SUMMARY
SETTING
In 2009, the World Health Organization (WHO) conducted a survey of the quality of four anti-tuberculosis drugs in the former Soviet Union countries. Kazakhstan had the highest proportion of substandard drugs.
OBJECTIVE
To assess the quality of anti-tuberculosis drugs used in Kazakhstan in 2014.
DESIGN
Fourteen anti-tuberculosis drugs from the Almaty Interdistrict TB Dispensary were randomly selected and screened for quality using Global Pharma Health Fund Minilab™ testing. First, the product and packaging were physically inspected to determine whether tablets/capsules were intact (i.e., whether they contained the full amount of the drug, and whether the packaging was genuine). Second, the tablets/capsules were dissolved in water to test whether they could be adequately absorbed by the body. Finally, semi-quantitive analyses were undertaken using thin-layer chromatography to verify the presence and concentration of the active pharmaceutical ingredient and to detect impurities.
RESULTS
We discovered no counterfeit medicines. However, 163 (19%) of the 854 anti-tuberculosis drugs sampled failed at least one of the three tests. These samples were found among 24/50 (48%) batches of 14 anti-tuberculosis drugs.
CONCLUSION
Our study identified a high proportion of poor-quality first- and second-line anti-tuberculosis drugs. Use of these medicines may lead to treatment failure and the development of drug resistance. Confirmatory testing should be performed to determine if they should be removed from the market.
Keywords: Minilab, MEDQUARD, drug resistance, tuberculosis, medicine quality
In a review carried out in 2007, substandard and counterfeit anti-tuberculosis drugs with an inadequate amount of the active pharmaceutical ingredient (API), reduced bioavailability, toxic impurities or incorrect labelling were identified in 28 countries.1 The use of such drugs could lead to adverse outcomes of tuberculosis (TB) treatment, treatment failure and the development of drug resistance.2–4 This is of particular concern in the newly independent states (NIS) of the former Soviet Union, where counterfeit medicines (excluding anti-tuberculosis drugs) constitute 20% of all drugs on the market, and where the highest global rates of drug-resistant TB occur.5
In 2009, the World Health Organization (WHO) conducted a survey of the quality of four anti-tuberculosis drugs in selected NIS countries using methods described in the International and US pharmacopoeias.6 Kazakhstan had the highest proportion of substandard drugs (23.3%), with a particularly high failure rate for rifampicin (RMP) produced in Kazakhstan.6
In 2014, Kazakhstan remained among the 10 countries that accounted for over 80% of the global number of cases of multidrug-resistant TB (MDR-TB, defined as TB resistant to at least isoniazid [INH] and RMP), with a prevalence of MDR-TB of 25% in newly treated and 55% in previously treated TB patients.7
We conducted a follow-up assessment of the quality of anti-tuberculosis drugs procured through the national budget and used in TB hospitals in Almaty, Kazakhstan. The study was performed with the National Center of Tuberculosis Problems (NCTP) Kazakhstan, Almaty, in collaboration with the Global Fund and the Centers for Disease Control and Prevention in Central Asia (CDC/CAR).
METHODS
Minilab system
During February–March 2014, drug quality was screened using the Global Pharma Health Fund (GPHF) Minilab™ test kit (Darmstadt, Germany). The kit contains the necessary testing apparatus, reagents and authentic reference standards of over 80 drugs on the WHO Essential Medicines list.
Three-stage screening
The GPHF-Minilab kit was used to screen the quality of study samples by conducting three tests.8 The first test was physical inspection of the solid dosage forms (shape, size and colour), which were compared with the reference standard drugs, and of the packaging to judge manufacturing quality and detect counterfeits. The second test was disintegration of the uncoated, normal-release, solid dosage forms to determine if they dissolved into soft fragments with no palpable core within 30 min, a prerequisite of drug release and appropriate absorption.9 The final test was semi-quantitative analysis by thin-layer chromatography (TLC), i.e., chemical analyses of the presence and relative concentration of the API and impurities in each sample. TLC was performed on both solid and liquid dosage forms. Details of testing are described in the Appendix.*
The number of samples used for testing was dependent upon which of the three types of screening was being assessed.8 If each batch underwent comprehensive testing (liquids were not subjected to disintegration testing), 6–10 dosage units/batch were used for visual inspection, 6 units/batch were used for the disintegration test and 2 units/batch were used for TLC testing. If a physical defect was found in a product, a second investigator examined the product and an agreement was reached. If at least one sample from a batch failed to pass the disintegration test, testing was repeated with six samples and the results of the retested samples were considered definitive. A group of samples was considered to have failed the disintegration test if all six tablets/capsules in the batch failed to disintegrate in water within 30 min.
TLC testing was performed in duplicate; in case of discrepancies, the results with the lower amount of API were considered definitive. TLC testing involved two steps: the first step was performed to determine the presence of the appropriate drug and the second to determine the concentration of the drug. The first step also helped determine whether the extent to which the TLC could travel was equivalent to that of the reference standard drug: a discrepancy of >5% (retention factor error) indicated a non-identical chemical composition. The second step of the test was used to ascertain the drug concentration by determining the intensity of the analyte on the gel; a sample with content < 80%of the API was considered to have failed the drug concentration assessment.
Testing was performed at the Kazakhstan Ministry of Health (MOH) Central Reference Laboratory within the State Enterprise Scientific and Practical Centre for Epidemiological Inspection and Monitoring, Almaty.8
Ethical approval
The Kazakhstan MOH provided permission to conduct the study. The Ethics Committee of the CDC, Atlanta, GA, USA, determined that this was a study to ascertain the proficiency of a laboratory test.10
Sampling procedure
Government purchase and supply of anti-tuberculosis drugs to NCPT pharmacy warehouses in Kazakhstan is centralised for subsequent nationwide use in TB dispensaries or polyclinics. The sale of first- and second-line anti-tuberculosis drugs in pharmacies is prohibited by decree.11 All anti-tuberculosis drugs are made available to patients at TB dispensaries or polyclinics by a single wholesale company, SK Pharmaceuticals, which is run by the MOH.6
Drugs assessed in this survey were registered with the National Drug Regulatory Authorities in Kazakhstan, were within their expiry date, were not WHO-prequalified and were randomly collected from the pharmacy warehouse of the Almaty Interdistrict TB Dispensary, which distributes anti-tuberculosis drugs to five district TB dispensaries and 39 polyclinics in Almaty.
To calculate the sample size, a list of all anti-tuberculosis drugs available at the Almaty Interdistrict TB Dispensary pharmacy warehouse was obtained from the NCPT. All anti-tuberculosis drugs procured through the national budget and for which we had reference standard drugs in the GPHF-Minilab kit were selected (Table 1). Sampling followed standard operational procedures (SOPs) developed by the US Pharmacopeia Drug Quality and Information Program and GPHF, and were specific to the medicine, its source and the size of the batch (Appendix).8,12 A ‘batch’ was defined as a particular production run by a company carrying an identification number. A ‘study sample’ was defined as a medicine with identical8 1) API (ethambutol [EMB], RMP, etc.); 2) brand; 3) dosage form (tablets, capsules, solutions for injections); 4) dose (200 mg, 50 mg/ml, etc.); 5) manufacturer (e.g., Lupin, Mumbai, India, etc.); and 6) serial/batch number (FEL 107A, 50212, etc.).
Table 1.
Anti-tuberculosis drugs assessed for quality using GPHF-Minilab™ screening (n = 14)
| Generic name | Brand name | Dose mg |
Name of manufacturer |
|---|---|---|---|
| Isoniazid | Isoniazid | 100 | PhC Romat* |
| Rifampicin | Rifampicin | 150 | PhC Romat* |
| Ethambutol | Ecox | 400 | Macleods† |
| Ethambutol | Combutol | 400 | Lupin† |
| Rifampicin | Rifampicin-Ferein§ | 150 | Brinsalov-A§ |
| Pyrazinamide | Pyzina | 500 | Lupin† |
| Ethionamide | Ethide | 250 | Lupin† |
| Prothionamide | Prothionamide | 250 | Lupin† |
| Cycloserine | Cycloserine | 250 | Global Pharm¶ |
| Ofloxacin | Oflox | 200 | Global Pharm¶ |
| Kanamycin | Kanamycin sulfate§ | 1000 | Santo# |
| Levofoxacin | Levofloxacin | 250 | Chimfarm# |
| Moxifloxacin | Floxsafe | 400 | MSN Laboratories** |
| Amoxicillin, clavulanic acid | Miclav | 625 | Unichem Laboratories** |
Pavlodar, Kazakhstan.
Mumbai, India.
Liquid forms.
Moscow, Russia.
Almaty, Kazakhstan.
Shymkent, Kazakhstan.
Telangana, India.
GPHF = Global Pharma Health Fund.
As recommended by the SOP, 50 dosage forms (e.g., tablets) of a single medicine were drawn from 10 containers taken from batches that included up to 500 containers (e.g., bottles) per batch.8 For batches of the remaining anti-tuberculosis drugs for which there were >500 containers/batch in the warehouse, 60 dosage forms were randomly sampled from 20 containers.
We collected 2779 study samples from 50 batches of 14 anti-tuberculosis drugs. Samples were collected in February 2014 and stored at room temperature (15–25°C), low humidity (<65%) and away from sunlight.13 Testing with the GPHF-Minilab kit was completed 30 days after collection.
Study definitions
Anti-tuberculosis drugs that failed at least one of the three GPHF-Minilab screening tests were considered ‘poor quality’. A ‘substandard drug’ is a pharmaceutical term for a medicine that does not comply with quality standards or specifications.14 ‘Counterfeit products’ is a category of substandard medicines that ‘deliberately do not conform with intellectual property rights or which violate trademark law’.15 Counterfeit products typically have the wrong or insufficient API (often no API) or have fake packaging.14
Statistical analysis
Statistical analyses were performed using Epi Info™ v3.5.4 (US CDC).
RESULTS
Of the 2779 study samples of the 14 brands of anti-tuberculosis drugs, 854 (31%) were screened. The remaining 1925 (69%) samples were retained for possible confirmatory quality-control testing and manufacturer investigations.
Of the 330 samples of 12 anti-tuberculosis drugs inspected physically (two medicines were not in solid form), 37 (11%) samples from 3/7 manufacturers failed the inspection test (Table 2). Of 14 batches of INH and RMP manufactured in Kazakhstan (PhC Romat, Pavlodar) 9 batches failed; of 3 batches of EMB manufactured in India (Macleods Pharmaceuticals, Mumbai) 2 batches failed; and of 7 batches of pyrazinamide (PZA) manufactured in India (Lupin), 2 batches failed. Of these 24 batches, 13 had physically damaged tablets/capsules, such as chips, which comprised an estimated 10–40% of the size of the tablet. An additional three batches of RMP (50212, 3013, 30212) from PhC Romat did not have the required registration information on the packaging, and differed in colour from the remaining batches of RMP.
Table 2.
Physical inspection of anti-tuberculosis drugs in solid dosage form, Almaty, Kazakhstan, 2014 (n = 12)
| Physical examination | |||||
|---|---|---|---|---|---|
|
|
|||||
| Generic name | Manufacturer | Batch number | Tested n |
Failed n |
Comments |
| Isoniazid | PhC Romat | 010113 | 10 | 3 | Fractures/chipped |
| 020113 | 6 | 1 | Chipped | ||
| 290213 | 6 | 1 | Chipped | ||
| 190213 | 10 | 0 | |||
| Rifampicin | PhC Romat | 50212, 30212, 20112 | 18 | 18 | No registration data, non-uniform colour |
| 30113 | 6 | 3 | Dents and fractures | ||
| 220513 | 6 | 1 | Fractured | ||
| 361212 | 6 | 1 | Fractured | ||
| 20113, 401212, 340913, 300613 | 24 | 0 | |||
| Ethambutol | Macleods | FEL 108A | 6 | 2 | Chipped, dented, non-homogeneous shell |
| FEL 107A | 6 | 2 | Chipped, non-homogeneous shell, abraded | ||
| FEL 105A | 6 | 0 | |||
| Ethambutol | Lupin | BL10018 | 10 | 0 | |
| Pyrazinamide | Lupin | DN20021 | 6 | 3 | Chipped, fractures and cracked |
| DN20022 | 6 | 2 | Chipped, dented, pinholes | ||
| DN10026, DN10036,DN10027, DN20028, DN20010A | 30 | 0 | |||
| Ethionamide | Lupin | CH 20002 A | 6 | 0 | |
| Prothionamide | Lupin | A300429, A300438,A300439, A300346, DN28030 | 50 | 0 | |
| Cycloserine | Global Pharm | 30113, 521212, 220513, 190513 | 40 | 0 | |
| Ofloxacin | Global Pharm | 40112 | 10 | 0 | |
| Levofloxacin | Chimfarm | 160712, 70512 | 12 | 0 | |
| Moxifloxacin | MSN Laboratories | 41302200, 41301212 | 20 | 0 | |
| Amoxicillin,clavulanic acid | Unichem Laboratories | BMVM12051,BMVM13020, BMVM13005 | 30 | 0 | |
| Total | 330 | 37 | |||
Of the 312 samples of 12 solid-form anti-tuberculosis drugs from 43 batches tested for disintegration, 90 (29%) samples of EMB (one batch from Lupin and three batches from Macleods), ethionamide (ETH) (one batch from Lupin) and ofloxacin (OFX) (one batch from Global Pharm, Almaty) failed the disintegration test in the required <30 min, taking as long as 58 min to disintegrate. In each case, all tablets tested in six batches of medicines from these manufacturers failed the disintegration test, suggesting a systematic problem (Table 3). The reference drugs, with analogous API and doses, disintegrated in water within 11–22 min.
Table 3.
Disintegration testing of anti-tuberculosis drugs in solid dosage form, Almaty, Kazakhstan, 2014 (n = 12)
| Disintegration test | |||||
|---|---|---|---|---|---|
|
|
|||||
| Generic name | Manufacturer | Batch number | Tested n |
Failed n |
Time range* min |
| Isoniazid | PhC Romat | 010113 | 24 | 0 | 12–15 |
| 020113 | |||||
| 290213 | |||||
| 190213 | |||||
| Rifampicin | PhC Romat | 50212, 30212, 20112, 30113, 220513, 361212 | 60 | 0 | 5–11 |
| 20113, 401212, 340913, 300613 | |||||
| Ethambutol | Macleods | FEL 105A† | 18 | 18 | 52–58 |
| FEL 107A† | 18 | 18 | 56–58 | ||
| FEL 108A† | 18 | 18 | 55–59 | ||
| Ethambutol | Lupin | BL10018‡ | 12 | 12 | 58 |
| Pyrazinamide | Lupin | DN20021, DN20022 | 42 | 0 | 3–5 |
| DN10026, DN10036 | |||||
| DN10027, DN20028 DN20010A | |||||
| Ethionamide | Lupin | CH 20002 A‡ | 12 | 12 | 50 |
| Prothionamide | Lupin | A300429, A300438 | 30 | 0 | 10–11 |
| A300439, A300346 DN28030 | |||||
| Cycloserine | Global Pharm | 30113, 521212 | 24 | 0 | 4–7 |
| 220513,190513 | |||||
| Ofloxacin | Global Pharm | 40112§ | 12 | 12 | 53–56 |
| Levofloxacin | Chimfarm | 160712, 70512 | 12 | 0 | 5–20 |
| Moxifloxacin | MSN Laboratories | 41302200, 41301212 | 12 | 0 | 6 |
| Amoxicillin, clavulanic acid | Unichem Laboratories | BMVM12051 BMVM13020 BMVM13005 | 18 | 0 | 22–30 |
| Total | 312 | 90 | |||
Should be <30 min.
Three re-tests of the batch.
Two re-tests of the batch.
TLC testing was performed on 50 batches of 14 anti-tuberculosis drugs in solid and liquid forms to confirm the authenticity of the API (retention factor error ≤ 5%) in all samples (n = 212). However, 36 (17%) samples in 9 batches of EMB (Lupin), RMP (PhC Romat) and kanamycin (KM) (Santo, Shymkent, Kazakhstan) contained <80% of the API (Table 4, Appendix Figure).
Table 4.
Thin-layer chromatography of anti-tuberculosis drugs in solid and liquid dosage forms, Almaty, Kazakhstan, 2014 (n = 14)
| Thin-layer chromatography | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Generic name | Manufacturer | Batch number | Tested n |
Retention factor error* % |
API content† % |
Failed n |
| Isoniazid | PhC Romat | 010113 | 16 | 0–1.52 | 100 | 0 |
| 020113 | ||||||
| 290213 | ||||||
| 190213 | ||||||
| Rifampicin | PhC Romat | 50212, 401212, 30113, 30212, 20112, 340913, 300613 | 28 | 0–0.23 | <80 | 28 |
| 20113, 220513, 361212 | 12 | 0 | <80 | 0 | ||
| Ethambutol | Macleods | FEL 105A | 12 | 0 | 100 | 0 |
| FEL 107A | ||||||
| FEL 108A | ||||||
| Ethambutol | Lupin | BL10018 | 4 | 0 | <80 | 4 |
| Rifampicin | Brinsalov-A | 170413, 40113 110213, 591214 | 16 | 0 | 100 | 0 |
| Pyrazinamide | Lupin | DN20021, DN20022, DN10026, DN10036, DN10027, DN20028 DN20010A | 28 | 0–0.93 | 80–100 | 0 |
| Ethionamide | Lupin | CH 20002 A | 4 | 0 | 100 | 0 |
| Prothionamide | Lupin | A300429, A300438, A300439, A300346 DN28030 | 20 | 0 | 100 | 0 |
| Cycloserine | Global Pharm | 30113, 521212, 220513,190513 | 16 | 0 | 100 | 0 |
| Kanamycin | Santo | 20313 | 4 | 1.28 | <80 | 4 |
| 10113, 10212 | 8 | 0 | 80–100 | 0 | ||
| Ofloxacin | Global Pharm | 40112 | 4 | 1.79 | 100 | 0 |
| Levofloxacin | Chimfarm | 160712, 70512 | 8 | 0–0.88 | 100 | 0 |
| Moxifloxacin | MSN Laboratories | 41302200, 41301212 | 8 | 0 | 100 | 0 |
| Amoxicillin, clavulanic acid‡ | Unichem Laboratories | BMVM12051 BMVM13020 BMVM13005 | 24 | 0 | 100 | 0 |
| Total | 212 | 36 | ||||
Relative retention factors of the spot with that of the standard to identify the presence of the API (should be ≤5%).
Should be 80–100%.
Amoxicillin and clavulanic acid samples/batch tested separately.
API = active pharmaceutical ingredient.
Overall, 163/854 (19%) samples failed at least one of the three GPHF-Minilab tests (Table 5). The most frequent reason for failure was poor disintegration (90/163, 55%), followed by failure on physical inspection (37/163, 23%) and failure on TLC analyses (36/163, 22%).
Table 5.
Overall assessment of the quality of anti-tuberculosis drugs, Almaty, Kazakhstan, 2014 (n = 14)
| Generic name | Manufacturer | Batch number | Physical inspection |
Disintegration test |
TLC | Assessment status |
|---|---|---|---|---|---|---|
| Isoniazid | PhC Romat | 010113, 020113 | —* | +† | + | Failed |
| 290213 | ||||||
| 190213 | + | + | + | Passed | ||
| Rifampicin | PhC Romat | 50212, 30113, 30212, | — | + | — | Failed |
| 401212, 20113, 300613, 340913, | + | + | — | Failed | ||
| 20112, 220513, 361212 | — | + | + | Failed | ||
| Ethambutol | Macleods | FEL 105A | + | — | + | Failed |
| FEL 107A, FEL 108A | — | — | + | Failed | ||
| Ethambutol | Lupin | BL10018 | + | — | — | Failed |
| Rifampicin | Brinsalov-A | 170413, 40113 110213, 591214 | Not performed for liquid forms | + | Passed | |
| Pyrazinamide | Lupin | DN20021, DN20022 | — | + | + | Failed |
| DN10026, DN10036 | + | + | + | Passed | ||
| DN10027, DN20028 DN20010A | ||||||
| Ethionamide | Lupin | CH 20002 A | + | — | + | Failed |
| Prothionamide | Lupin | A300429, A300438 | + | + | + | Passed |
| A300439, A300346 DN28030 | ||||||
| Cycloserine | Global Pharm | 30113, 521212 | + | + | + | Passed |
| 220513,190513 | ||||||
| Kanamycin | Santo | 20313 | Not performed for liquid forms | — | Failed | |
| 10113, 10212 | + | Passed | ||||
| Ofloxacin | Global Pharm | 40112 | + | — | + | Failed |
| Levofloxacin | Chimfarm | 160712, 70512 | + | + | + | Passed |
| Moxifloxacin | MSN Laboratories | 41302200, 41301212 | + | + | + | Passed |
| Amoxicillin, clavulanic acid | Unichem Laboratories | BMVM12051 BMVM13020 BMVM13005 | + | + | + | Passed |
— Failed testing.
+ Passed testing.
TLC = thin-layer chromatography.
DISCUSSION
Eight of the first- and second-line anti-tuberculosis drugs used for treatment in Almaty, procured through the national budget for use throughout Kazakhstan and manufactured in Kazakhstan and India, failed our basic quality-control requirements. We detected no counterfeit drugs, suggesting that there is active regulation of pharmaceuticals in Kazakhstan even if the quality control of the regulated drugs is not optimal. Our findings can be extrapolated to anti-tuberculosis drugs used in other regions of the country.
We believe our work is valid, as samples were randomly selected for testing and sampling was not biased. All technical work was performed by trained analysts following GPHF-Minilab guidelines. We consulted experts from the US Pharmacopeia (Rockville, MD, USA) before the start of the study, and received guidance throughout the study from GPHF-Minilab experts. Medicine Quality Assessment Reporting Guidelines on the reporting of drug quality were followed.16
The GPHF-Minilab is an effective, simple, cost-effective and rapid tool for the identification of substandard medicines, and an excellent screening tool for many medicines.2,17–20 Not all aspects of the tool, however, have equivalent sensitivity and specificity, and the GPHF-Minilab is best thought of as a tool for the detection of gross product failures. In particular, disintegration, although an important forerunner to dissolution (and subsequent bioavailability), may not accurately predict the bioavailability of all drugs or forms, notably RMP. According to the semi-quantitative TLC component of GPHF-Minilab,21,22 drugs with at least 80% of the API would pass the test. Given that ±10%variations in TLC readings can be seen with the naked eye, medicines with 70–80% of the API that otherwise meet quality standards could be acceptable.21,23,24 However, as TLC may mistakenly identify a form as having too little or sufficient API, reader experience is important for accurate interpretation.24 Conversely, the detection of breakdown products of the API by TLC due to, for example, incorrect storage, can lend confidence to an interpretation of too little drug. GPHF-Minilab screening is thus useful only for identifying gross quality failures, and is not an indicator of adequate product quality, as some products would require further testing with independent pharmacopoeia-based tests (if possible): we recommend this approach.
Our assessment has implications for policy and practice. First, failure in the tests can be due to poor manufacturing practices, counterfeiting or poor storage conditions. There was no evidence of counterfeiting, as no products with poor packaging or lack of API were found. All the drugs evaluated were made by approved manufacturers and had passed assessment by the National Center for Medicines, Medical Devices and Medical Equipment Expertise before acceptance for use in Kazakhstan. We therefore assumed that discrepancies between our GPHF-Minilab screening and the initial testing of these anti-tuberculosis drugs upon registration were due to poor manufacturing practices, leading to variations in drug quality over time, or poor storage conditions.
The stability of any medicine for the duration of its claimed shelf-life can be ensured under 1) appropriate manufacturing conditions and 2) subsequent storage at the correct temperature and humidity. We cannot comment on the first point, as we do not have information on the manufacturing practices of the different manufacturers. Some deterioration in the quality of the medicines may have occurred during distribution from the manufacturer to the health facilities. The pharmacy warehouse where the samples were collected in January 2014 was not heated (outdoor temperatures in Almaty range from about 30°C in the summer to −15°C in the winter), although anti-tuberculosis drugs are required to be stored at room temperature (15–25°C).13 Studies have shown that the stability of anti-tuberculosis drugs in tropical environments can withstand high external humidity.1,25,26 However, the humidity trapped during manufacturing and packaging may lead to accelerated degradation, a situation that can be aggravated by the wide temperature differences between day and night in Almaty.27
Second, substandard medications can harm patients. Although we identified no counterfeit medication other than RMP lacking registration information, defective tablets of INH, RMP, EMB and PZA indicate non-compliance with good manufacturing practices and can deliver less than the required amount of medication. Tablets of EMB, ETH and OFX that failed to disintegrate within the appropriate time limit are unlikely to release the drug in the body within the appropriate time, which would likely affect absorption and lead to lower blood levels of the drug than required. Products containing <100% of the API as tested using TLC will deliver less than the purported amounts of the drug.
The results of our study are consistent with the findings of the 2009 WHO survey of anti-tuberculosis drugs in the NIS, which found substandard forms of RMP and OFX among the four drugs tested and marketed in Kazakhstan (RMP, INH, KM and OFX).6 We also found additional drugs of substandard quality.
Anti-tuberculosis drugs in Kazakhstan should be of high quality. The National Drug Authority should ensure that standards are met, and approval of drugs that are pre-qualified by the WHO is one way of achieving this aim. Post-marketing surveillance should also be considered to ensure that the drugs remain of high quality; periodic use of the Minilab kit could therefore be useful. The cost of the Minilab kit is around US$5000, and one kit was needed for our study. Given the current budget of US$21 million in Kazakhstan for the procurement of anti-tuberculosis drugs, such an investment, if it can detect substandard drugs, would be a wise one. Manufacturers who continually fail quality-control assessments should be banned from participating in national tenders.2 Nevertheless, even high-quality drugs can deteriorate under poor storage conditions, and the storage conditions of the pharmacy warehouses need to be monitored in Kazakhstan.
In conclusion, poor-quality anti-tuberculosis drugs may lead to treatment failure and the development of drug resistance. Confirmation of our results, including the use of high-performance liquid chromatography,9,22,28 is required to ensure fairness to manufacturers and to evaluate the true utility of the Minilab system. The use of poor-quality drugs should be prohibited.
Acknowledgments
This research was conducted by the National Center of Tuberculosis Problems, Kazakhstan, with the collaboration of the Global Fund and the Centers for Disease Control and Prevention in Central Asia (CDC/CAR). The study was funded by CDC/CAR.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
RWOJ is employed by the Global Pharma Health Fund (GPHF). The purchase of the GPHF-Minilab system occurred prior to RWOJ providing technical advice for this project.
APPENDIX
The GPHF-Minilab™ system evaluates three aspects of drug quality. Details of the evaluation procedures are presented below.
Physical inspection
For the physical inspection, 6–10 samples were drawn from each batch of anti-tuberculosis drug and assessed based on 24 visual criteria. These criteria were: packaging (labelling, presence of serial and license numbers, etc.); examination of the sample for physical defects (fractures, fissures, chips, abrasion, stickiness, etc.); examination of the sample for other characteristics (appropriate closing of the shells, uniformity of size and colour in a single product batch, presence of dirty spots, foreign particles or dents, etc.).
Inspection was performed in broad daylight using sterile gloves and spatulas. If a sample failed the physical inspection by one of the researchers, it was re-examined by two other researchers to verify the result. If a sample failed to meet at least 1 of the 24 testing criteria by all researchers, it was considered to have failed.
Disintegration test
A disintegration test was performed by immersing anti-tuberculosis drugs in warm distilled water (35–39°C) using an apparatus operated to raise and lower the bottle with the immersed drug at a constant frequency of 29–32 cycles/min, per US Pharmacopeia guidelines (http://www.pharmacopeia.cn/v29240/usp29nf24s0_c701h.html Accessed August 2017).
All tested anti-tuberculosis products are labeled as ‘quick-release’, and were supposed to disintegrate in water in <30 min (i.e., until the tablet/capsule became a soft mass or dissolved completely).
Before testing the study samples, we performed quality control by disintegrating the GPHF-Minilab reference standard drugs containing the active pharmaceutical ingredient (API) and doses analogous to the study samples in warm distilled water. After the reference standards disintegrated in <30 min, the disintegration test was performed on six samples per batch of the anti-tuberculosis products studied.
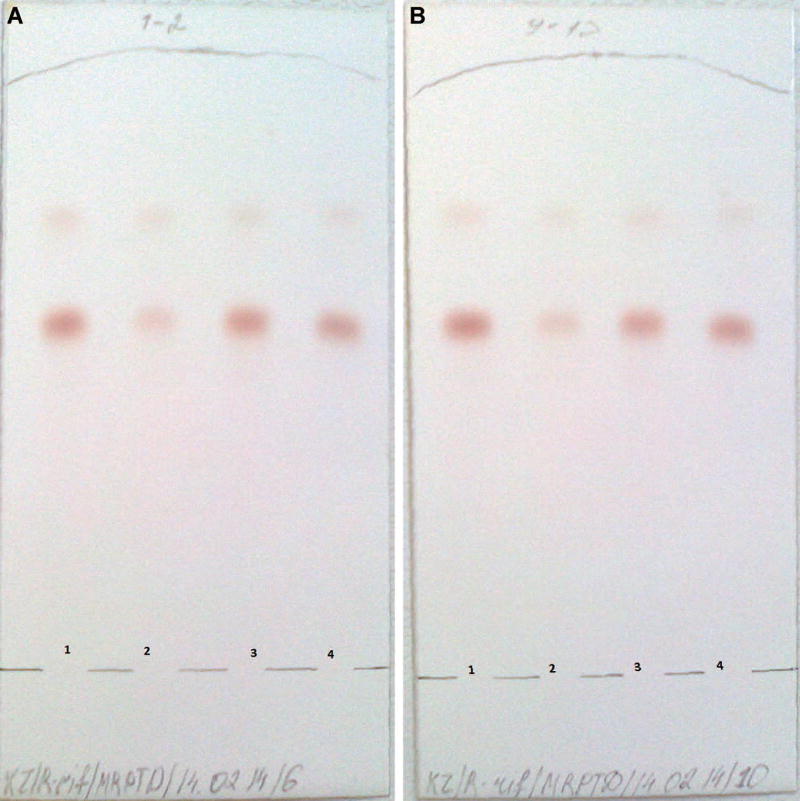
Figure A.
Chromatoplates observed in the thin-layer chromatography assessment of rifampicin samples. A) Lane 1 from the left=upper working limit representing 100% of the total drug; lane 2 from the left = poor-quality drug product with <80% of the drug; lane 3 from the left = good-quality drug product; lane 4 on the right=lower working limit representing 80% of the total drug. B) As above for a rifampicin sample from a different batch.
Table A.
Sampling scheme for batch assessment
| A | If the number of items in the batch consists of <100 containers, for example, patient packs or boxes, then the minimum number of items to be tested is 10% or four containers, whichever is greater. Draw from the total number of containers and sample ≥50 tablets or capsules. | 1 container per batch delivered. Draw 50 tablets as sample |
| 2 containers per batch delivered. Draw 25 tablets from each container | ||
| 10 containers per batch delivered. Sample 4 containers and draw 13 tablets from each container | ||
| 40 containers per batch delivered. Sample 4 containers only and draw 13 tablets from each item | ||
| 50 containers per batch delivered. Sample 5 containers equivalent to 10% and draw 10 tablets from each container | ||
| 97 containers per batch delivered. Sample 10 containers (about equivalent to 10%) and draw 5 tablets only from each container | ||
| B | If the number of items in the batch consists of >100 but ≤500 containers, for example, patient packs or boxes, then the minimum number of items to be tested is 10 containers. Draw from the total number of containers and sample ≥50 tablets or capsules. | 150 containers per batch delivered. Sample 10 containers and draw 5 tablets from each container |
| 375 containers per batch delivered. Sample 10 containers and draw 5 tablets from each container | ||
| 490 containers per batch delivered. Sample 10 containers and draw 5 tablets from each container | ||
| C | If the number of items in the batch consists of >500 containers, for example, patient packs or boxes, the minimum number of items to be tested are 2% or 20 containers, whichever is less. Draw from the total number of containers and sample ≥50 tablets or capsules. | 540 containers per batch delivered. Sample 11 containers (about 2%) and draw 5 tablets from each container |
| 800 containers per batch delivered. Sample 16 containers (precisely 2%) and draw 3 tablets from each container | ||
| 1000 containers per batch delivered. Sample 20 containers and draw 3 tablets from each container | ||
| 2000 containers per batch delivered. Sample 20 containers and draw 3 tablets from each container | ||
| 9000 containers per batch delivered. Sample 20 containers and draw 3 tablets from each container |
A group of samples was considered to have ‘passed’ the test if all six tablets/capsules disintegrated. If at least one tablet/capsule did not disintegrate within 30 min, the test was repeated. If the second test had one or more units of samples that did not disintegrate, the sample was considered to have failed the test.
Chemical analyses: thin-layer chromatography
The GPHF-Minilab thin-layer chromatography (TLC) test identifies the API qualitatively by comparing the distance of travel (retention factor [Rf] value) between the sample spot and reference standard spot on the same plate. If the Rf of the reference standard and the study sample are identical, the medicines have the same chemical composition. The API was also identified by estimating the Rf error:
If the Rf error is ≤ 5%, the sample can be considered to have passed the test.
If the Rf error is > 5% but <10%, the sample was considered ‘suspicious’.
If the Rf error is ≥ 10%, the sample is considered to have failed the test.
Semi-quantitative proof of the API concentration is performed visually by observing the colour, size and intensity of the sample and two reference standard spots, one containing 80% and one 100% of the API concentration; this shows if the sample falls below, or between, 80% and 100% of the declared concentration.
A sample that demonstrated content that was 80–100% of the API was considered to have passed the test. A sample that demonstrated content of <80% of the API was considered to have failed the test.
Quality control of TLC analyses was performed prior to testing of the study samples by TLC-testing the reference standards of the selected anti-tuberculosis drugs. Duplicate TLC tests of the study samples (two samples per test and batch of the anti-tuberculosis drug) were then performed, and the Rf from both tests compared. The results of the second analysis were considered final.
GPHF-Minilab sampling procedures8
The amount of samples to be drawn and the costs of sampling and workload of testing can be streamlined and minimised following statistical quality-control methods to be found, for example, in the standard handbooks of the International Standards Organization or batch analysis guidelines in pharmacopoeias for sterility testing. Taking these rules into account, sampling should take place in accordance with the schedule in Table A. In our study, the sample size was, on average, 50 tablets or capsules per batch. This covered the maximum requirement of 24 tablets/capsules per examination with the GPHF-Minilab and a minimum of 26 tablets/capsules kept aside for further confirmatory testing.
Footnotes
The appendix is available in the online version of this article, at http://www.ingentaconnect.com/content/iuatld/ijtld/2017/00000021/00000010/art00018
Conflict of interest: No other conflicts.
References
- 1.Kelesidis T, Kelesidis I, Rafailidis PI, Falagas ME. Counterfeit or substandard antimicrobial drugs: a review of the scientific evidence. J Antimicrob Chemother. 2007;60:214–236. doi: 10.1093/jac/dkm109. [DOI] [PubMed] [Google Scholar]
- 2.Laserson KF, Kenyon AS, Kenyon TA, Layloff T, Binkin NJ. Substandard tuberculosis drugs on the global market and their simple detection. Int J Tuberc Lung Dis. 2001;5:448–454. [PubMed] [Google Scholar]
- 3.Okeke IN, Lamikanra A, Edelman R. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg Infect Dis. 1999;5:18–27. doi: 10.3201/eid0501.990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caminero JA. Multidrug-resistant tuberculosis: epidemiology, risk factors and case finding. Int J Tuberc Lung Dis. 2010;14:382–390. [PubMed] [Google Scholar]
- 5.Kelesidis T, Falagas ME. Substandard/counterfeit antimicrobial drugs. Clin Microbiol Rev. 2015;28:443–464. doi: 10.1128/CMR.00072-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO/EMP/QSM/2011.2. Geneva, Switzerland: WHO; 2011. [Accessed June 2017]. Survey of the quality of anti-tuberculosis medicines circulating in selected newly independent states of the former Soviet Union. Quality assurance and safety: medicines, essential medicines and pharmaceutical policies. http://apps.who.int/medicinedocs/documents/s19053en/s19053en.pdf. [Google Scholar]
- 7.World Health Organization. WHO/HTM/TB/2015.22. Geneva, Switzerland: WHO; 2015. [Accessed August 2017]. Global tuberculosis report, 2015. http://www.who.int/tb/publications/global_report/gtbr15_main_text.pdf. [Google Scholar]
- 8.Jähnke R. Thin Layer Chromatographic Tests. Darmstadt, Germany: GPHF; 2008. A concise quality control guide on essential drugs and other medicines. Volume II. [Google Scholar]
- 9.Jähnke R. Counterfeit medicines and the GPHF-Minilab for rapid drug quality verification. Pharmazeutische Industrie. 2004:1187–1193. [Google Scholar]
- 10.National Health & Medical Research Council. Advice to Institutions, Human Research Ethics Committees and Health Care Professionals. Canberra, Australia: NHMRC; 2003. [Accessed June 2017]. When does quality assurance in health care require independent ethical review? http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/e46.pdf. [Google Scholar]
- 11.Government of the Republic of Kazakhstan. Almaty, Kazakhstan: Government of the Republic of Kazakhstan; 2009. Decree No. 1729 of the Government of the Republic of Kazakhstan of 30 October 2009 ‘On Approval of the Rules for the Organization and Conduct of Procurement of Medicines, Preventive (Immunobiological, Diagnostic, Disinfecting) Drugs, Medical Devices and Medical Equipment, Pharmaceutical Services for the Provision of a Guaranteed Volume of Free Medical Care and Medical care in the system of compulsory social insurance. [Google Scholar]
- 12.United States Pharmacopeia Drug Quality and Information Program and Collaborators. Ensuring the quality of medicines in resource-limited countries: an operational guide. Rockville, MD, USA: United States Pharmacopeial Convention; 2007. [Google Scholar]
- 13.Order of the Minister of Health and Social Development of the Republic of Kazakhstan from 24 April 2015. Astana, Kazakhstan: Minister of Health and Social Development; 2015. [Accessed August 2017]. No 262. On approval of rules of storage and transportation of drugs, medical devices and medical equipment. https://www.pharm.reviews/dokumenty/item/190-prikaz-ministra-zdravookhraneniya-i-sotsialnogo-razvitiya-rk-ot-24-aprelya-2015-goda-262. [Google Scholar]
- 14.World Health Organization. Geneva, Switzerland: WHO; 2013. What are substandard and counterfeit medicines? [Google Scholar]
- 15.World Health Organization. WHO/EDM/QSM/99.1. Geneva, Switzerland: WHO; 1999. Counterfeit drugs: guidelines for the development of measures to combat counterfeit drugs. [Google Scholar]
- 16.Newton PN, Lee SJ, Goodman C, et al. Guidelines for field surveys of the quality of medicines: a proposal. PLOS Med. 2009;6:e52. doi: 10.1371/journal.pmed.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bate R, Jensen P, Hess K, Mooney L, Milligan J. Substandard and falsified anti-tuberculosis drugs: a preliminary field analysis. Int J Tuberc Lung Dis. 2013;17:308–311. doi: 10.5588/ijtld.12.0355. [DOI] [PubMed] [Google Scholar]
- 18.Bate R, Tren R, Mooney L, et al. Pilot study of essential drug quality in two major cities in India. PLOS ONE. 2009;4:e6003. doi: 10.1371/journal.pone.0006003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visser BJ, Meerveld-Gerrits J, Kroon D, et al. Assessing the quality of anti-malarial drugs from Gabonese pharmacies using the MiniLab®: a field study. Malar J. 2015;14:273. doi: 10.1186/s12936-015-0795-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Risha PG, Msuya Z, Clark M, Johnson K, Ndomondo-Sigonda M, Layloff T. The use of Minilabs to improve the testing capacity of regulatory authorities in resource limited settings: Tanzanian experience. Health Policy (Amsterdam, Netherlands) 2008;87:217–222. doi: 10.1016/j.healthpol.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Fadeyi I, Lalani M, Mailk N, Van Wyk A, Kaur H. Quality of the antibiotics—amoxicillin and cotrimoxazole from Ghana, Nigeria, and the United Kingdom. Am J Trop Med Hyg. 2015;92(Suppl):87–94. doi: 10.4269/ajtmh.14-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ministry of Public Health and Sanitation & Ministry of Medical Services. Nairobi, Kenya: Pharmacy and Poisons Board; 2017. [Accessed June 2017]. Monitoring the quality of antimalarial medicines circulating in Kenya. http://pharmacyboardkenya.org/?p=554. [Google Scholar]
- 23.Khuluza F, Kigera S, Heide L. Low prevalence of substandard and falsified antimalarial and antibiotic medicines in public and faith-based health facilities of Southern Malawi. Am J Trop Med Hyg. 2017;96:1124–1135. doi: 10.4269/ajtmh.16-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. WHO/EMP/QSM/2011.1. Geneva, Switzerland: WHO; 2011. [Accessed June 2017]. Survey of the quality of selected antimalarial medicines circulating in six countries of sub-Saharan Africa. http://www.who.int/medicines/publications/WHO_QAMSA_report.pdf. [Google Scholar]
- 25.Ashokraj Y, Kohli G, Kaul CL, Panchagnula R. Quality control of anti-tuberculosis FDC formulations in the global market: part II-accelerated stability studies. Int J Tuberc Lung Dis. 2005;9:1266–1272. [PubMed] [Google Scholar]
- 26.Agrawal S, Panchagnula R. In vitro evaluation of fixed dose combination tablets of anti-tuberculosis drugs after real time storage at ambient conditions. Die Pharmazie. 2004;59:782–785. [PubMed] [Google Scholar]
- 27.World Health Organization. Quality of misoprostol products. [Accessed June 2017];WHO Drug Information. 2016 30:35–39. http://www.who.int/medicines/publications/druginformation/WHO_DI_30-1_Quality.pdf. [Google Scholar]
- 28.Newton P, Proux S, Green M, et al. Fake artesunate in southeast Asia. Lancet. 2001;357:1948–1950. doi: 10.1016/S0140-6736(00)05085-6. [DOI] [PubMed] [Google Scholar]