Abstract
Accumulating evidence indicates that endostatin inhibits fibrosis. However, the mechanism is yet to be clarified. The aim of this study is to evaluate the effect of endostatin on platelet-derived growth factor-BB (PDGF-BB)-or transforming growth factor β1 (TGF-β1)-induced fibrosis in cultured human skin fibroblasts, and to further examine the molecular mechanisms involved. Human dermal fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM) and serum-starved for 48 h before treatment. Cells were grouped as follows: “PDGF-BB”, “PDGF-BB+endostatin”, “TGF-β1”, “TGF-β1+endostatin”, “endostatin”, and “blank control”. The fibroblasts were stimulated with either TGF-β1 or PDGF-BB for 72 h in order to set up the fibrosis model in vitro. The cells were co-cultured with either TGF-β1 or PDGF-BB and endostatin and were used to check the inhibiting effect of endostatin. A blank control group and an endostatin group were used as negative control groups. The biomarkers of fibrosis, including the expression of collagen I, hydroxyproline, and α-smooth muscle actin (α-SMA), were evaluated using an enzyme-linked immunosorbent assay (ELISA) and Western blot. The expression of phosphorylated PDGF receptor β (p-PDGFRβ), PDGFRβ, phosphorylated extracellular signal-regulated kinase (p-ERK), and ERK was detected using Western blot and immunofluorescent staining was used to explore the mechanisms. Both PDGF-BB and TGF-β1 significantly up-regulated the expression of collagen I, hydroxyproline, and α-SMA. Endostatin significantly attenuated both the PDGF-BB-and TGF-β1-induced over-expression of collagen I, hydroxyproline, and α-SMA. PDGF-BB and TGF-β1 both promoted the expression of PDGFR, ERK, and p-ERK. Endostatin inhibited the expression of PDGFR and p-ERK but did not affect the expression of total ERK. Endostatin inhibited hypertrophic scar by modulating the PDGFRβ/ERK pathway. Endostatin could be a promising multi-target drug in future fibrosis therapy.
Keywords: Endostatin, Hypertrophic scar, Phosphorylated platelet-derived growth factor receptor (p-PDGFR), Extracellular signal-regulated kinase (ERK), Signal pathway
1. Introduction
Cutaneous scarring is an inevitable result of deep skin injury. Hypertrophic scar is characterized by excessive collagen deposition and impaired functional or cosmetic outcomes. There are plenty of therapeutic interventions to treat hypertrophic scars, including operative and conservative treatments (Friedstat and Hultman, 2014); however, there are no effective ways to completely cure hypertrophic scar.
Endostatin is a strong endogenous angiogenesis inhibitor and commonly used as an anti-angiogenic therapy targeted against tumor vasculature. Recently, multiple other functions of endostatin have been discovered beyond its anti-angiogenic properties. For example, it can modulate androgen receptor function (Lee et al., 2015) and prevent sepsis or acute kidney injury (Peng et al., 2015; Mårtensson et al., 2016).
Accumulating evidence indicates that endostatin inhibits organ and skin fibrosis. Our previous study confirmed that systemic application of endostatin could prevent hypertrophic scar formation in the rabbit ear model (Ren et al., 2013). Yamaguchi et al. (2012) found that endostatin-derived peptide ameliorates organ fibrosis that is triggered by transforming growth factor-β (TGF-β) and bleomycin in human and mouse tissues. However, the molecular mechanisms by which endostatin mediates fibrosis in fibroblasts remain poorly understood.
TGF-β is the primary factor that drives fibrosis (Meng et al., 2016). Platelet-derived growth factor (PDGF) also plays a pivotal role in fibrosis (Andrae et al., 2008). As a subtype of PDGF, PDGF-BB has a strong mitogenic effect on fibroblasts (Lin et al., 2015). TGF-β and PDGF-BB have usually been employed as the fibrosis models in previous studies for their fibrosis-promoting effect (Cho et al., 2016; Choi et al., 2016). The PDGF receptor (PDGFR) signal pathway is involved in both angiogenesis and fibrosis and has become a new target for treating fibrosis. Interestingly, endostatin is also involved in these two important pathological processes. It was our hypothesis that endostatin has a close relationship with the PDGFR signal pathway.
In this study, we evaluated the effect of endostatin on fibrosis by using PDGF-BB-and TGF-β1-induced human skin fibroblast fibrosis models and further elucidated its underlying mechanisms.
2. Materials and methods
2.1. Reagents and antibodies
Primary antibodies against PDGFR-β, phosphorylated PDGF receptor β (p-PDGFRβ), α-smooth muscle actin (α-SMA), phosphorylated extracellular signal-regulated kinase (p-ERK), ERK, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Cell Signaling Technology (Beverly, MA, USA) and recombinant endostatin (rE, Endostar™) was purchased from the Simcere Pharmaceutical Company (Nanjing, China). Both PDGF-BB and TGF-β1 were purchased from the Prospech Bio, Rehovot, Israel. The enzyme-linked immunosorbent assay (ELISA) kits of human hydroxyproline and collagen I were purchased from the Abbexa Company (Cambridge, UK) and the MTS kit from the Promega Company (Madison, WI, USA).
2.2. Culture of human skin fibroblasts
Human dermal fibroblasts were purchased from ATCC (Manassas, VA, USA) and cultured according to their recommendations. All experimental procedures were conducted in accordance with the relevant guidelines and regulations of the hospital’s central labs. Cell passages 3–5 were used in this experiment and were cultured in Dulbecco’s modified Eagle’s medium (DMEM), then were seeded in 12-well plates at a density of 3×103 cells/well for 24 h and were serum-starved for 48 h before treatment.
2.3. Treatments and groups
The cells were divided into 6 groups: (1) control group; (2) endostatin group; (3) PDGF group; (4) “PDGF+endostatin” group; (5) TGF-β1 group; (6) “TGF-β1+endostatin” group. In the control group, fibroblasts were treated with serum-free DMEM for 72 h. In the endostatin group, cells were treated with endostatin for 72 h. The fibroblasts were treated for 72 h with 200 ng/ml PDGF-BB in the PDGF-treated group and with 10 ng/ml TGF-β1 in the TGF-β1-treated group. In the “PDGF+endostatin” group, fibroblasts were treated with 200 ng/ml PDGF-BB for 72 h and 5 μg/ml endostatin was added at the 24th hour. Lastly, in the “TGF-β1+endostatin” group, fibroblasts were treated with 10 ng/ml TGF-β1 for 72 h and 5 μg/ml endostatin was added at the 24th hour. All cells were cultured in serum-free DMEM.
2.4. MTT cytotoxicity assay
The toxicity of endostatin on fibroblasts was evaluated using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Human fibroblasts (3×103) were grown in 96-well plates in a medium containing 10% fetal bovine serum for 24 h. The fibroblasts were washed three times with serum-free DMEM and were serum-starved for 24 h to make cells quiescent. Subsequently, endostatin (0, 1.25, 2.5, 5, and 10 μg/ml in DMEM) was added to each well for either 24, 48, or 72 h. The MTT solution was added and then incubated at 37 °C for 3 h. The MTT solvent solution was added and incubated for 15 min; absorbance was recorded at 420 nm on an MR7000 microplate reader (Dynatech, NV, USA).
2.5. ELISA assay
The concentrations of collagen I and hydroxyproline proteins were determined using commercially available ELISA kits following the manufacturer’s guidance and as described by Hu et al. (2012). Either a monoclonal antibody of collagen type I or hydroxyproline was added to the assay diluents (100 μl). Then 50 μl of either control or sample was added and incubated for two hours. Each well was washed three times with wash buffer. Two hundred microliters of either collagen type I or hydroxyproline conjugate was then added to each well for two hours. The wells were then washed further three times. The substrate solution (200 μl) was added for 30 min and the reaction was then stopped. The optical density was tested with a microplate reader. The concentrations of collagen I and hydroxyproline proteins were determined by comparing the optical densities with the standard curves. The supernatant fluid was harvested and measured three times.
2.6. Western blot analysis
The expression of PDGFR, p-PDGFRβ, ERK, p-ERK, and α-SMA was detected using Western blot analysis according to the standard protocol as described by Okada et al. (2013). Cells were scraped into the lysis buffer before separating the target proteins in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferring them onto a polyvinylidene fluoride (PVDF) membrane. Membranes were probed overnight at 4 °C with the following primary antibodies: PDGFR-β (1:1000 dilution), p-PDGFR-β (1:1000 dilution), ERK1/2 (1:500 dilution), and p-ERK1/2 (1:500 dilution). GAPDH (1:2000 dilution) was used as an internal control. The levels of target protein bands were analyzed with ImageJ software (v.1.60) normalized using GAPDH.
2.7. Immunofluorescent staining
Fibroblasts were treated with 4% (0.04 g/ml) paraformaldehyde solution followed by 0.2% (v/v) Triton X-100. Immunofluorescent staining with the antibody against PDGFRβ was performed according to the manufacturer’s instruction as described by Hu et al. (2012). DAPI reagent (4',6-diamidino-2-phenylindole, MP Biomedicals, 1:7500) was used to stain the nucleus.
2.8. Statistical analysis
Each experiment was repeated three times and data were recorded and analyzed with Prism 6.0 software. A one-way analysis of variance (ANOVA) was the chosen statistical technique for testing the hypotheses and results with a P-value <0.05 considered statistically significant.
3. Results
3.1. Toxicity of endostatin with MTT cytotoxicity assay
The toxicity of endostatin was tested by exposing human fibroblasts, for durations of 24, 48, and 72 h, to gradient concentrations of endostatin (0, 1.25, 2.5, 5, and 10 μg/ml) before assessment by MTT assay. No significant reduction of absorbance was observed at 0–5 μg/ml of endostatin after 24–48 h. A concentration of 5 μg/ml was therefore chosen as the working concentration and 48 h as the acting time. The dose–response curves are shown in Fig. 1.
Fig. 1.
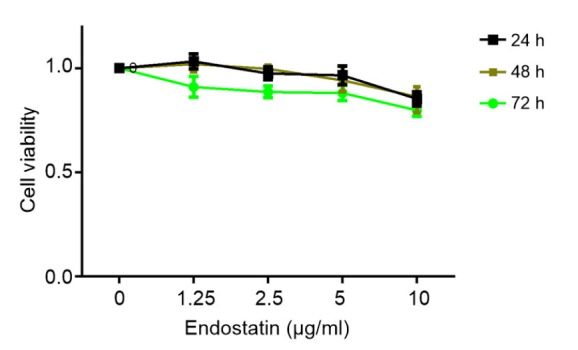
MTT cytotoxicity assay (the dose–response curves)
The toxicity of endostatin on fibroblasts was evaluated by MTT assay. No significant reduction in absorbance was observed at 0–5 μg/ml of endostatin after 24–72 h. We therefore chose 5 μg/ml as the working concentration and 48 h as the acting time
3.2. Effect of endostatin on fibrosis
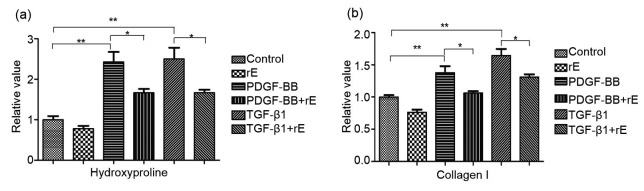
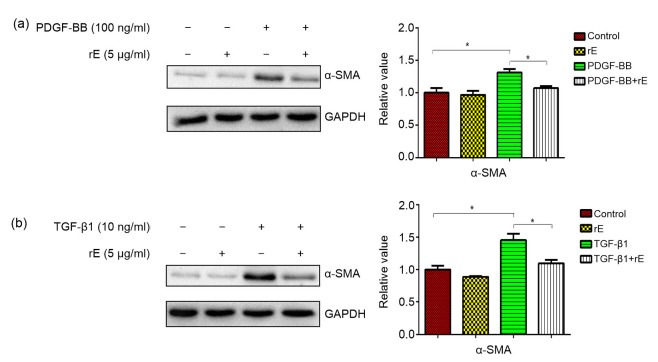
Hydroxyproline was significantly decreased in the endostatin-treated groups compared with both the PDGF-BB and TGF-β1 groups (Fig. 2a) and the results were similar for collagen I (Fig. 2b). Endostatin significantly inhibited the overexpression of α-SMA in both the PDGF and TGF-β1 groups (Fig. 3).
Fig. 2.
Expression of collagen I and hydroxyproline with ELISA
Fibroblasts were grouped as follows: “PDGF-BB”, “PDGF-BB+endostatin”, “TGF-β1”, “TGF-β1+endostatin”, “endostatin”, and “blank control” groups. The protein concentrations of collagen I and hydroxyproline proteins were determined using ELISA. (a) The hydroxyproline content in both PDGF-BB-and TGF-β1-induced groups was markedly higher than that in the control group (P<0.01), whereas the hydroxyproline content was significantly decreased in the endostatin-treated groups (P<0.01). (b) The results for the collagen I content were similar to the hydroxyproline results. For the statistics, data are expressed as mean±standard deviation (SD) (n=3). * P<0.05, ** P<0.01. rE: recombinant endostatin
Fig. 3.
Expression of SMA protein with Western blot analysis
Exposure of human fibroblasts to PDGF (a) or TGF-β1 (b) led to a significantly higher expression of the SMA protein. PDGF-BB-and TGF-β1-induced SMA expression was markedly inhibited by endostatin. Results were analyzed using ImageJ software (v.1.60) and the bands were quantified. For the statistics in this figure, data are expressed as mean±SD (n=3). * P<0.05. rE: recombinant endostatin
3.3. Inhibitive effect of endostatin on the expression of PDGFR, p-PDGFR, and p-ERK
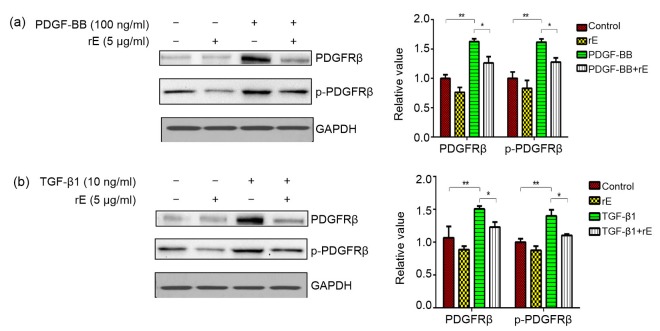
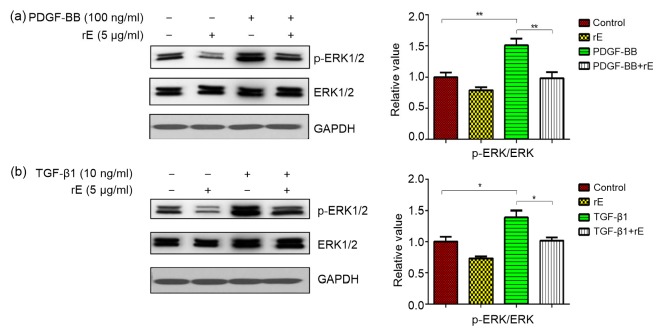
Endostatin inhibited both the PDGF-BB-and TGF-β1-induced expression of PDGFR and p-PDGFR (Fig. 4). The expression of PDGFRβ was investigated using immunofluorescent staining (Fig. 5) and the result is consistent with that of Western blot. The p-ERK/ERK ratio was significantly increased in both PDGF-BB and TGF-β1 groups and decreased in the endostatin-treated group (Fig. 6).
Fig. 4.
Expression of PDGFRβ and p-PDGFRβ proteins with Western blot analysis
The expression of PDGFRβ and p-PDGFRβ was upregulated by both PDGF-BB (a) and TGF-β1 (b), but was significantly inhibited by endostatin. Results were analyzed using ImageJ software (v.1.60) and the bands were quantified. For the statistics in this figure, data are expressed as mean±SD (n=3). * P<0.05, ** P<0.01. rE: recombinant endostatin
Fig. 5.
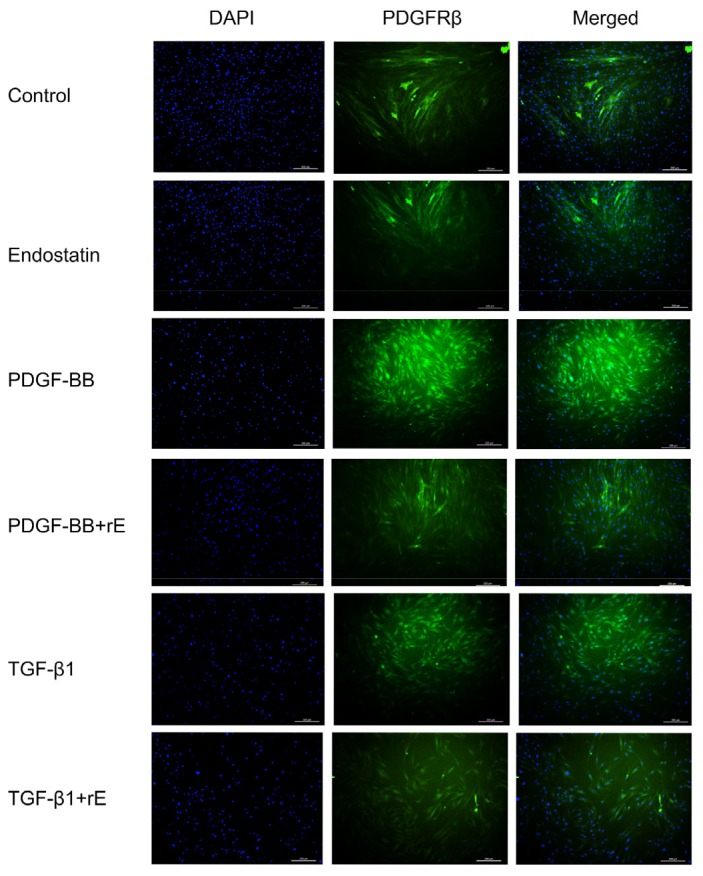
Expression of PDGFRβ with immunofluorescent staining
Immunofluorescence using antibodies against PDGFRβ (green). DAPI was used to stain the nucleus (blue) in cultured human skin fibroblasts. The quantitation of the fluorescent images of PDGFRβ-positive cells was performed with the ImageJ software (v.1.60). The PDGFRβ-positive ratio in the PDGF-BB-and TGF-β1-induced groups is significantly higher than that in the blank control and endostatin groups (P<0.01). Endostatin markedly inhibited the expression of PDGFRβ compared with the PDGF-BB or TGF-β1 groups (P<0.05). Scale bar: 200 μm (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
Fig. 6.
Expression of ERK and p-ERK proteins with Western blot analysis
The p-ERK/ERK ratio was significantly upregulated by PDGF-BB (a) and TGF-β1 (b), but significantly decreased after treatment with endostatin. Results were analyzed using ImageJ software (v.1.60) and the bands were quantified. For the statistics in this figure, data are expressed as mean±SD (n=3). * P<0.05, ** P<0.01. rE: recombinant endostatin
4. Discussion
This study demonstrated that endostatin inhibits the protein expression of PDGFR and p-ERK, resulting in a reduction of scarring in a fibroblast fibrosis model.
Fibroblasts are activated by injury and other growth factors in the pathogenesis of fibrosis. Fibroblasts can differentiate into myofibroblasts to produce collagen (Bochaton-Piallat et al., 2016). The productions of collagen and hydroxyproline are indicators for the degree of fibrosis (Tanaka et al., 2010). Endostatin alleviated both PDGF-BB-and TGF-β1-induced overexpression of hydroxyproline and collagen I in human fibroblasts. α-SMA is a marker of myofibroblasts and plays a key role in wound healing and fibrosis (Bochaton-Piallat et al., 2016). The results of the present study showed that the expression of α-SMA was increased by TGF-β1 and PDGF-BB and inhibited by endostatin. Endostatin was therefore thought to inhibit the transition from fibroblast to myofibroblast. To the best of our knowledge, this is the first study to investigate endostatin inhibition of human fibroblast fibrosis in vitro by modulating the PDGFR/ERK pathway.
The PDGFR signal pathway plays a critical role during epithelial-mesenchymal transition (EMT) in the progress of fibrosis and the overexpression of PDGF contributes to fibrotic disease (Tan et al., 1995). Scar-derived fibroblasts express more PDGFR than those from normal skin (Haisa et al., 1994). The PDGF/PDGFR signal pathway plays a pivotal role in angiogenesis and fibrosis (Tan et al., 1995; Bonner, 2004; Wynn, 2008; Borkham-Kamphorst and Weiskirchen, 2015) by activation of transmembrane receptor tyrosine kinases (Heldin et al., 2002; Mori et al., 2008). The PDGF signaling pathway has become an effective target for inhibiting fibrosis (Fang et al., 2013; Richeldi et al., 2016).
The results of the present study showed that the expression of PDGFR was significantly increased in both TGF-β1-and PDGF-induced fibrosis. The activation of PDGFRβ resulted in increased collagen production. Endostatin inhibited the expression of PDGFR. The activation of the PDGF-BB/PDGFRβ-axis plays a role in fibrosis pathogenesis. Binding to PDGF could induce the dimerization and autophosphorylation of PDGFR (Jechlinger et al., 2006), which could then transfer the signal by the downstream ERK cascade (Kaltalioglu and Coskuncevher, 2015).
ERK is in the downstream of the PDGFR signaling pathway and has a close relationship with cellular proliferation and migration (Reif et al., 2003). PDGF-BB activates ERK by phosphorylation in mitogenesis (Tangkijvanich et al., 2002). A high level of ERK phosphorylation has been demonstrated in scar-derived fibroblasts. In the present study, endostatin did not affect the expression of total ERK but inhibited the phosphorylation of ERK. These findings suggest that endostatin ameliorated dermal fibroblast fibrosis by suppressing the activation of ERK.
TGF-β1 and PDGF have close relationships with each other and both contribute to fibrotic disease (Tan et al., 1995). PDGF promotes the expression of TGF-β receptor I/II and leads to a chain reaction of fibrosis (Tiede et al., 2009). The expression of PDGF/PDGFR is significantly upregulated in TGF-β1-induced EMT (Erawan et al., 2008; Lehembre et al., 2008). The results of the present study suggest that the expression of PDGFR is significantly increased in both TGF-β1-and PDGF-induced fibrosis, and decreases after treatment with endostatin.
There are some limitations to this study: (1) our study only investigated the mechanism of endostatin on fibrosis in vitro but not included the in vivo samples; (2) the downstream signal pathway of TGF-β1 was not investigated.
5. Conclusions
This study demonstrated that endostatin remarkably alleviated TGF-β1-and PDGF-BB-induced fibrosis in human fibroblasts by inhibiting the PDGFR/ERK pathway. Endostatin could be a promising multi-target drug in future fibrosis therapy.
Footnotes
Project supported by the Zhejiang Provincial Natural Science Foundation of China (No. LY15H150004) and the Teaching Department of the Zhejiang Province (No. Y201330073), China
Compliance with ethics guidelines: Yuan LI and Hai-tao REN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Gene Dev. 2008;22(10):1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bochaton-Piallat ML, Gabbiani G, Hinz B. The myofibroblast in wound healing and fibrosis: answered and unanswered questions. F1000Research. 2016;5:752. doi: 10.12688/f1000research.8190.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth F R. 2004;15(4):255–273. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Borkham-Kamphorst E, Weiskirchen R. The PDGF system and its antagonists in liver fibrosis. Cytokine Growth Factor Rev. 2015;28:53–61. doi: 10.1016/j.cytogfr.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Cho JS, Fang TC, Reynolds TL, et al. PDGF-BB promotes type I IFN-dependent vascular alterations and monocyte recruitment in a model of dermal fibrosis. PLoS ONE. 2016;11(9):e0162758. doi: 10.1371/journal.pone.0162758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi HI, Ma SK, Bae EH, et al. Peroxiredoxin 5 protects TGF-β induced fibrosis by inhibiting Stat3 activation in rat kidney interstitial fibroblast cells. PLoS ONE. 2016;11(2):e0149266. doi: 10.1371/journal.pone.0149266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erawan BK, Evgenia K, Roeyen CRC, et al. Platelet-derived growth factor isoform expression in carbon tetrachloride-induced chronic liver injury. Lab Invest. 2008;88(10):1090–1100. doi: 10.1038/labinvest.2008.71. [DOI] [PubMed] [Google Scholar]
- 8.Fang L, Zhan S, Huang C, et al. TRPM7 channel regulates PDGF-BB-induced proliferation of hepatic stellate cells via PI3K and ERK pathways. Toxicol Appl Pharmacol. 2013;272(3):713–725. doi: 10.1016/j.taap.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Friedstat JS, Hultman CS. Hypertrophic burn scar management: what does the evidence show? A systematic review of randomized controlled trials. Ann Plastic Surg. 2014;72(6):S198–S201. doi: 10.1097/SAP.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 10.Haisa M, Okochi H, Grotendorst GR. Elevated levels of PDGF α receptors in keloid fibroblasts contribute to an enhanced response to PDGF. J Invest Dermatol. 1994;103(4):560–563. doi: 10.1111/1523-1747.ep12396856. [DOI] [PubMed] [Google Scholar]
- 11.Heldin CH, Eriksson U, Östman A. New members of the platelet-derived growth factor family of mitogens. Arch Biochem Biophys. 2002;398(2):284–290. doi: 10.1006/abbi.2001.2707. [DOI] [PubMed] [Google Scholar]
- 12.Hu JG, Wu XJ, Feng YF, et al. PDGF-AA and bFGF mediate B104CM-induced proliferation of oligodendrocyte precursor cells. Int J Mol Med. 2012;30(5):1113–1118. doi: 10.3892/ijmm.2012.1110. [DOI] [PubMed] [Google Scholar]
- 13.Jechlinger M, Sommer A, Moriggl R, et al. Autocrine PDGFR signaling promotes mammary cancer metastasis. J Clin Invest. 2006;116(6):1561–1570. doi: 10.1172/JCI24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaltalioglu K, Coskuncevher S. A bioactive molecule in a complex wound healing process: platelet-derived growth factor. Int J Dermatol. 2015;54(8):972–977. doi: 10.1111/ijd.12731. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Isayeva T, Larson MR, et al. Endostatin: a novel inhibitor of androgen receptor function in prostate cancer. Proc Natl Acad Sci USA. 2015;112(5):1392–1397. doi: 10.1073/pnas.1417660112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehembre F, Yilmaz M, Wicki A, et al. NCAM-induced focal adhesion assembly: a functional switch upon loss of E-cadherin. EMBO J. 2008;27(19):2603–2615. doi: 10.1038/emboj.2008.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin X, Kong LN, Huang C, et al. Hesperetin derivative-7 inhibits PDGF-BB-induced hepatic stellate cell activation and proliferation by targeting Wnt/β-catenin pathway. Int Immunopharmacol. 2015;25(2):311–320. doi: 10.1016/j.intimp.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Mårtensson J, Jonsson N, Glassford NJ, et al. Plasma endostatin may improve acute kidney injury risk prediction in critically ill patients. Ann Intens Care. 2016;6(1):1–9. doi: 10.1186/s13613-016-0108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 20.Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med. 2008;205(1):43–51. doi: 10.1084/jem.20071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okada M, Suzuki A, Yamawaki H, et al. Levosimendan inhibits interleukin-1β-induced cell migration and MMP-9 secretion in rat cardiac fibroblasts. Eur J Pharmacol. 2013;718(1-3):332–339. doi: 10.1016/j.ejphar.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Peng Y, Gao M, Jiang Y, et al. Angiogenesis inhibitor endostatin protects mice with sepsis from multiple organ dysfunction syndrome. Shock. 2015;44(4):357–364. doi: 10.1097/SHK.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 23.Reif S, Lang A, Lindquist JN, et al. The role of focal adhesion kinase-phosphatidylinositol 3-kinase-Akt signaling in hepatic stellate cell proliferation and type I collagen expression. J Biol Chem. 2003;278(10):8083–8090. doi: 10.1074/jbc.M212927200. [DOI] [PubMed] [Google Scholar]
- 24.Ren HT, Hu H, Li Y, et al. Endostatin inhibits hypertrophic scarring in a rabbit ear model. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2013;14(3):224–230. doi: 10.1631/jzus.B1200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richeldi L, Cottin V, du Bois RM, et al. Nintedanib in patients with idiopathic pulmonary fibrosis: combined evidence from the TOMORROW and INPULSIS® trials. Resp Med. 2016;113:74–79. doi: 10.1016/j.rmed.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Tan E, Qin H, Kennedy S, et al. Platelet-derived growth factors-AA and -BB regulate collagen and collagenase gene expression differentially in human fibroblasts. Biochem J. 1995;310(2):585–588. doi: 10.1042/bj3100585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka KI, Ishihara T, Azuma A, et al. Therapeutic effect of lecithinized superoxide dismutase on bleomycin-induced pulmonary fibrosis. Am J Physiol-Lung C. 2010;298(3):L348–L360. doi: 10.1152/ajplung.00289.2009. [DOI] [PubMed] [Google Scholar]
- 28.Tangkijvanich P, Santiskulvong C, Melton AC, et al. p38 MAP kinase mediates platelet-derived growth factor-stimulated migration of hepatic myofibroblasts. J Cell Physiol. 2002;191(3):351–361. doi: 10.1002/jcp.10112. [DOI] [PubMed] [Google Scholar]
- 29.Tiede S, Ernst N, Bayat A, et al. Basic fibroblast growth factor: a potential new therapeutic tool for the treatment of hypertrophic and keloid scars. Ann Anat. 2009;191(1):33–44. doi: 10.1016/j.aanat.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Wynn T. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaguchi Y, Takihara T, Chambers RA, et al. A peptide derived from endostatin ameliorates organ fibrosis. Sci Transl Med. 2012;4(136):136ra71. doi: 10.1126/scitranslmed.3003421. [DOI] [PMC free article] [PubMed] [Google Scholar]