous Paleozoic and Mesozoic amniotes (Maxwell et al. 2011; LeBlanc & Reisz, 2013; Pretto et al. 2014; Sassoon et al. 2015; Dumont et al. 2016; Fong et al. 2016; García & Zurriaguz, 2016; LeBlanc et al. 2016a,b), further emphasizing the evolutionary conservatism in amniote tooth attachment tissues. The similarity in tooth attachment tissue types between squamates and crocodilians, coupled with mounting evidence of phylogenetic congruence across Amniota, strongly suggest that tooth attachment tissues in crocodilians, squamates, and mammals are homologous. We reject the hypothesis that mammals and crocodilians independently evolved a three‐part tooth attachment system (including a PDL), and provide an alternative, echoed by several recent studies: cementum, PDL, and alveolar bone are plesiomorphic features for the major amniote clades, including squamates.
Figure 10.
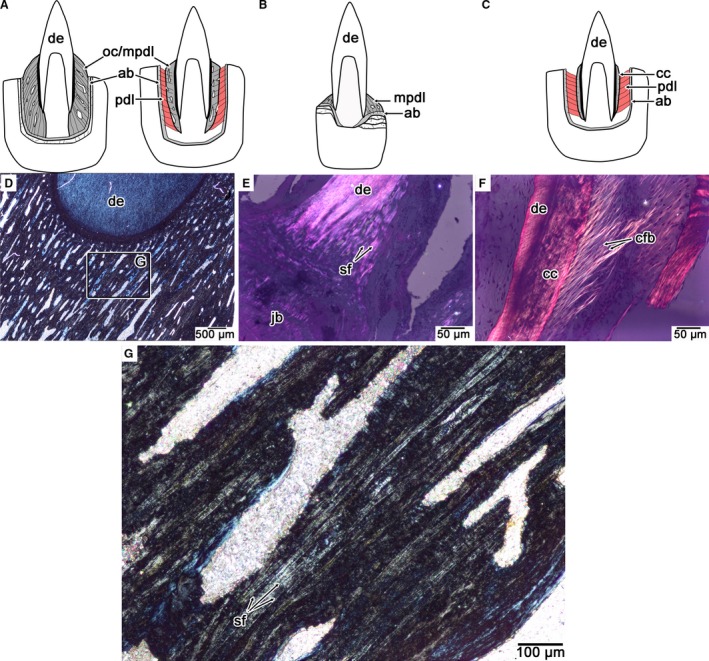
Comparisons of PDL orientation and calcification in mosasaurids, snakes, and crocodilians. (A) Calcified PDL in an ankylosed mosasaurid tooth (thecodont ankylosis), exemplified by Platecarpus (left) and the non‐ankylosed tooth (thecodont gomphosis) of Globidens phosphaticus (right). (B) Calcified PDL in an ankylosed snake tooth (pleurodont ankylosis). (C) Partly calcified PDL in a crocodilian tooth (thecodont gomphosis). (D) Cross‐polarized light image of the calcified PDL in an ankylosed mosasaurid tooth, highlighting the network of calcified PDL fiber bundles (stage and image slightly rotated in order to see the Sharpey's fibers). (E) Cross‐polarized light image of the calcified PDL in the snake Cylindrophis highlighting the network of calcified PDL fiber bundles (stage and image slightly rotated in order to see the Sharpey's fibers). (F) Cross‐polarized light image of the partially calcified PDL of Caiman highlighting the network of non‐calcified PDL fiber bundles. (G) Closeup of the calcified PDL in (A) highlighting the organized network of Sharpey's fibers that form the periodontium in ankylosed mosasaurid teeth. ab, alveolar bone; cc, cellular cementum; cfb, collagen fiber bundle; mpdl, mineralized periodontal ligament; oc, osteocementum; pdl, periodontal ligament; sf, Sharpey's fibers.
Thirdly, tooth implantation and attachment describe two separate features of a vertebrate dentition and have separate evolutionary significance. As noted by previous authors (Owen, 1840; Caldwell et al. 2003; Maxwell et al. 2011; LeBlanc & Reisz, 2013; Dumont et al. 2016; Fong et al. 2016) thecodonty is a geometric term related to the amount of bony support around a tooth, whereas tooth attachment describes the degree of calcification of the tooth to the jaw. Thecodonty displays extensive homoplasy across Amniota (Dumont et al. 2016) and occurs when the bony support for the tooth is symmetrical on the labial and lingual sides, encapsulating the long root of a tooth. As such, we distinguish mosasaur and crocodilian tooth implantation and attachment as thecodont ankylosis and thecodont gomphosis, respectively, thus acknowledging their topological similarities and their differences in degree of calcification of the supporting tissues (the tissues themselves being homologous; Fig. 8). The one exception among mosasaurids would be the teeth of G. phosphaticus, which possesses a thecodont gomphosis, similar to crocodilians (Fig. 4). This would not represent an independent acquisition of a true PDL attachment within Mosasauridae but simply a lack of the complete calcification of the mosasaurid PDL (Fig. 10A). Snakes, however, have a much shallower form of implantation that approaches a pleurodont ankylosis in most forms (Zaher & Rieppel, 1999). Most snake teeth show evidence of extremely rapid calcification of the attachment tissues, but the highly organized calcified matrix strongly suggests the presence of a transient ligamentous connection. The difference between snakes and mosasaurs are related to geometry of the attachment tissues and the supporting jawbone (tooth implantation) and not attachment tissue identity (tooth attachment; Fig. 10).
Timing and extent of PDL calcification determines tooth attachment mode in reptiles
Crocodilian periodontia consist of a large contingent of calcified tissue (cellular cementum) but are still attached to the jaws by PDL, leading some to refer to crocodilian tooth attachment as an intermediate between a mammalian gomphosis and reptilian ankylosis (Kvam, 1960; Miller, 1968; McIntosh et al. 2002; Luan et al. 2009). Indeed, compared with the teeth of humans and other mammals (Fig. 1A), crocodilian teeth are coated in an extensive amount of cellular cementum that seemingly entombs the PDL space as the tissue continues to calcify (Fig. 9G–I). McIntosh et al. (2002) also demonstrated that the caiman PDL exhibits multiple mineralization foci, suggesting that the PDL itself also retains the ability to calcify. This is the normal condition for crocodilian periodontal tissue formation and maintenance, but in mammals a similar condition can occur pathologically and to a greater extent. Following trauma or infection, or even as an effect of ageing (Ive et al. 1980; Lim et al. 2014), the mammalian PDL can be encroached upon by bone or cementum, forming an ankylotic attachment (Atrizadeh et al. 1971; Andersson et al. 1984; Beertsen et al. 1997; Andreasen, 2012). Whereas many pathological forms of dental ankylosis in mammals are largely destructive and bear little resemblance to dental ankylosis in other amniotes, experimental manipulations of PDL homeostasis in mouse models have shown that alveolar bone or cementum can continue to mineralize and reduce the space for the PDL (Lim et al. 2014, 2016). Moreover, the mammalian PDL consists of small populations of osteoblasts and cementoblasts and thus retains the capacity to form mineralized tissues (Beertsen et al. 1997; Handa et al. 2002; Andreasen, 2012). This latent ability of the mammalian PDL to calcify led LeBlanc et al. (2016a) to hypothesize that the ancestral condition for Synapsida (the amniote group to which mammals belong) was for the PDL to calcify completely and be entombed by extensive formation of alveolar bone, forming a stable ankylosis of the teeth to the jaws in non‐mammalian synapsids.
The data presented here highlight an alternative strategy for forming a stable ankylosis (i.e. one that does not lead to pathological root resorption) in snakes and mosasaurids. Studying mosasaurid dental ontogeny reveals two possibilities: (i) the cellular cementum rapidly calcifies centrifugally, entombing the PDL, and eventually reaching a thin layer of alveolar bone; or (ii) the PDL itself calcifies centrifugally from the cementum surface outwards (Figs 2 and 3). Concerning the former possibility, McIntosh et al. (2002) proposed that the crocodilian PDL develops calcified masses within the ligament itself as well as along the cementum surface. We found supporting evidence for calcification of the PDL in the form of Sharpey's fibers that extended past the cementum mineralization front (Fig. 9C). Alternatively, Zahradnicek et al. (2014) showed that small populations of cementoblasts formed the attachment tissue in an extant species of gecko.
It is currently unclear which is more likely in mosasaurids. Regardless of the origin of the mineralization front in mosasaurids, the end result is the same: a calcified mass coating the root of the tooth that is composed almost entirely of calcified fiber bundles from the PDL (Figs 2, 3, 4). The difference between early synapsid ankylosis (LeBlanc et al. 2016a) and that of mosasaurids is therefore not related to tissue identity, but simply to the direction of the PDL mineralization front, with the direction of mineralization being centrifugal (outwards from the cementum surface) in mosasaurids and centripetal (from the alveolar bone inwards) in synapsids (LeBlanc et al. 2016a).
Unfortunately, the rate of calcification of the PDL in most snakes must be so rapid as to obscure which tissue, cementum or alveolar bone, forms the dental ankylosis. However, the arrangements of the tooth attachment tissues in Xenopeltis (Savitsky, 1981), other squamates with hinged teeth (Patchell & Shine, 1986; Budney et al. 2006), and Caiman (Berkovitz & Sloan, 1979) lead us to hypothesize that delaying or inhibiting the calcification of the PDL, rather than repeated independent acquisitions of cementum, PDL, and alveolar bone, results in a gomphosis‐type tooth attachment in reptiles. Even among mosasaurids, there appears to be variation in tooth attachment mode, with some species showing evidence of a gomphosis‐type tooth attachment (Polcyn et al. 2010; Fig. 4). Under the classical paradigm, such species would have to convergently evolve cementum, PDL, and alveolar bone, whereas our alternative hypothesis posits that these instances simply represent losses of the end stage of reptilian dental ontogeny: ankylosis. The squamate periodontium therefore retains the ability to form the three tooth attachment tissues common to mammals and crocodilians, a feature that is probably the plesiomorphic condition for all amniotes (LeBlanc & Reisz, 2013).
Whereas our hypothesis would seem to be at odds with the traditional classification of squamate dental attachment (Tomes, 1882; Peyer, 1968; Zaher & Rieppel, 1999) and more recent developmental studies in chameleonids and Sphenodon (Kieser et al. 2009; Dosedělová et al. 2016), where a single, ‘bone of attachment’‐like tissue has been described, there are two important reasons for this type of histological study to be pursued further in other squamate lineages. First, the acrodont dentitions of chameleonids and Sphenodon consist of an extremely reduced periodontium and an apomorphic relationship of the teeth to their attachment tissues and the jawbones (Kieser et al. 2009; Buchtová et al. 2013; Dosedělová et al. 2016). This in turn may confound our understanding of dental tissue complexity in modern reptiles and should therefore be explored further in other squamates. Secondly, previous histological studies of pleurodont dentitions in some modern squamates show evidence of cementum‐like root tissues and ligamentous tooth connections (Patchell & Shine, 1986; Gaengler & Metzler, 1992; Gaengler, 2000). More recently, histological work has demonstrated the presence of a PDL along the base of the tooth in modern gecko (McIntosh et al. 2002) and root cementum in extant iguanians (Luan et al. 2009), and developmental work by Zahradnicek et al. (2014) has shown the presence of cementoblast‐like cells forming a portion of the mineralized attachment tissue complex in a species of extant gecko. These findings challenge the conventional, single‐tissue model of reptilian tooth attachment, where ‘bone of attachment’ fuses the tooth to the jaws. Given the underlying fibrous network of the mosasaurid (Figs 2, 3, 4) and snake (Savitsky, 1981; Hermyt et al. 2017; Figs 5 and 6) periodontal tissues, squamate tooth attachment tissue development should be re‐examined under the evolutionary and developmental paradigms we propose here.
Conclusions
Evolutionary and developmental studies have independently shown that the amniote periodontium is a modular entity consisting of three attachment tissues (cementum, PDL, and alveolar bone; Ten Cate & Mills, 1972; Ten Cate, 1997; Gaengler, 2000; Luan et al. 2009; Maxwell et al. 2011; LeBlanc & Reisz, 2013; Dumont et al. 2016; Fong et al. 2016; LeBlanc et al. 2016a). These tissues are variably developed across Amniota to produce the observed diversity of tooth attachment and implantation modes (Gaengler, 2000; Luan et al. 2009; LeBlanc & Reisz, 2013; LeBlanc et al. 2016a). Squamates have until recently been treated as the exception to this rule, because they seemingly possess a simple, single‐tissue attachment system consisting of ‘bone of attachment’ (Tomes, 1882; Peyer, 1968; Zaher & Rieppel, 1999). Mosasaurids have served as a pivotal taxon in helping to unravel the underlying complexity of ankylosed teeth in squamates, but even the identity of these attachment tissues has remained a point of contention (Caldwell et al. 2003; Rieppel & Kearney, 2005; Luan et al. 2009). Our re‐interpretation of mosasaurid and snake periodontia and comparisons with periodontal ontogeny in extant crocodilians reveals that the differences lie not in the identity of the tissues themselves but in attachment geometry (dictated by jawbone and accumulations of old dental tissues) and whether the periodontium completely calcifies. We conclude that, similar to extinct synapsids (LeBlanc et al. 2016a), evolutionary changes to the relative timing of dental tissue formation and calcification dictate tooth attachment mode (gomphosis or ankylosis) in extinct and modern reptiles.
Author contributions
A.R.H.L. conceived the study, A.R.H.L., M.W.C. contributed data, A.R.H.L., M.W.C., D.O.L. analyzed the data, A.R.H.L. drafted the manuscript, M.W.C., D.O.L. provided critical revisions to the manuscript.
Acknowledgements
A.R.H.L. thanks the Killam Trustees for financial support through an Izaak Walton Killam Memorial Postdoctoral Fellowship. Additional financial support was provided by an NSERC Discovery Grant (#23458) and Research Chair's Allowance to M.W.C. The authors also thank E. Maxwell and L. Budney for their assistance in making thin sections that were used in this study. Lastly we thank two anonymous reviewers for their constructive comments and suggestions, which improved this manuscript.
References
- Andersson L, Blomlöf L, Lindskog S, et al. (1984) Tooth ankylosis: Clinical, radiographic and histological assessments. Int J Oral Surg 13, 423–431. [DOI] [PubMed] [Google Scholar]
- Andreasen JO (2012) Pulp and periodontal tissue repair – regeneration or tissue metaplasia after dental trauma. A review: Pulp and periodontal tissue repair. Dent Traumatol 28, 19–24. [DOI] [PubMed] [Google Scholar]
- Atrizadeh F, Kennedy J, Zander H (1971) Ankylosis of teeth following thermal injury. J Periodontal Res 6, 159–167. [DOI] [PubMed] [Google Scholar]
- Bardet N, Suberbiola XP, Iarochene M, et al. (2005) Durophagous Mosasauridae (Squamata) from the Upper Cretaceous phosphates of Morocco, with description of a new species of Globidens . Netherlands J Geosci 84, 167–175. [Google Scholar]
- Beertsen W, McCulloch CA, Sodek J (1997) The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000 13, 20–40. [DOI] [PubMed] [Google Scholar]
- Berkovitz BKB, Sloan P (1979) Attachment tissues of the teeth in Caiman sclerops (Crocodilia). J Zool 187, 179–194. [Google Scholar]
- Buchtová M, Zahradníček O, Balková S, et al. (2013) Odontogenesis in the Veiled Chameleon (Chamaeleo calyptratus). Arch Oral Biol 58, 118–133. [DOI] [PubMed] [Google Scholar]
- Budney LA (2004) A Survey of Tooth Attachment Histology in Squamata: The Evaluation of Tooth Attachment Classifications and Characters. Edmonton: University of Alberta. [Google Scholar]
- Budney LA, Caldwell MW, Albino A (2006) Tooth socket histology in the Cretaceous snake Dinilysia, with a review of Amniote dental attachment tissues. J Vert Paleontol 26, 138–145. [Google Scholar]
- Caldwell MW (2007) Ontogeny, anatomy and attachment of the dentition in mosasaurs (Mosasauridae: Squamata). Zool J Linn Soc 149, 687–700. [Google Scholar]
- Caldwell MW, Budney LA, Lamoureux DO (2003) Histology of tooth attachment tissues in the Late Cretaceous mosasaurid Platecarpus . J Vert Paleontol 23, 622–630. [Google Scholar]
- Dosedělová H, Štěpánková K, Zikmund T, et al. (2016) Age‐related changes in the tooth‐bone interface area of acrodont dentition in the chameleon. J Anat 229, 356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont M, Tafforeau P, Bertin T, et al. (2016) Synchrotron imaging of dentition provides insights into the biology of Hesperornis and Ichthyornis, the ‘last’ toothed birds. BMC Evol Biol 16, 178. doi 10.1186/s12862‐016‐0753‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong RKM, LeBlanc ARH, Berman DS, et al. (2016) Dental histology of Coelophysis bauri and the evolution of tooth attachment tissues in early dinosaurs. J Morphol 277, 916–924. [DOI] [PubMed] [Google Scholar]
- Gabe M (1976) Histological Techniques. New York: Springer‐Verlag. [Google Scholar]
- Gaengler P (2000) Evolution of tooth attachment in lower vertebrates to tetrapods In: Development, Function and Evolution of Teeth. (eds Teaford MF, Smith MM, Ferguson MWJ.), pp. 173–185, Cambridge: Cambridge University Press. [Google Scholar]
- Gaengler P, Metzler E (1992) The periodontal differentiation in the phylogeny of teeth – an overview. J Periodontal Res 27, 214–225. [DOI] [PubMed] [Google Scholar]
- García RA, Zurriaguz V (2016) Histology of teeth and tooth attachment in titanosaurs (Dinosauria; Sauropoda). Cretac Res 57, 248–256. [Google Scholar]
- Gilmore CW (1912) A new mosasauroid reptile from the Cretaceous of Alabama. Proc U S Nat Mus 40, 484–489. [Google Scholar]
- Handa K, Saito M, Yamauchi M, et al. (2002) Cementum matrix formation in vivo by cultured dental follicle cells. Bone 31, 606–611. [DOI] [PubMed] [Google Scholar]
- Hermyt M, Kaczmarek P, Kowalska M, et al. (2017) Development of the egg tooth – The tool facilitating hatching of squamates: Lessons from the grass snake Natrix natrix . Zool Anz 266, 61–70. [Google Scholar]
- Ive JC, Shapiro PA, Ivey JL (1980) Age‐related changes in the periodontium of pigtail monkeys. J Periodontal Res 15, 420–428. [DOI] [PubMed] [Google Scholar]
- Kearney M, Rieppel O, Wood RM (2006) An investigation into the occurrence of plicidentine in the teeth of squamate reptiles. Copeia 2006, 337–350. [Google Scholar]
- Kieser JA, Tkatchenko T, Dean MC, et al. (2009) Microstructure of dental hard tissues and bone in the Tuatara dentary, Sphenodon punctatus (Diapsida: Lepidosauria: Rhynchocephalia). Dent Tiss 13, 80–85. [DOI] [PubMed] [Google Scholar]
- Kvam T (1960) The teeth of Alligator mississippiensis (Daud) VI. Periodontium. Acta Odontol Scand 18, 67–82. [DOI] [PubMed] [Google Scholar]
- LeBlanc ARH, Reisz RR (2013) Periodontal ligament, cementum, and alveolar bone in the oldest herbivorous tetrapods, and their evolutionary significance. PLoS One 8, e74697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc ARH, Reisz RR, Brink KS, et al. (2016a) Mineralized periodontia in extinct relatives of mammals shed light on the evolutionary history of mineral homeostasis in periodontal tissue maintenance. J Clin Periodontol 43, 323–332. [DOI] [PubMed] [Google Scholar]
- LeBlanc ARH, Reisz RR, Evans DC, et al. (2016b) Ontogeny reveals function and evolution of the hadrosaurid dinosaur dental battery. BMC Evol Biol 16, 152. doi 10.1186/s12862‐016‐0721‐1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MSY (1997) The phylogeny of varanoid lizards and the affinities of snakes. Philos Trans R Soc Lond B Biol Sci 352, 53–91. [Google Scholar]
- Lim WH, Liu B, Mah SJ, et al. (2014) The molecular and cellular effects of ageing on the periodontal ligament. J Clin Periodontol 41, 935–942. [DOI] [PubMed] [Google Scholar]
- Lim WH, Liu B, Mah SJ, et al. (2016) Alveolar bone turnover and periodontal ligament width are controlled by Wnt. J Periodontol 86, 319–326. [DOI] [PubMed] [Google Scholar]
- Liu M, Reed DA, Cecchini GM, et al. (2016) Varanoid tooth eruption and implantation modes in a late cretaceous mosasaur. Front Physiol 7, 145. doi 10.3389/fphys.2016.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan X, Walker C, Dangaria S, et al. (2009) The mosasaur tooth attachment apparatus as paradigm for the evolution of the gnathostome periodontium. Evol Dev 11, 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JE (2007) A new species of the durophagous mosasaur Globidens (Squamata: Mosasauridae) from the Late Cretaceous Pierre Shale Group of central South Dakota, USA. Geol Soc Am Spec Pap 427, 177–198. [Google Scholar]
- Martin JE, Fox JE (2007) Stomach contents of Globidens, a shell‐crushing mosasaur (Squamata), from the Late Cretaceous Pierre Shale Group, Big Bend area of the Missouri River, central South Dakota. Geol Soc Am Spec Pap 427, 167–176. [Google Scholar]
- Maxwell EE, Caldwell MW, Lamoureux DO (2011) Tooth histology, attachment, and replacement in the Ichthyopterygia reviewed in an evolutionary context. Paläontol Zeitsch 86, 1–14. [Google Scholar]
- McIntosh JE, Anderton X, Flores‐De‐Jacoby L, et al. (2002) Caiman periodontium as an intermediate between basal vertebrate ankylosis‐type attachment and mammalian ‘true’ periodontium. Microsc Res Tech 59, 449–459. [DOI] [PubMed] [Google Scholar]
- Miller WA (1968) Periodontal attachment apparatus in the young Caiman sclerops . Arch Oral Biol 13, 735–743. [DOI] [PubMed] [Google Scholar]
- Nanci A (2013) Ten Cate's Oral Histology: Development, Structure, and Function. St. Louis: Elsevier Mosby. [Google Scholar]
- Osborn JW (1984) From reptile to mammal: evolutionary considerations of the dentition with emphasis on tooth attachment. Symp Zool Soc Lond 52, 549–574. [Google Scholar]
- Owen R (1840) Odontography; or, a Treatise on the Comparative Anatomy of the Teeth; Their Physiological Relations, Mode of Development, and Microscopic Structure, in the Vertebrate Animals. London: Hippolyte Bailliere. [PMC free article] [PubMed] [Google Scholar]
- Patchell FC, Shine R (1986) Hinged teeth for hard‐bodied prey: a case of convergent evolution between snakes and legless lizards. J Zool 208, 269–275. [Google Scholar]
- Peyer B (1968) Comparative Odontology. Chicago: The University of Chicago Press. [Google Scholar]
- Polcyn MJ, Jacobs LL, Schulp AS, et al. (2010) The North African Mosasaur Globidens phosphaticus from the Maastrichtian of Angola. Hist Biol 22, 175–185. [Google Scholar]
- Pretto F, Cabreira SF, Schultz CL (2014) Tooth microstructure of the Early Permian aquatic predator Stereosternum tumidum and paleobiological implications. Acta Palaeontol Pol 59, 125–133. [Google Scholar]
- Reeder TW, Townsend TM, Mulcahy DG, et al. (2015) Integrated analyses resolve conflicts over squamate reptile phylogeny and reveal unexpected placements for fossil taxa. PLoS One 10, e0118199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisz RR (1997) The origin and early evolutionary history of amniotes. Trends Ecol Evol 12, 218–222. [DOI] [PubMed] [Google Scholar]
- Rieppel O (2001) Tooth implantation and replacement in Sauropterygia. Paläontol Zeitsch 75, 207–217. [Google Scholar]
- Rieppel O, Kearney M (2005) Tooth replacement in the Late Cretaceous mosasaur Clidastes. J Herpetol 39, 688–692. [Google Scholar]
- Sassoon J, Foffa D, Marek R (2015) Dental ontogeny and replacement in Pliosauridae. R Soc Open Sci 2, 150384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitsky AH (1981) Hinged teeth in snakes: an adaptation for swallowing hard‐bodied prey. Science 212, 346–349. [DOI] [PubMed] [Google Scholar]
- Scheyer TM, Sander PM (2004) Histology of ankylosaur osteoderms: implications for systematics and function. J Vert Paleontol 24, 874–893. [Google Scholar]
- Ten Cate AR (1997) The development of the periodontium – a largely ectomesenchymally derived unit. Periodontol 2000 13, 9–19. [DOI] [PubMed] [Google Scholar]
- Ten Cate AR, Mills C (1972) The development of the periodontium: the origin of alveolar bone. Anat Rec 173, 69–77. [DOI] [PubMed] [Google Scholar]
- Tomes CS (1882) A Manual of Dental Anatomy: Human and Comparative. Philadelphia: Presley Blakiston. [Google Scholar]
- Vickaryous MK, Sire JY (2009) The integumentary skeleton of tetrapods: origin, evolution, and development. J Anat 214, 441–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaher H, Rieppel O (1999) Tooth implantation and replacement in squamates, with special reference to mosasaur lizards and snakes. Am Mus Nov (3271), 1–19. [Google Scholar]
- Zahradnicek O, Buchtova M, Dosedelova H, et al. (2014) The development of complex tooth shape in reptiles. Front Physiol 5, 74. doi 10.3389/fphys.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]


