Abstract
Objective: The aim of this study was to formulate polymer-based artesunate nanoparticles for malaria treatment. Methods: Artesunate was loaded with poly(D,L-lactic-co-glycolic acid) (PLGA) by solvent evaporation from an oil-in-water single emulsion. Nanoparticles were characterized by X-ray diffraction and differential scanning calorimetry analyses. In vivo antimalarial studies at 4 mg/kg were performed on Swiss male albino mice infected with Plasmodium berghei. Hematological and hepatic toxicity assays were performed. In vitro cytotoxicity of free and encapsulated artesunate (Art-PLGA) to cell line RAW 264.7 was determined at concentrations of 7.8–1000 µg/ml. Results: The particle size of the formulated drug was (329.3±21.7) nm and the entrapment efficiency was (38.4±10.1)%. Art-PLGA nanoparticles showed higher parasite suppression (62.6%) compared to free artesunate (58.2%, P<0.05). Platelet counts were significantly higher in controls (305 000.00±148 492.40) than in mice treated with free artesunate (139 500.00±20 506.10) or Art-PLGA (163 500.00±3535.53) (P<0.05). There was no sign of hepatic toxicity following use of the tested drugs. The half maximal inhibitory concentration (IC50) of Art-PLGA (468.0 µg/ml) was significantly higher (P<0.05) than that of free artesunate (7.3 µg/ml) in the in vitro cytotoxicity assay. Conclusions: A simple treatment of PLGA-entrapped artesunate nanoparticles with dual advantages of low toxicity and better antiplasmodial efficacy has been developed.
Keywords: Poly(D,L-lactic-co-glycolic acid) (PLGA); Artesunate-PLGA delivery system; Antiplasmodial; Toxicity
1. Introduction
Malaria is a life threatening disease, which has attracted significant attention in terms of developing or improving drug regimens. The problems of toxicity and poor bio-distribution of most of the mainstay drugs for most parasitic diseases have necessitated the search for better alternatives. Artesunate, a partially synthetic derivative of artemisinin, has been approved for the treatment of malaria and has also been extensively documented for its anticancer properties (Woerdenbag et al., 1993; Efferth, 2007; Liu et al., 2011). Despite the therapeutic uses of artesunate, it has the disadvantage of a short half-life, therefore requiring frequent administration (Chadha et al., 2012; Nguyen et al., 2015). Its instability often causes easy degradation, resulting in poor pharmacokinetics and low bioavailability and pharmacological activity (Agnihotri et al., 2013; Meng et al., 2014). Therefore, development of a carrier that can maintain a sustained release profile and avoid rapid degradation of the drug is essential for its effective therapeutic usage.
The high bioavailability, good encapsulation, controlled release, and low toxicity of biodegradable nanoparticles have resulted in their frequent use as drug delivery vehicles (Nguyen et al., 2015). Poly(D,L-lactic-co-glycolic acid) (PLGA) is one of the most successful biodegradable polymers used for the development of nanoparticle treatments. Its hydrolysis within the body produces glycolic acid, which is a non-toxic biodegradable metabolite monomer (Kumari et al., 2010). Along with approval for use in humans by the US Food and Drug Administration (Jain, 2000), this polymer is a good candidate for preparation of drug delivery systems for diseases like cancer, schistosomiasis, malaria, and other infectious agents (Mainardes and Evangelista, 2005; Acharya and Sahoo, 2011; Pradhan et al., 2013; Nguyen et al., 2015). While polymeric particle delivery of artesunate has undeniably enjoyed wide therapeutic application, its antimalarial potency and toxicological effects have not been studied.
Therefore, the aim of this study was to develop a polymeric drug delivery system for artesunate that has proper physical properties for improved malaria therapy and low toxicity.
2. Materials and methods
2.1. Materials
Artesunate (from Artemisia annua L. (Asteraceae)), polyvinyl alcohol (PVA; M W=30–70 kDa), D-mannitol, dimethyl sulfoxide (DMSO), and 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). PLGA (intrinsic viscosity η=0.41 dl/g, copolymer ratio 50:50, 45 kDa) was purchased from Purac Biochem, the Netherlands. Dichloromethane and acetone were procured from Merck Serono Ltd., Middlesex, UK. Water was purified using a Milli-Qplus system from Millipore (MQ water, Ontario, Canada). Culture medium RPMI-1640, fetal calf serum (FCS), and antibiotic-antimycotic were obtained from GIBCO Invitrogen (Grand Island, NY, USA). All other chemicals were of analytical grade.
2.2. Preparation of nanoparticles
Artesunate-loaded PLGA (Art-PLGA) nanoparticles were formulated by solvent evaporation from an oil-in-water single emulsion (Chittasupho et al., 2009). Briefly, 5 mg of artesunate was added to the organic phase containing 50 mg of polymer dissolved in 3.5 ml of dichloromethane and 0.5 ml of acetone to constitute a 1:10 (drug to polymer, w/w) ratio. The organic phase was then added drop-wise to 16 ml aqueous solution (2% PVA as emulsifier) with sonication using a sonicator (Qsonica sonicators, Newtown, USA) at 30 W with a 40% duty cycle for 3 min in ice-cold water. The emulsion was continuously stirred by magnetic stirrer (Thermo Scientific, Massachusetts, USA) at 300 r/min for 6 h to evaporate the solvent, leaving behind a colloidal suspension of the drug-encapsulated nanoparticles in aqueous phase. The formulation was centrifuged at 16 000 r/min for 15 min using an ultracentrifuge (Beckman Coulter, Atlanta, USA) and then washed three times with MQ water. Dry powders were obtained by lyophilization of frozen samples in a freeze dryer (Labconco FreeZone 2.5, Kansas City, USA) in the presence of 5% mannitol as a cryoprotectant.
2.3. Measurement of particle size and zeta potential
The particle diameter, zeta potential, and polydispersity index (PDI) of the Art-PLGA-entrapped nanoparticulate drug were measured by a dynamic light scattering method using a Zetasizer Nano-ZS system (Malvern Instruments, UK). A homogenous solution obtained from dispersal of an appropriate amount of the formulated particle in MQ water was transferred to a clear disposable sizing cuvette for size and PDI measurements. A clear zeta cell was used for zeta potential analysis. Readings were taken in triplicate and results were expressed as mean±standard deviation (SD).
2.4. X-ray diffraction analysis and differential scanning calorimetry
X-ray diffraction (XRD) analysis was conducted on lyophilized Art-PLGA nanoparticles using an X’Pert-Pro multipurpose X-ray diffractometer (PANalytical, the Netherlands). The CuKα radiation was generated at 45 kV and 40 mA in the diffraction angle (2θ) range of 5°–40°.
Differential scanning calorimetry (DSC) was conducted on Art-PLGA nanoparticles to characterize the physical state of the drug. Thermograms were obtained using a Pyris 1 differential scanning calorimeter (PerkinElmer, USA). Dry nitrogen gas was used to purge the DSC cell at a flow rate of 40 ml/min. About 6–8 mg of the formulation was sealed in a standard aluminum pan with a lid and heated at a rate of 5 °C/min from 50 °C to 300 °C.
2.5. Drug entrapment and encapsulation efficiency
Drug entrapment and encapsulation efficiency were determined using a modified form of the method described by Anitha et al. (2011). Lyophilized drug-loaded nanoparticles (10 mg) were dissolved in 1 ml acetonitrile (a common solvent for polymeric particles and drugs). The solution was thoroughly mixed and subjected to solvent evaporation for 9–10 h at 50 °C using a heater (CH-100, Biosan Ltd., Cambridge, UK). The residue was resuspended in 500 µl methanol, vortexed and centrifuged at 13 000 r/min for 20 min. The supernatant (500 µl) was collected and stored. The process was repeated with 500 µl of acetonitrile.
A stock solution (1000 µg/ml) of artesunate was prepared by dissolving 5 mg of the drug in methanol (5 ml). The drug concentration of non-entrapped artesunate was determined using an ultraviolet-visible (UV-vis) spectrophotometer (Ultrospec 2100 pro, Amersham Biosciences, USA) at λ max of 222 nm. Calibration plots of UV-vis spectrophotometer analysis of non-entrapped drug were obtained at concentrations ranging from 5 to 70 µg/ml. The supernatant containing encapsulated drug was analyzed by the same method. Then the drug content and encapsulation efficiency of the formulation were estimated.
2.6. Murine model and parasite strain
In vivo assays adhered to the Principles of Laboratory Animal Care (National Research Council, 2010). Swiss male albino mice were obtained from the Animal House Centre of the Department of Pharmacology, University of Ibadan, Nigeria. The rodent malaria line used was Plasmodium berghei NK-65 obtained from the Institute of Advanced Medical Research and Training (IAMRAT), University College Hospital, University of Ibadan, Nigeria.
2.7. Animals and conditions
A total of 15 Swiss male albino mice ((20.0±2.0) g) of 5–6 weeks old were used in a Peters’ 4-d suppressive test on three groups with five mice each group. Animals were maintained in standard pathogen-free conditions and fed ad libitum.
2.8. Antiplasmodial evaluation using the Peters’ 4-d suppressive test
On Day 0, P. berghei-infected erythrocytes (pEry) were obtained from an infected donor Swiss male mouse and diluted in physiological saline to 1×107 pEry/ml. Mice were infected intraperitoneally (ip) with an aliquot of 0.2 ml of the parasite suspension. The lyophilized Art-PLGA-entrapped nanoparticles were reconstituted in distilled water containing 10% Tween 80. At 2 h post-infection, the mice were treated orally with 0.1 ml of the nanoparticulate drug suspension with an equivalent artesunate composition of 4 mg/kg of body weight (Chinaeke et al., 2015). The positive control group was treated orally with free artesunate at a dose of 4 mg/kg. The negative control group received 0.2 ml of the vehicle. On Days 1–3 post-infection, the Day 0 treatments were repeated in the experimental groups of mice. On Day 4 post-infection, blood smears prepared from the tail vein were Giemsa-stained and examined microscopically (Cheesbrough, 1998). The percentage parasitemia was determined microscopically by counting the number of infected erythrocytes among total erythrocytes in four fields of view. The percentage suppression of P. berghei was evaluated on the 4th day after treatment using the formula: (A−B)/A×100%, where A is the mean parasitemia in the untreated group (negative control) and B is the mean parasitemia in each treated group (Tona et al., 2001). Animals were monitored daily for clinical signs and weight loss.
2.9. Rectal temperature and changes in weight
The rectal temperature and body weight of the mice and their baseline mean parasitemia before treatment were measured. The same measurements were repeated 4 d post treatment with free artesunate or the PLGA-encapsulated artesunate nanoparticulate drug.
2.10. Hematological and hepatic toxicity assays
Acclimatized albino rats (weight 94–105 g) were administered with drugs at similar concentrations to those used for the antiplasmodial study (i.e. 4 mg/kg for free and nano-formulated artesunate) for 4 d. Blood samples (2 ml) were collected from the rats through a retro-orbital puncture, into ethylene diamine tetraacetic acid (EDTA) tubes. Hematological parameters including packed cell volume (PCV), red blood cell (RBC) count, white blood cell (WBC) count, neutrophils, eosinophils and hemoglobin (Hb) levels were evaluated in accordance with standard procedures. The cyanomethemoglobin method was used for the measurement of Hb levels; a hemocytometer was used to estimate the RBC and WBC counts, while PCV was measured by the conventional method of filling capillary tubes with blood and centrifuging using a microhematocrit centrifuge. The concentrations of plasma enzymes like aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphate (ALP) were determined using Randox diagnostic kits.
2.11. Cell lines and culture
Murine RAW 264.7 macrophages were obtained from Product Development Cell-1 (National Institute of Immunology, New Delhi, India). The equilibrated cells were incubated in RPMI-1640 (Sigma, St. Louis, MO, USA) containing 10% FCS and 1% penicillin G/streptomycin/amphotericin B (PSA) at 37 °C and 5% CO2 in an air humidified incubator. Cells were harvested when confluence was reached, and were washed with RPMI medium. The cells were diluted to 5×104 cells/ml with 200 µl of the cell suspension seeded per well in sterile 96-well plates and incubated for 24 h to allow cell attachment.
2.12. In vitro cell viability
The method of Zhang and Feng (2006) was adopted with some modifications. Briefly, the seeded RAW 264.7 cells (density, 5×104 viable cells/well) were incubated with free drug or the drug-loaded PLGA nanoparticle suspension at concentrations ranging from 7.8 to 1000 μg/ml for 24 h. At designated time intervals, the medium was removed and 100 µl of culture medium and 10 µl of MTT (5 mg/ml in phosphate-buffered saline (PBS)) were added to the wells. After incubation for 3–4 h, the culture solution was removed, leaving the precipitate of formazan crystals. DMSO (100 µl) was then added to each well before the plate was analyzed by a microplate reader at 570 nm. Cell viability was calculated using the following equation:
Cell viability (%)=Abss/Abscontrol×100%,
where Abss is the absorbance of the cells incubated with the PLGA-loaded nanoparticle suspension, and Abscontrol is the absorbance of the control containing cells incubated with the culture medium only. IC50 is the concentration at which the free drug or nanoparticle-entrapped drug inhibits cell growth by 50%, and is calculated using GraphPad Prism 6 statistical software (GraphPad Software Inc., La Jolla, CA, USA).
2.13. Statistical analysis
Data were entered in an MS-Excel spreadsheet and transferred to GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA) for analysis. Two-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests were used to test for significant differences. A P-value of <0.05 was considered to be statistically significant. Cytotoxicity was expressed as the mean inhibition relative to the unexposed control±SD for three parallel readings.
3. Results
3.1. Characterization of nanoparticles
The size, PDI, and zeta potential of Art-PLGA-entrapped nanoparticles were (329.3±21.7) nm, 0.355±0.04, and (−17.4±7.1) mV, respectively. The drug entrapment efficiency (%EE) was (38.4±10.1)%. The effects of surfactants and drug/polymer ratios on nanoparticle characteristics are presented in Table 1.
Table 1.
Effects of surfactants and drug/polymer ratios on nanoparticle characteristics
| Surfactant | OP:EAP | Drug:PLGA | Particle size (nm)* | Zeta potential (mV)* | PDI* |
| 1% PVA | 1:5 (4 ml:20 ml) | 1:5 (10 mg:50 mg) | 3330.00±1.88 | −13.3±3.3 | 0.869±0.094 |
| 2% SLS | 1:4 (4 ml:16 ml) | 1:10 (2.5 mg:25 mg) | 340.60±5.15 | −14.1±3.9 | 0.322±0.124 |
| 2% PVA | 1:5 (4 ml:20 ml) | 1:5 (10 mg:50 mg) | 7300.00±1.70 | −24.5±12.4 | 1.000±0.000 |
| 2% PVA | 1:4 (4 ml:16 ml) | 1:10 (5 mg:50 mg) | 329.30±21.70 | −17.4±7.1 | 0.355±0.040 |
| 1% SLS | 1:4 (4 ml:16 ml) | 1:10 (5 mg:50 mg) | 3700.00±0.60 | −16.6±22.5 | 0.653±0.122 |
Data are expressed as mean±SD (n=3); OP: organic phase; EAP: external aqueous phase; PVA: polyvinyl alcohol; SLS: sodium lauryl sulfate; PDI: polydispersity index
The crystalline contents of the formulated nanoparticles were determined by powder X-ray diffraction (P-XRD) analysis. The P-XRD diffractograms for Art-PLGA showed two peaks representing 2θ of 42.1° and 43.7°, respectively (Fig. 1). The DSC thermogram of PLGA-entrapped artesunate showed no intensive endothermic peak for artesunate. However, a sharp PLGA relaxation peak at 47 °C was observed along with a diminished PLGA glass transition peak from 55 °C to 65 °C (Fig. 2). A very weak thermal event was present between 140 °C and 150 °C. Intensive endothermic events occurred from 260 °C to 300 °C.
Fig. 1.
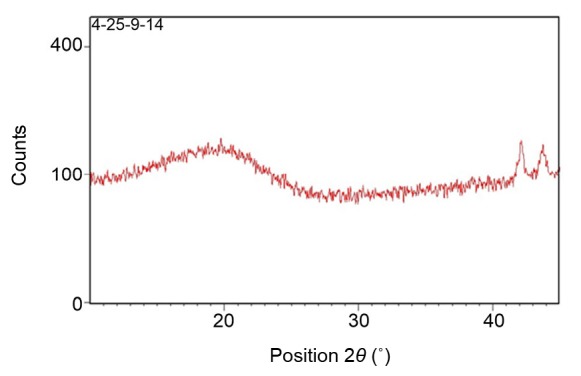
XRD spectra of formulated polymeric nanoparticles
Fig. 2.
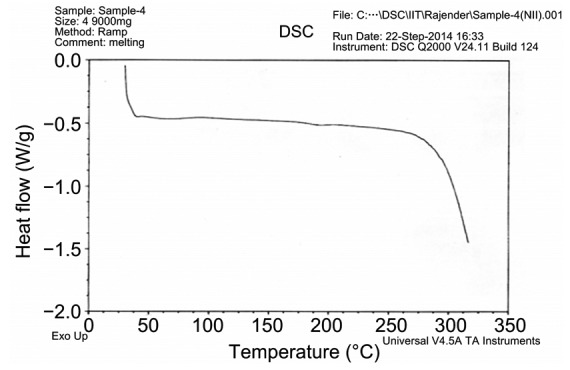
Thermogram of Art-PLGA nanoparticles
3.2. Parasite suppression
The percentage parasite suppression was lower (58.2%) in the free artesunate group than in the group treated with the nano-synthesized form (62.6%) at 4 d post-treatment. No parasite reduction was observed in the control group (Table 2).
Table 2.
Response to treatment in Swiss albino mice
| Group | Parasitemia* | Parasite suppression (%) | Weight (g)*
|
Temperature (°C)*
|
||||
| Before | After | P | Before | After | P | |||
| Art-free | 1.9±0.7a | 58.2 | 28.0±3.2 | 26.0±1.3 | >0.05 | 34.5±1.0 | 36.8±1.0 | <0.05 |
| Art-PLGA | 1.7±0.1a | 62.6 | 28.2±2.7 | 29.2±1.7 | >0.05 | 33.7±0.4 | 36.2±0.9 | <0.05 |
| Control | 4.5±0.6b | 24.2±3.2 | 23.2±1.2 | >0.05 | 36.1±0.5 | 36.3±1.0 | >0.05 | |
Similar superscript letters in the same column denote no significant difference while different letters denote a significant difference. Parasitemia denotes mean of parasite counts in 5 mice representing each experimental group.
Data are expressed as mean±SD (n=5)
3.3. Variation in body weight and rectal temperature
The body weight of mice decreased from (28.0±3.2) g on Day 0 to (26.0±1.3) g on Day 4 after treatment with free artesunate, and from (24.2±3.2) g to (23.2±1.2) g in the control group during the same period (P>0.05). However, there was a weight gain from (28.2±2.7) g to (29.2±1.7) g in mice treated with the Art-PLGA nanoparticulate drug (P>0.05) (Table 2). There was a significant temperature gain from (34.5±1.0) °C to (36.8±1.0) °C in mice treated with free artesunate and from (33.7±0.4) °C to (36.2±0.9) °C in mice treated with Art-PLGA nanoparticulate drugs (P<0.05) (Table 2). No temperature gain was observed in the control group.
3.4. Variation in hematological and liver function parameters
There was no significant variation in most of the observed hematological parameters in free or nano-formulated artesunate exposed groups compared with the control group (P>0.05). However, the platelet counts (305 000.00±148 492.40) observed in the control group were significantly higher than those in the free (139 500.00±20 506.10) and nano-formulated drug (163 500.00±3535.53) treatment groups (Table 3). There were no significant differences in AST or ALT concentrations in all experimented groups, whereas the ALP concentration was significantly lower in the free artesunate group ((70.5±19.1) U/L) than in the control ((120.5±6.4) U/L) and the nano-synthesised groups ((110.5±4.9) U/L) (P<0.05; Fig. 3).
Table 3.
Hematological variation between free and nano-synthesised artesunate treatments
| Group | PCV (%) | Hb (g/dl) | RBC (×106/cm3) | WBC (×103/cm3) | Platelet (×103/cm3) | Lymphocyte (%) | Neutrophil (%) | Monocyte (%) |
| Artesunate | 37.00±2.83a | 12.20±0.92a | 5.80±0.61a | 6.10±1.17a | 139.50±20.51a | 64.00±2.83a | 31.50±3.54a | 2.50±0.71a |
| Art-PLGA | 37.50±2.12a | 12.00±0.57a | 6.20±0.08a | 8.80±2.47a | 163.50±3.54a | 70.00±0.00a | 26.50±0.71a | 1.50±0.71a |
| Control | 37.00±2.83a | 12.10±0.78a | 5.70±0.59a | 12.00±8.94a | 305.00±148.49b | 63.50±2.12a | 32.00±1.41a | 2.00±1.41a |
Similar superscript letters in the same column denote no significant difference while different letters denote a significant difference. PCV: packed cell volume; Hb: hemoglobin; RBC: red blood cell; WBC: white blood cell. Data are expressed as mean±SD (n=5)
Fig. 3.
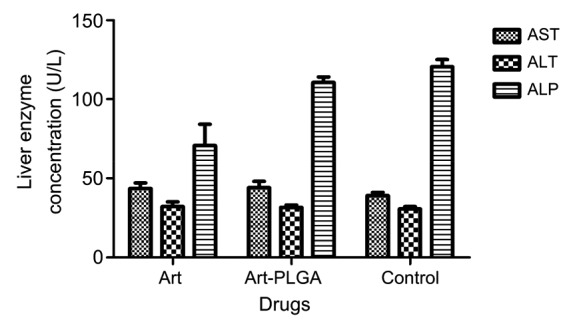
Hepatic toxicity assessment of free and nanoparticulate artesunate
AST: aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphate; Art: artesunate; Art-PLGA: artesunate entrapped PLGA nanoparticles. Data are expressed as mean±SD (n=5)
3.5. In vitro cell viability
Art-PLGA nanoparticles showed lower toxicity than the free drug. Cell viability data following treatment with free artesunate or Art-PLGA-entrapped nanoparticles are presented in Fig. 4. Cell viability varied in a concentration-dependent manner (P<0.001). Generally, cells became less viable at higher doses. The IC50 of Art-PLGA (468.0 µg/ml) was significantly higher than that of free artesunate (7.3 µg/ml).
Fig. 4.
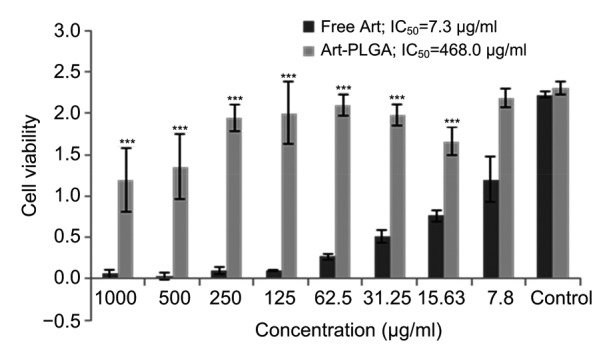
Cell viability and inhibitory concentrations in free and entrapped drugs
IC50: half maximal inhibitory concentration; Art: artesunate; Art-PLGA: artesunate-entrapped PLGA nanoparticles. Data are expressed as mean±SD (n=3). *** P<0.001, vs. free Art
4. Discussion
The method employed in the formulation yielded particles which appeared soluble and finely dispersed in water when compared to the free non-entrapped drugs. Dry lyophilized nanoparticles have been shown to have good physical and chemical stability and can be stored at room temperature for over 6 months without decomposition or aggregation (Bhawana et al., 2011). The influence of the larger surface area of nanoparticles in promoting dissolution has been emphasized (McNeil, 2005). The negative zeta potential denotes non-toxicity and the values for all formulations fall within the acceptable range for easy charge neutralization, thus favoring less interference in in vivo studies (Nordström, 2011). The PDI of our formulation, which was less than 0.4, will enhance moderate distribution (Nobbmann, 2014). The nanoparticles could, however, be further optimised to give a narrow range of monodispersed PDI for better drug distribution. The mean Art-PLGA nanoparticle size ((329.3±21.7) nm) observed in our study was larger than that found in a related study ((289.6±4.6) nm) (Nguyen et al., 2015). Our formulation also showed lower drug entrapment efficiency ((38.4±10.1)%) than that shown in that study ((42.36±0.73)%). The PLGA concentration, volume of the aqueous phase, and type of surfactants and stability agents used are known to affect particle size, PDI, and encapsulation efficiency (Wohlfart et al., 2011; Cooper and Harirforoosh, 2014). An increase in aqueous phase volume produces small nanoemulsion droplets, which decrease the size and PDI of the particles while an increase in PLGA concentration increases the viscosity of the polymer solution, resulting in poorer dispersibility of the organic phase in the aqueous phase (Nguyen et al., 2015).
The diffractogram patterns showing peaks at 42.1° and 43.7° 2θ suggest the amorphous nature of Art-PLGA (Panda et al., 2016). The absence of a decomposition exotherm indicates the increased physical stability of the drug in the formulation (Chadha et al., 2012). The thermogram of the Art-PLGA nanoparticles, which did not show any intensive endothermic peaks, indicates that chemical interactions which could alter the drug or the polymer properties did not occur during particle formulation (Panda et al., 2016). However, the presence of endothermic events at 260–300 °C could be due to residual excipients or the presence of impurities in the drug or polymer. It is also possible that polymers are corrosive in nature and their melting and interaction with the metal pan in previous samples may have resulted in the generation of peaks in non-specific regions. The very weak thermal event between 140 °C and 150 °C could have been due to the melting of artesunate. However, in the Art-PLGA formulation, the amount of artesunate was small compared to that in PLGA, and hence no significant endotherm was visible.
The increase in suppression of P. berghei in Art-PLGA-treated mice compared with those in the free drug and control groups was consistent with the findings of a report on the antimalarial properties of artesunate-loaded solid lipid microparticles, in which improved activity was linked to the drug’s sustained release action (Chinaeke et al., 2015). There was no parasite suppression in the control group since no drug was administered. The P. berghei murine model has been widely adopted for systematic screening of potential antimalarial drugs at the early stages of drug discovery. The screening results, however, do not always translate to the occurrence of Plasmodium falciparum malaria in man. Thus, there is a need to perform similar studies in a P. falciparum animal model (Ibrahim et al., 2014). The efficacy of Art-PLGA nanoparticles may be related to their ability to penetrate mucus and microvilli barriers. Nanoparticles as large as 500 nm encapsulated with a muco-inert polymer have been observed to traverse physiological human mucus rapidly, with diffusivities only 4-fold lower than their rates in pure water (Lai et al., 2007). The capacity of the polymeric nanoparticles to protect the encapsulated drug and its stability in the digestive tract have been reported to facilitate improved cellular uptake and controlled drug release at the target (des Rieux et al., 2006).
The negative association between P. berghei parasitemia and rectal temperature as observed in free artesunate and Art-PLGA-treated groups was consistent with earlier reports (Dikasso et al., 2006; Chinaeke et al., 2015). While weight loss in the control group could be associated with parasite burden, the loss of weight in the group treated with free artesunate could also result from toxic effects of the drug. There were no significant differences in most of the observed hematological parameters between mice administered with free artesunate or Art-PLGA nanoparticles and the control groups. However, the significantly lower platelet counts in the treated groups compared with the control group were consistent with the claim that the best side effect of artemisinin-based compounds is that they lower reticulocyte counts (Clark, 2012). Artesunate’s interaction with erythrocyte plasma membranes initiates a reaction between the drug and ferrous iron in the heme prosthetic group of hemoglobin, thus activating the drug to form toxic free radicals (Clark, 2012). The mechanism of action of artesunate and its ability to accumulate in the erythrocytes are associated with hemotoxicity (Bigoniya et al., 2015). The controlled release of artesunate from PLGA, however, could have resulted in the higher reticulocyte counts compared with the free drug. The lack of significant differences in transaminases, the important markers of hepatocellular toxicity and damage (Faber et al., 1981), suggests that free and nanoparticulate drugs are not toxic to the liver at the tested concentrations.
With the growing evidence of artesunate-associated toxicity to normal cells or tissues (Mesembe et al., 2004; White et al., 2006) and poor delivery, our method of polymer encapsulation of artesunate with reduced toxicity and effective delivery to target cells becomes more promising. The results of the in vitro cytotoxicity assay on the RAW 264.7 cell line further support the safety of PLGA nanoparticulate artesunate compared with the free drug.
A simple formulation of PLGA-entrapped artesunate nanoparticles with dual advantages of low toxicity and better antiplasmodial efficacy has been developed. The formulated nanoparticulate drugs will augment research efforts towards developing anti-malarial drugs with enhanced delivery and efficacy.
Acknowledgments
Oyetunde OYEYEMI acknowledges the Centre for Science and Technology of the Non-Aligned and Other Developing Countries (NAM S&T Centre) in collaboration with the Department of Science & Technology (DST), the Government of India for “Research Training Fellowship for Developing Country Scientists (RTF-DCS)” award, undertaken at the National Institute of Immunology, New Delhi, India.
Footnotes
Compliance with ethics guidelines: Kabiru DAUDA, Zulaikha BUSARI, Olajumoke MORENIKEJI, Funmilayo AFOLAYAN, Oyetunde OYEYEMI, Jairam MEENA, Debasis SAHU, and Amulya PANDA declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Del Rev. 2011;63(3):170–183. doi: 10.1016/j.addr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Agnihotri J, Singh S, Bigonia P. Formal chemical stability analysis and solubility analysis of artesunate and hydroxychloroquinine for development of parenteral dosage form. J Pharm Res. 2013;6:117–122. doi: 10.1016/j.jopr.2012.11.025. [DOI] [Google Scholar]
- 3.Anitha A, Deepagan VG, Rani VVD, et al. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate-chitosan nanoparticles. Carbohyd Poly. 2011;84(3):1158–1164. doi: 10.1016/j.carbpol.2011.01.005. [DOI] [Google Scholar]
- 4.Bhawana RK, Basniwal HS, Buttal VK, et al. Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J Agric Food Chem. 2011;59(5):2056–2061. doi: 10.1021/jf104402t. [DOI] [PubMed] [Google Scholar]
- 5.Bigoniya P, Sahu T, Tiwari V. Hematological and biochemical effects of sub-chronic artesunate exposure in rats. Toxicol Rep. 2015;2:280–288. doi: 10.1016/j.toxrep.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chadha R, Gupta S, Pathak N. Artesunate-loaded chitosan/lecithin nanoparticles: preparation, characterization, and in vivo studies. Drug Dev Ind Pharm. 2012;38(12):1538–1546. doi: 10.3109/03639045.2012.658812. [DOI] [PubMed] [Google Scholar]
- 7.Cheesbrough M. District Laboratory Practice in Tropical Countries Part 1. London: Cambridge University Press; 1998. [Google Scholar]
- 8.Chinaeke EE, Chime SA, Onyishi VI, et al. Formulation development and evaluation of the anti-malaria properties of sustained release artesunate-loaded solid lipid microparticles based on phytolipids. Drug Deliv. 2015;22(5):652–665. doi: 10.3109/10717544.2014.881633. [DOI] [PubMed] [Google Scholar]
- 9.Chittasupho C, Xie SX, Baoum A, et al. ICAM-1 targeting of doxorubicinloaded PLGA nanoparticles to lung epithelial cells. Eur J Pharm Sci. 2009;37(2):141–150. doi: 10.1016/j.ejps.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark RL. Effects of artemisinins on reticulocyte counts and and relationship to possible embryotoxicity in confirmed and unconfirmed malarial patients. Birth Def Res Part A: Clin Mol Teratol. 2012;94(2):61–75. doi: 10.1002/bdra.22868. [DOI] [PubMed] [Google Scholar]
- 11.Cooper DL, Harirforoosh S. Design and optimization of PLGA-based diclofenac loaded nanoparticles. PLoS ONE. 2014;9(1):e87326. doi: 10.1371/journal.pone.0087326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.des Rieux A, Fievez V, Garinot M, et al. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006;116(1):1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Dikasso D, Makonnen E, Debella A, et al. In vivo anti-malaria activity of hydroalcoholic extract from Asparaginus africanus in mice infected with Plasmodium berghei . Ethiop J Health Dev. 2006;20(2):112–118. doi: 10.4314/ejhd.v20i2.10021. [DOI] [Google Scholar]
- 14.Efferth T. Willmar Schwabe Award 2006: antiplasmodial and antitumor activity of artemisinin-from bench to bedside. Planta Med. 2007;73(4):299–309. doi: 10.1055/s-2007-967138. [DOI] [PubMed] [Google Scholar]
- 15.Faber JL, Chein KR, Mitlnacht S. Myocardial ischemia: the pathogenesis of irreversible cell injury in ischemia. Am J Pathol. 1981;102(2):271–281. [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim N, Ibrahim H, Dormoi J, et al. Albumin-bound nanoparticles of practically water-soluble antimalarial lead greatly enhance its efficacy. Int J Pharm. 2014;464(1-2):214–224. doi: 10.1016/j.ijpharm.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21(23):2475–2490. doi: 10.1016/S0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 18.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Coll Surf B: Biointer. 2010;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Lai SK, O'Hanlon DE, Harrold S, et al. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci USA. 2007;104(5):1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu WM, Gravett AM, Dalgleish AG. The antimalarial agent artesunate possesses anticancer properties that can be enhanced by combination strategies. Int J Cancer. 2011;128(6):1471–1480. doi: 10.1002/ijc.25707. [DOI] [PubMed] [Google Scholar]
- 21.Mainardes RM, Evangelista RC. PLGA nanoparticles containing praziquantel effect of formulation variables on size distribution. Int J Pharm. 2005;290(1-2):137–144. doi: 10.1016/j.ijpharm.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 22.McNeil SE. Nanotechnology for the biologist. J Leuk Biol. 2005;78(3):585–592. doi: 10.1189/jlb.0205074. [DOI] [PubMed] [Google Scholar]
- 23.Meng H, Xu K, Xu Y, et al. Nanocapsules based on mPEGylated artesunate prodrug and its cytotoxicity. Coll Surf B: Biointer. 2014;115:164–169. doi: 10.1016/j.colsurfb.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 24.Mesembe OE, Ivang AE, Udo-Attah G, et al. A morphometric study of the teratogenic effect of artesunate on the central nervous system of the Wistar rats foetus. Nig J Physiol Sci. 2004;19(1):92–97. [Google Scholar]
- 25.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press; 2010. [Google Scholar]
- 26.Nguyen HT, Tran TH, Kim JO, et al. Enhancing the in vitro anti-cancer efficacy of artesunate by loading into poly D,L-lactide-co-glycolide (PLGA) nanoparticles. Arch Pharm Res. 2015;38(5):716–724. doi: 10.1007/s12272-014-0424-3. [DOI] [PubMed] [Google Scholar]
- 27.Nobbmann ULF. Polydispersity–what does it mean for DLS and chromatography?. http://www.materials-talks. com/blog/2014/10/23/polydispersity-what-does-it-mean-for-dls-and-chromatography [accessed on Oct. 24, 2016].2014. [Google Scholar]
- 28.Nordström P. Formulation of Polymeric Nanoparticles Encapsulating and Releasing a New Hydrophobic Cancer Drug. MS T. Chalmers University of Technology, Göteborg, Sweden; 2011. [Google Scholar]
- 29.Panda A, Meena J, Katara R, et al. Formulation and characterization of clozapine and risperidone co-entrapped spray-dried PLGA nanoparticles. Pharm Dev Technol. 2016;21(1):43–53. doi: 10.3109/10837450.2014.965324. [DOI] [PubMed] [Google Scholar]
- 30.Pradhan R, Poudel BK, Ramasamy T, et al. Docetaxel loaded polylactic acid-co-glycolic acid nanoparticles: formulation, physicochemical characterization and cytotoxicity studies. J Nanosci Nanotechnol. 2013;13(8):5948–5956. doi: 10.1166/jnn.2013.7735. [DOI] [PubMed] [Google Scholar]
- 31.Tona L, Mesia K, Ngimbi NP, et al. In vivo antimalarial activity of Cassia occindentalis, Morinda morindoides and Phyllanthus niruri . Ann Trop Med Parasitol. 2001;95(1):47–57. doi: 10.1080/00034983.2001.11813614. [DOI] [PubMed] [Google Scholar]
- 32.White TE, Bushdid PB, Ritter S, et al. Artesunate-induced depletion of embryonic erythroblasts precedes embryolethality and teratogenicity in vivo. Birth Def. Res. Part B: Dev Reprod Toxicol. 2006;77(5):413–429. doi: 10.1002/bdrb.20092. [DOI] [PubMed] [Google Scholar]
- 33.Woerdenbag HJ, Moskal TA, Pras N, et al. Cytotoxicity of artemisinin-related endoperoxides to Ehrlich ascites tumor cells. J Nat Prod. 1993;56(6):849–856. doi: 10.1021/np50096a007. [DOI] [PubMed] [Google Scholar]
- 34.Wohlfart S, Khalansky AS, Gelperina S, et al. Efficient chemotherapy of rat glioblastoma using doxorubicin-loaded PLGA nanoparticles with different stabilizers; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang ZP, Feng SS. The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly(lactide)-tocopheryl polyethylene glycol succinate nanoparticles. Biomaterials. 2006;27(21):4025–4033. doi: 10.1016/j.biomaterials.2006.03.006. [DOI] [PubMed] [Google Scholar]


