Abstract
Attempts to repair myocardial infarcts by transplanting cardiomyocytes or skeletal myoblasts have failed to reconstitute healthy myocardium and coronary vessels integrated structurally and functionally with the remaining viable portion of the ventricular wall. The recently discovered growth and transdifferentiation potential of primitive bone marrow cells (BMC) prompted us, in an earlier study, to inject in the border zone of acute infarcts Lin− c-kitPOS BMC from syngeneic animals. These BMC differentiated into myocytes and vascular structures, ameliorating the function of the infarcted heart. Two critical determinants seem to be required for the transdifferentiation of primitive BMC: tissue damage and a high level of pluripotent cells. On this basis, we hypothesized here that BMC, mobilized by stem cell factor and granulocyte-colony stimulating factor, would home to the infarcted region, replicate, differentiate, and ultimately promote myocardial repair. We report that, in the presence of an acute myocardial infarct, cytokine-mediated translocation of BMC resulted in a significant degree of tissue regeneration 27 days later. Cytokine-induced cardiac repair decreased mortality by 68%, infarct size by 40%, cavitary dilation by 26%, and diastolic stress by 70%. Ejection fraction progressively increased and hemodynamics significantly improved as a consequence of the formation of 15 × 106 new myocytes with arterioles and capillaries connected with the circulation of the unaffected ventricle. In conclusion, mobilization of primitive BMC by cytokines might offer a noninvasive therapeutic strategy for the regeneration of the myocardium lost as a result of ischemic heart disease and, perhaps, other forms of cardiac pathology.
Sudden occlusion of a major coronary artery and acute myocardial ischemia lead to rapid death of myocytes (M) and vascular structures in the supplied region of the ventricle. Despite the demonstration that a subpopulation of cardiac muscle cells is able to replicate (1), and new vessels are formed (2), this regeneration is restricted to the viable myocardium. The loss of M, arterioles, and capillaries in the infarcted area is irreversible, resulting with time in the formation of scarred tissue. For this reason, most experimental and clinical therapies have mainly focused on limiting infarct size. Attempts to replace the necrotic zone of the heart by transplanting cardiomyocytes or skeletal myoblasts (3–7), although successful in the survival of many of the grafted cells, have invariably failed to reconstitute healthy myocardium and coronary vessels integrated structurally and functionally with the spared ventricular wall.
The recognition that stem cells, particularly those from the bone marrow, have the capacity to colonize different tissues, proliferate, and transdifferentiate into cell lineages of the host organ (8, 9), prompted us in an earlier study (10) to inject Lin− c-kitPOS bone marrow cells (BMC) in the contracting myocardium bordering acute infarcts. Surprisingly, the implanted BMC differentiated into M and coronary vessels ameliorating the function of the injured heart (10). This approach, however, required a surgical intervention that was accompanied by high mortality and a grafting success rate of 40%. Therefore, the identification and utilization of a noninvasive method would be highly desirable. Two main determinants seem to be critical for colonization and transdifferentiation of BMC into a variety of tissues: recent damage and a high number of circulating stem cells (8, 9, 11, 12). On this basis, we hypothesized that a sufficient number of BMC mobilized by stem cell factor (SCF) and granulocyte-colony-stimulating factor (G-CSF; refs. 13 and 14) would home to the infarcted heart and promote cardiac repair. To test this possibility, mice were injected with SCF and G-CSF to increase the number of circulating stem cells from 29 in nontreated controls to 7,200 in cytokine-treated mice (13).
Materials and Methods
Myocardial Infarction (MI) and Cytokines.
C57BL/6 male mice at 2 months of age were splenectomized and 2 weeks later were injected s.c. with recombinant rat SCF, 200 μg/kg/day, and recombinant human G-CSF, 50 μg/kg/day (Amgen Biologicals), once a day for 5 days (13, 14). Under ether anesthesia, the left ventricle (LV) was exposed and the coronary artery was ligated (10, 15, 16). SCF and G-CSF were given for 3 more days. Controls consisted of splenectomized infarcted and sham-operated (SO) mice injected with saline. BrdUrd, 50 mg/kg body weight, was given once a day for 13 days before the mice were killed; mice were killed at 27 days. Protocols were approved by New York Medical College.
Echocardiography and Hemodynamics.
Echocardiography was performed in conscious mice by using a Sequoia 256c (Acuson, Mountain View, CA) equipped with a 13-MHz linear transducer (15L8). The anterior chest area was shaved and two- dimensional (2D) images and M-mode tracings were recorded from the parasternal short axis view at the level of papillary muscles. From M-mode tracings, anatomical parameters in diastole and systole were obtained (17). Ejection fraction (EF) was derived from LV cross-sectional area in 2D short axis view (17): EF = [(LVDA − LVSA)/LVDA] × 100, where LVDA and LVSA correspond to LV areas in diastole and in systole. Mice were anesthetized with chloral hydrate (400 mg/kg body weight, i.p.), and a microtip pressure transducer (SPR-671; Millar Instruments, Houston) connected to a chart recorder was advanced into the LV for the evaluation of pressures and + and − dP/dt in the closed-chest preparation (10, 15, 16).
Cardiac Anatomy and Infarct Size.
After hemodynamic measurements, the abdominal aorta was cannulated, the heart was arrested in diastole with CdCl2, and the myocardium was perfused with 10% (vol/vol) formalin. The LV chamber was filled with fixative at a pressure equal to the in vivo measured end-diastolic pressure (15, 16). The LV intracavitary axis was measured, and three transverse slices from the base, mid-region, and apex were embedded in paraffin. The mid-section was used to measure LV thickness, chamber diameter, and volume (15, 16). Infarct size was determined by the number of M lost from the left ventricular free wall (LVFW; refs. 18 and 19).
Newly Formed M.
The volume of regenerating myocardium was determined by measuring in each of three sections the area occupied by the restored tissue and section thickness. The product of these two variables yielded the volume of tissue repair in each section. Values in the three sections were added, and the total volume of formed myocardium was obtained. Additionally, the volume of 400 M was measured in each heart. Sections were stained with desmin and laminin Abs and propidium iodide (PI). Only longitudinally oriented cells with centrally located nuclei were included. The length and diameter across the nucleus were collected in each M to compute cell volume, assuming a cylindrical shape (18, 19). M were divided in classes, and the number of M in each class was calculated from the quotient of total M class volume and average cell volume (20, 21). The number of arteriole and capillary profiles per unit area of myocardium was measured as described (18, 19).
BrdUrd and Ki67.
Sections were incubated with BrdUrd or Ki67 Ab. M were recognized with a mouse monoclonal anti-cardiac myosin, endothelial cells (EC) were recognized with rabbit polyclonal anti-factor VIII, and smooth muscle cells (SMC) were recognized with a mouse monoclonal anti-α-smooth muscle actin. The fractions of M, EC, and SMC nuclei labeled by BrdUrd and Ki67 were obtained by confocal microscopy (10). Nuclei sampled in 11 cytokine-treated mice for BrdUrd were M = 3,541; EC = 2,604; SMC = 1,824; and for Ki67 were M = 3,096; EC = 2,465; SMC = 1,404.
Cell Differentiation.
Cytoplasmic and nuclear markers were used; M nuclei, rabbit polyclonal Csx/Nkx2.5, MEF2, and GATA4 Abs (10, 22, 23); cytoplasm, mouse monoclonal nestin (24), rabbit polyclonal desmin (25), cardiac myosin, mouse monoclonal α-sarcomeric actin, and rabbit polyclonal connexin 43 Abs (10); EC cytoplasm, mouse monoclonal flk-1, vascular endothelial (VE)-cadherin, and factor VIII Abs (10, 26, 27); and SMC cytoplasm, flk-1 and α-smooth muscle actin Abs (10, 28). Scar was detected by a mixture of collagen type I and type III Abs.
Statistics.
Results are mean ± SD. Significance was determined by the Student's t test and Bonferroni method (16). Mortality was computed with a log-rank test. P < 0.05 was significant.
Results
BMC Mobilization by Cytokines Reduces Mortality and Induces Myocardial Repair After Infarction.
Given the ability of bone marrow Lin− c-kitPOS cells to transdifferentiate into the cardiogenic lineage (10), we used a protocol to maximize their number in the peripheral circulation to increase the probability of their homing to the region of dead myocardium. In normal animals, the frequency of Lin− c-kitPOS cells in the blood is only a small fraction of similar cells present in the bone marrow (13, 14). We have documented previously that the cytokine treatment used here promotes a marked increase of Lin− c-kitPOS cells in the bone marrow and a redistribution of these cells from the bone marrow to the peripheral blood. This protocol leads to a 250-fold increase in Lin− c-kitPOS cells in the circulation (13, 14).
In the current study, BMC mobilization by SCF and G-CSF resulted in a dramatic increase in survival of infarcted mice; with cytokine treatment, 73% of mice (11 of 15) survived 27 days, whereas mortality was very high in untreated infarcted mice (Fig. 1A). A large number of animals in this group died from 3 to 6 days after MI and only 17% (9 of 52) reached 27 days (P < 0.001). Mice that died within 48 h post-MI were not included in the mortality curve to minimize the influence of the surgical trauma. Infarct size was similar in the cytokine- [64 ± 11% (n = 11)] and saline- [62 ± 9% (n = 9)] injected animals as measured by the number of M lost in the LVFW at 27 days (see Fig. 5, which is published as supplemental data on the PNAS web site, www.pnas.org).
Figure 1.
Mortality and myocardial regeneration. (A) Cytokine-treated infarcted mice, n = 15; untreated infarcted mice, n = 52; log-rank test, P < 0.0001. (B) Large infarct (MI) in a cytokine-treated mouse; forming myocardium (arrowheads) at higher magnification (adjacent panel). (C) MI in a nontreated mouse. Healing comprises the entire infarct (arrowheads). Scarring at higher magnification (adjacent panel). Red = cardiac myosin; yellow-green = propidium iodide (PI) labeling of nuclei; blue-magenta = collagen types I and III. (B and C, ×20; Insets, ×80.)
BMC mobilization promoted myocardial regeneration in all 11 cytokine-treated infarcted mice, killed 27 days after surgery (Fig. 1B). Myocardial growth within the infarct was also seen in the 4 mice that died prematurely at day 6 (n = 2) and at day 9 (n = 2). Cardiac repair was characterized by a band of newly formed myocardium occupying most of the damaged area. The developing tissue extended from the border zone to the inside of the injured region and from the endocardium to the epicardium of the LVFW. In the absence of cytokines, myocardial replacement was never observed, and healing with scar formation was apparent (Fig. 1C). Conversely, only small areas of collagen accumulation were detected in treated mice.
BMC Mobilization Partially Restored Myocardial Mass.
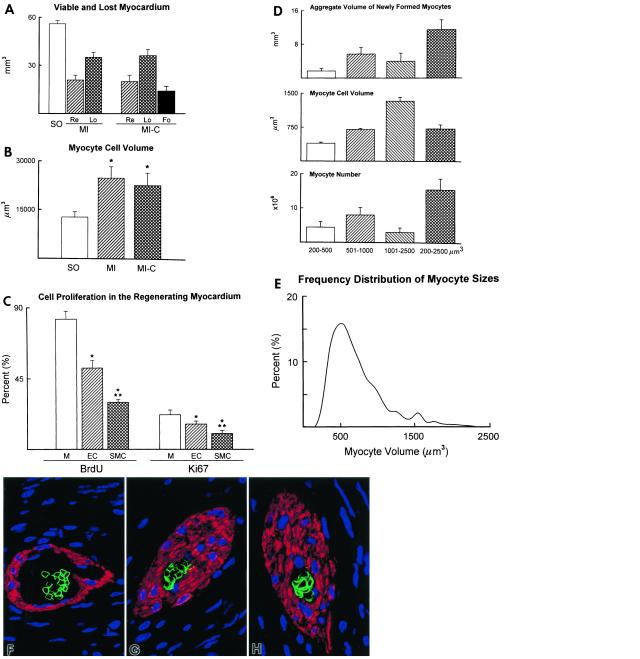
To quantify the contribution of the developing band to the ventricular mass, we first determined the volume of the LVFW (weight divided by 1.06 g/ml) in each group of mice. These data were 56 ± 2 mm3 in SO, 62 ± 4 mm3 (viable FW = 41 ± 3; infarcted FW = 21 ± 4) in infarcted nontreated animals, and 56 ± 9 mm3 (viable FW = 37 ± 8; infarcted FW = 19 ± 5) in infarcted cytokine-treated mice. These values were compared with the expected values of spared and lost myocardium at 27 days, given the size of the infarct in the nontreated and cytokine-treated animals. From the volume of the LVFW (56 mm3) in SO and infarct size in nontreated (62%) and treated (64%) mice, it was possible to calculate the volume of myocardium destined to remain (nontreated = 21 mm3; treated = 20 mm3) and destined to be lost (nontreated = 35 mm3; treated = 36 mm3) 27 days after coronary occlusion (Fig. 2A). The volume of newly formed myocardium was detected exclusively in cytokine-treated mice and found to be 14 mm3 (Fig. 2A). Thus, the repair band reduced infarct size from 64% (36 mm3/56 mm3 = 64%) to 39% [(36 mm3 − 14 mm3)/56 mm3 = 39%]. Because the spared portion of the LVFW at 27 days was 41 and 37 mm3 in nontreated and treated mice (see above), the remaining myocardium, shown in Fig. 2A, underwent 95% (P < 0.001) and 85% (P < 0.001) hypertrophy, respectively. Consistently, M cell volume increased 94% and 77% (Fig. 2B).
Figure 2.
Myocardial regeneration. (A) Remaining viable (Re), lost (Lo), and newly formed (Fo) myocardium in LVFW at 27 days in MI and MI-C; SO, myocardium without infarct. (B) Cellular hypertrophy in spared myocardium. (C) M, EC, and SMC labeled by BrdUrd and Ki67; n = 11. * and **, P < 0.05 vs. M and EC. (D and E) Volume, number (n = 11), and class distribution (bucket size, 100 μm3; n = 4,400) of M within the formed myocardium. (F–H) Arterioles with TER-119-labeled erythrocyte membrane (green fluorescence); blue fluorescence = propidium iodide (PI) staining of nuclei; red fluorescence = α-smooth muscle actin in SMC. F, ×800; G and H, ×1,200.
Myocardial Regeneration Is Characterized by Dividing Myocytes and Forming Vascular Structures.
Ki67 and BrdUrd were used to evaluate the growth stage of the cells in the regenerating band (Fig. 6 A–D, which is published as supplemental data on the PNAS web site). BrdUrd was injected daily between days 14–26 to measure the cumulative extent of cell proliferation while Ki67 was assayed to determine the number of cycling cells at the time of death. Ki67 identifies cells in G1, S, G2, prophase, and metaphase, decreasing in anaphase and telophase (10). The percentages of BrdUrd- and Ki67-positive M were 1.6- and 1.4-fold higher than EC, and 2.8- and 2.2-fold higher than SMC, respectively (Fig. 2C). The forming myocardium occupied 76 ± 11% of the infarct; M constituted 61 ± 12%, new vessels 12 ± 5%, and other components 3 ± 2%. The band contained 15 × 106 regenerating M that were in an active growing phase and had a wide size distribution (Fig. 2 D and E). EC and SMC growth resulted in the formation of 15 ± 5 arterioles and 348 ± 82 capillaries per mm2 of new myocardium. Thick wall arterioles with several layers of SMC and luminal diameters of 10–30 μm represented vessels in early differentiation. At times, incomplete perfusion of the coronary branches within the repairing myocardium during the fixation procedure led to arterioles and capillaries containing erythrocytes (Fig. 2 F–H). This observation provided evidence that the new vessels were functionally competent and connected with the coronary circulation. Therefore, tissue repair reduced infarct size and M growth exceeded angiogenesis; muscle mass replacement was the prevailing feature of the infarcted heart.
Five cytoplasmic proteins were identified to establish the state of differentiation of M (10, 24, 25): nestin, desmin, α-sarcomeric actin, cardiac myosin, and connexin 43. Nestin was recognized in individual cells scattered across the forming band (Fig. 3A). With this exception, all other M expressed desmin (Fig. 3B), α-sarcomeric actin, cardiac myosin, and connexin 43 (Fig. 3C). Three transcription factors implicated in the activation of the promoter of several cardiac muscle structural genes were examined (10, 22, 23): Csx/Nkx2.5, GATA-4, and MEF2 (Fig. 7 A–C, which is published as supplemental data on the PNAS web site). Single cells positive for flk-1 and VE-cadherin (26, 27), two EC markers, were present in the repairing tissue (Fig. 3 D and E); flk-1 was detected in SMC isolated or within the arteriolar wall (Fig. 3F). This tyrosine kinase receptor promotes migration of SMC during angiogenesis (28). Therefore, repair of the infarcted heart involved growth and differentiation of all cardiac cell populations, resulting in de novo myocardium.
Figure 3.
Markers of differentiating cardiac cells. (A–F) Labeling of M by nestin (A, yellow), desmin (B, red), and connexin 43 (C, green); red fluorescence = cardiac myosin (A and C). (D and E) Yellow-green fluorescence reflects labeling of EC by flk-1 (arrows, D) and VE-cadherin (arrows, E); red fluorescence = factor VIII in EC (D and E). (F) Green fluorescence labeling of SMC cytoplasms by flk-1; endothelial lining is also labeled by flk-1; red fluorescence = α-smooth muscle actin; blue fluorescence = propidium iodide (PI) labeling of nuclei. (A and E, ×1,200; B and F ×800; C, ×1,400; D, ×1,800.)
Myocardial Repair Improved Anatomical Remodeling and Ventricular Function.
Myocardial regeneration attenuated cavitary dilation and mural thinning during the evolution of the infarcted heart in vivo. Echocardiographically, LV end-systolic (LVESD) and end-diastolic diameters (LVEDD) increased more in nontreated than in cytokine-treated mice at 9, 16, and 26 days after infarction (Fig. 8 A and B, which is published as supplemental data on the PNAS web site). Infarction prevented the evaluation of anterior wall systolic thickness (AWST) and anterior wall diastolic thickness (AWDT) . When measurable, posterior wall thickness in systole (PWST) and diastole (PWDT) was greater in treated mice (Fig. 8 C and D). Anatomically, the wall bordering and remote from infarction was 26% and 22% thicker in cytokine-injected mice (Fig. 8 E). BMC-induced repair resulted in a 42% higher wall thickness to chamber radius ratio (Fig. 4A). Additionally, tissue regeneration decreased the expansion in cavitary diameter (−14%), longitudinal axis (−5%; Fig. 8 F and G), and chamber volume (−26%; Fig. 4B). Importantly, ventricular mass to chamber volume ratio was 36% higher in treated animals (Fig. 4C). Therefore, BMC mobilization that led to proliferation and differentiation of a new population of M and vascular structures attenuated the anatomical variables which define cardiac decompensation.
Figure 4.
MI, cardiac anatomy, and function. (A–C) LV dimensions at time of death, 27 days after surgery; SO (n = 9), nontreated infarcted (MI, n = 9), and cytokine-treated infarcted (MI-C, n = 10). (D) EF by echocardiography (SO, n = 9; MI, n = 9; and MI-C, n = 9). (E–M) M-mode echocardiograms of SO (E–G), MI (H–J), and MI-C (K–M); newly formed contracting myocardium (arrows). Detailed echocardiograms are shown in Fig. 8. (N) Wall stress, SO (n = 9); MI (n = 8); and MI-C (n = 9). Results are mean ± SD. * and **, P < 0.05 vs. SO and MI, respectively.
Measurements of EF during the evolution of infarction and hemodynamics at the time of death showed that repair improved ventricular performance. EF was 48, 62, and 114% higher in treated than in nontreated mice at 9, 16, and 26 days after coronary occlusion, respectively (Fig. 4D). In mice exposed to cytokines, contractile function developed with time in the infarcted region of the wall (Fig. 4 E–M; Fig. 8 H–P). Conversely, LV end-diastolic pressure (LVEDP) increased 76% more in nontreated mice. The changes in LV systolic pressure (not shown), developed pressure (LVDP), and + and − dP/dt were also more severe in the absence of cytokine treatment (Fig. 9 A–D, which is published as supplemental data on the PNAS web site). Additionally, the increase in diastolic stress in the zone bordering and remote from infarction was 69–73% lower in cytokine-treated mice (Fig. 4N). Therefore, cytokine-mediated infarct repair restored a noticeable level of contraction in the regenerating myocardium, decreasing diastolic wall stress and increasing ventricular performance.
Discussion
On the basis of the results presented above, we conclude that cytokine administration, with the consequent mobilization of BMC into the circulation and, presumably, their translocation to the infarcted portion of the heart, led to a significant magnitude of myocardial repair. Tissue regeneration comprised parenchymal cells and vascular structures. This anatomical restoration was accompanied by a dramatic reduction in post-MI mortality and a remarkable recovery in ventricular performance. Such a high degree of anatomical and functional improvement was accomplished in 100% of the treated animals by using a noninvasive procedure. The importance of these observations for the potential treatment of ischemic heart disease in humans is apparent.
The ability of exogenous BMC to home to the damaged area of the myocardium and differentiate into cells of the cardiogenic lineage, including coronary arterioles and capillaries, was shown previously by local transplantation of Lin− c-kitPOS cells into the border zone of an acute infarct (10). However, thoracic surgery and injection of foreign cells were required. Because of the complexity of the protocol, the rate of success with this invasive approach was only 40%. Additionally, this procedure required the availability of syngeneic donors as the source of the transplanted cells. In contrast, the methodology described here succeeded in all cases, eliminated the mortality and morbidity of thoracic surgery and, most importantly, obviated the use of foreign cells with the risk of transmission of infectious agents and the activation of an immunological reaction. Obviously, the generation of true myocardium and coronary vessels is superior to the use of skeletal myoblasts as a replacement for dead cardiac tissue (4, 5, 29). Although skeletal myoblasts survive and differentiate into skeletal muscle when injected into the myocardium, they never become electrically coupled with the rest of the heart because they do not express connexin 43 (4, 5, 29). Moreover, the diastolic properties of skeletal muscle cells are different from those of cardiomyocytes. Similarly, the formation of vessels only severely limits the possibility of complete functional repair after a segmental loss of ventricular mass (30).
Connexin 43 was clearly detectable in the newly formed M derived from BMC at 9 days after implantation (10) and it acquired a more mature pattern of distribution at 27 days. Consistent with the identification of contractile activity in the repairing myocardium, the expression of connexin 43 suggests that operative gap junctions were developed between M. To our knowledge, with the exception of this and our previous report using BMC, none of the published attempts to repair cardiac tissue post-MI have resulted in the production of functional, healthy myocardium. Although not investigated here, this contention implies that extracellular matrix supporting parenchymal cells and coronary vessels had to be formed. Additionally, scar formation was minimal in the treated animals but this does not exclude the notion that groups of myofibroblasts were present at the edges of the regenerating tissue.
The long-term unfavorable outcome of the infarcted heart is directly related to the initial infarct size that determines the degree of impaired pump function and the magnitude of dilation and wall thinning (31, 32). The changes in cardiac anatomy acutely after infarction, in combination with elevated filling pressure and decreased systolic pressure, induce large increases in diastolic stress and modest increases in systolic stress (18, 31). These structural-functional modifications promote chronic remodeling and the evolution of the myopathy to terminal failure (19, 31). Formation of new myocardium within the infarct attenuated the anatomical alterations, led to chronic increases in EF, and reduced the abnormalities in cavitary pressure, contractility, and loading. Longer intervals after homing of primitive BMC may result in complete repair of the infarcted heart.
Despite the success of the protocol used here, there are questions that we are currently addressing. Administration of SCF and G-CSF mobilizes pluripotent Lin− c-kitPOS cells from the bone marrow to the peripheral blood (13). The number of circulating Lin− c-kitPOS cells increased 250-fold. Donor BMC injected intravenously home to injured organs including liver (8) and skeletal muscle (33). Because of previous results with Lin− c-kitPOS cells (10), we propose that Lin− c-kitPOS cells are responsible for cardiac repair. Our results do not provide unambiguous information about the origin of the cells reconstituting the myocardium. It could be argued that cytokine treatment mobilized bone marrow stem cells and resident cardiac stem cells, which together participated in tissue regeneration. We have begun gene-marking studies in an effort to document the plasticity of adult Lin− c-kitPOS bone marrow cells in myocardial repair. This issue could also be addressed by treatment of irradiated animals with cytokines or by transfusing infarcted animals with BMC from syngeneic sex-mismatched donors.
The efficacy of cytokine treatment starting 5 days before MI followed by 3 more days postcoronary occlusion raises the question of the most effective therapeutic window. Additionally, it is not clear whether tissue repair is a result of the homing of BMC to the lesion or whether BMC, once mobilized, nest randomly throughout the organism and only those in the damaged myocardium rapidly proliferate and transdifferentiate. The former possibility is more attractive and supported by the rapid induction of SCF in a number of tissues, including the myocardium (34), in response to injury (34–37). SCF could be responsible for migration, accumulation, and multiplication of primitive BMC in the infarcted zone where they acquire the heart muscle phenotype reaching functional competence.
In conclusion, BMC injected or mobilized to the damaged myocardium behave as cardiac stem cells, giving rise to M, endothelial cells, and smooth muscle cells. Such behavior is no longer surprising given the remarkable plasticity of adult bone marrow stem cells (8–12, 30). New evidence offers clues as to some of the biochemical pathways implicated in this transdifferentiation. The interplay between the signal transduction pathways of bone morphogenetic proteins and the Wnt family of genes is responsible for the expression of lineage-determining genes that condition whether a mesodermal precursor cell becomes a blood cell or a cardiac cell (38, 39). During development, the differentiation into a M or a hemopoietic cell is an opposing and mutually exclusive choice. This choice, established by the nature of the cell, seems to be influenced by cues from the environment where the cell resides. Such environmental cues have proven to be difficult to elucidate in the maturing embryo. Surprisingly, as shown here and previously (10, 13), adult BMC remain open to both developmental pathways and readily reprogram themselves in response to the habitat. The absence of hematopoietic islands in the regenerating myocardium as a result of BMC localization is further testimony of the responsiveness of these cells to environmental factors. Thus, this system offers a favorable experimental setting to uncover the biology of this intriguing and potentially clinically useful transdifferentiation.
We believe that the approach presented here might bring myocardial regeneration closer to clinical reality and might also offer the opportunity to uncover the molecular mechanisms involved.
Supplementary Material
Acknowledgments
We thank Dr. Seigo Izumo (Beth Israel Deaconess Medical Center, Boston) for providing us with the Csx/Nkx2.5 Ab. The mAb Rat-401 (anti-nestin) developed by S. Hockfield was obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the National Institute of Child Health and Human Development, and maintained by the University of Iowa, Iowa City. This work was supported by National Institutes of Health Grants HL-38132, HL-39902, HL-43023, AG-15756, AG-17042, HL-66923, and HL-65577.
Abbreviations
- BMC
bone marrow cells
- SCF
stem cell factor
- G-CSF
granulocyte-colony stimulating factor
- LV
left ventricle
- EC
endothelial cells
- SMC
smooth muscle cells
- EF
ejection fraction
- SO
sham-operated
- LVFW
LV free wall
- M
myocyte
- MI
myocardial infarction
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Beltrami A, Urbanek K, Kajstura J, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami C A, Anversa P. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 2.Linzbach A J. Am J Cardiol. 1960;5:370–382. doi: 10.1016/0002-9149(60)90084-9. [DOI] [PubMed] [Google Scholar]
- 3.Leor J, Patterson M, Quinones M J, Kedes L H, Kloner R A. Circulation. 1996;94,Suppl.:II331–II336. [PubMed] [Google Scholar]
- 4.Murry C E, Wiseman R W, Schwartz S M, Hauschka S D. J Clin Invest. 1996;98:2512–2523. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor D A, Atkins B Z, Hungspreugs P, Jones T R, Reedy M C, Hutcheson K A, Glower D D, Kraus W E. Nat Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 6.Tomita S, Li R-K, Weisel R D, Mickle D A G, Kim E-J, Sakai T, Jia Z-Q. Circulation. 1999;100,Suppl.:II247–II256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 7.Menasche P, Hagege A A, Scorsin M, Pouzzet B, Desnos B, Duboc D, Scwartz K, Vilquin J-T, Marolleau J-P. Lancet. 2000;357:279–280. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 8.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman I L, Grompe M. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 9.Brazelton T R, Rossi F M V, Keshet G I, Blau H M. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 10.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Pickel J, McKay R, Nadal-Ginard B, Bodine D M, Leri A, Anversa P. Nature (London) 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 11.Bjornson C R R, Rietze R L, Reynolds B A, Magli M C, Vescovi A L. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- 12.Mezey E, Chandross K J, Harta G, Maki R A, McKercher S R. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 13.Bodine D M, Seidel N E, Gale M S, Nienhuis A W, Orlic D. Blood. 1994;84:1482–1491. [PubMed] [Google Scholar]
- 14.Orlic D, Fischer R, Nishikawa S-I, Nienhuis A W, Bodine D M. Blood. 1993;91:3247–3254. [PubMed] [Google Scholar]
- 15.Li Q, Li B, Wang X, Leri A, Jana K P, Liu Y, Kajstura J, Baserga R, Anversa P. J Clin Invest. 1997;100:1991–1999. doi: 10.1172/JCI119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li B, Setoguchi M, Wang X, Andreoli A M, Leri A, Malhotra A, Kajstura J, Anversa P. Circ Res. 1999;84:1007–1019. doi: 10.1161/01.res.84.9.1007. [DOI] [PubMed] [Google Scholar]
- 17.Pollick C, Hale S L, Kloner R A. J Am Soc Echocardiogr. 1995;8:602–610. doi: 10.1016/s0894-7317(05)80373-6. [DOI] [PubMed] [Google Scholar]
- 18.Olivetti G, Capasso J M, Meggs L G, Sonnenblick E H, Anversa P. Circ Res. 1991;68:856–869. doi: 10.1161/01.res.68.3.856. [DOI] [PubMed] [Google Scholar]
- 19.Beltrami C A, Finato N, Rocco M, Feruglio G A, Puricelli C, Cigola E, Quaini F, Sonnenblick E H, Olivetti G, Anversa P. Circulation. 1994;89:151–163. doi: 10.1161/01.cir.89.1.151. [DOI] [PubMed] [Google Scholar]
- 20.Kajstura J, Zhang X, Liu Y, Szoke E, Cheng W, Olivetti G, Hintze T H, Anversa P. Circulation. 1995;92:2306–2317. doi: 10.1161/01.cir.92.8.2306. [DOI] [PubMed] [Google Scholar]
- 21.Reiss K, Cheng W, Ferber A, Kajstura J, Li P, Li B, Olivetti G, Homcy C J, Baserga R, Anversa P. Proc Natl Acad Sci USA. 1996;93:8630–8635. doi: 10.1073/pnas.93.16.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Q, Schwarz J, Bucana C, Olson E N. Science. 1997;276:1404–1407. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasahara H, Bartunkova S, Schinke M, Tanaka M, Izumo S. Circ Res. 1998;82:936–946. doi: 10.1161/01.res.82.9.936. [DOI] [PubMed] [Google Scholar]
- 24.Kachinsky A M, Dominov J A, Boone Miller J. J Histochem Cytochem. 1995;43:843–847. doi: 10.1177/43.8.7542682. [DOI] [PubMed] [Google Scholar]
- 25.Hermann H, Aebi U. In: Subcellular Biochemistry: Intermediate Filaments. Herrmann H, Harris E, editors. Vol. 31. New York: Plenum; 1998. pp. 319–362. [PubMed] [Google Scholar]
- 26.Yamaguchi T P, Dumont D J, Conlon R A, Breitman M L, Rossant J. Development (Cambridge, UK) 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- 27.Breier G, Breviario F, Caveda L, Berthier R, Schnurch H, Gotsch U, Vestweber D, Risau W, Dejana E. Blood. 1996;87:630–641. [PubMed] [Google Scholar]
- 28.Couper L L, Bryant S R, Eldrup-Jorgensen J, Bredenberg C E, Lindner V. Circ Res. 1997;81:932–939. doi: 10.1161/01.res.81.6.932. [DOI] [PubMed] [Google Scholar]
- 29.Reinecke H, Murry C E. Cardiovasc Pathol. 2000;9:337–344. doi: 10.1016/s1054-8807(00)00055-7. [DOI] [PubMed] [Google Scholar]
- 30.Kocher A A, Schuster M D, Szabolcs M J, Takuma S, Burkhoff D, Wang J, Homma S, Edwards N M, Itescu S. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 31.Pfeffer M A, Braunwald E. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 32.Braunwald E, Bristow M R. Circulation. 2000;102:IV14–IV23. doi: 10.1161/01.cir.102.suppl_4.iv-14. [DOI] [PubMed] [Google Scholar]
- 33.Gussoni E, Soneoka Y, Strickland C D, Buzney E A, Khan M K, Flint A F, Kunkel L M, Mulligan R C. Nature (London) 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 34.Frangogiannis N G, Perrard J L, Mendoza L H, Burns A R, Lindsey M L, Ballantyne C M, Lloyd M H, Smith C W, Entman M L. Circulation. 1998;98:687–698. doi: 10.1161/01.cir.98.7.687. [DOI] [PubMed] [Google Scholar]
- 35.Gorospe J R, Nishikawa B K, Hoffman E P. J Neurol Sci. 1996;135:10–17. doi: 10.1016/0022-510x(95)00255-z. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto T, Katayama I, Nishioka K. Dermatology. 1998;197:109–114. doi: 10.1159/000017979. [DOI] [PubMed] [Google Scholar]
- 37.Gaca M D, Pickering J A, Arthur M J, Benyon R C. J Hepatol. 1999;30:850–858. doi: 10.1016/s0168-8278(99)80139-1. [DOI] [PubMed] [Google Scholar]
- 38.Winnier G, Blessing M, Labosky P A, Hogan B L. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 39.Marvin M J, Di Rocco G, Gardiner A, Bush S M, Lassar A B. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.