Abstract
Gallstone disease (GSD) is related to several diabetes risk factors. The present study was to examine whether GSD was independently associated with type 2 diabetes in the China Kadoorie Biobank study. After excluding participants with prevalent diabetes and prior histories of cancer, heart disease, and stroke at baseline, 189,154 men and 272,059 women aged 30–79 years were eligible for analysis. The baseline prevalence of GSD was 5.7% of the included participants. During 4,138,687 person-years of follow-up (median, 9.1 years), a total of 4,735 men and 7,747 women were documented with incident type 2 diabetes. Compared with participants without GSD at baseline, the multivariate-adjusted hazard ratios (HRs) for type 2 diabetes for those with GSD were 1.09 (95% CI: 0.96–1.24; P = 0.206), 1.21 (95% CI: 1.13-1.30; P < 0.001), and 1.17 (95% CI: 1.10-1.25; P < 0.001) in men, women, and the whole cohort respectively. There was no statistically significant heterogeneity between men and women (P = 0.347 for interaction). The association between GSD and type 2 diabetes was strongest among participants who reported ≥5 years since the first diagnosis and were still on treatment at baseline (HR = 1.48; 95% CI: 1.16-1.88; P = 0.001). The present study highlights the importance of developing a novel prevention strategy to mitigate type 2 diabetes through improvement of gastrointestinal health.
Introduction
Type 2 diabetes has become epidemic worldwide1. In China, a rapid increase in diabetes incidence was observed in recent decades, with a prevalence of 11.6% in 20102. Gallstone disease (GSD) remains a common gastrointestinal disorder in both developed countries3 and Asian populations such as Chinese4,5. GSD is related to several cardiometabolic risk factors such as obesity, dyslipidemias (hypertriglyceridemia and low high-density lipoprotein cholesterol), unhealthy diet, and sedentary lifestyle3,6. Several prospective studies have supported that the presence of GSD was associated with increased risk of coronary heart disease, which could not be explained by traditional risk factors7. Whether there is a similar association between GSD and type 2 diabetes remains unclear. Previous studies which have related GSD to diabetes were limited by cross-sectional design8–10. To our knowledge, only one prospective study on the relation between GSD and increased risk of type 2 diabetes has been reported11.
We have previously shown an association between GSD and ischemic heart disease in a large prospective cohort of 0.5 million adults − the China Kadoorie Biobank (CKB) study12. We aimed to examine the association between GSD and the risk of incident type 2 diabetes in the same population. We also assessed potential interactions between GSD and conventional risk factors for type 2 diabetes.
Results
Baseline characteristics of study participants
At baseline, 5.7% of the 461,213 participants reported the presence of GSD (men, 3.6%; women, 7.1%). Compared with participants without GSD, those with GSD were older, more likely to be urban residents, had higher body mass index (BMI) and waist circumference (WC) values, had a higher prevalence of chronic hepatitis/cirrhosis and peptic ulcer, and had a higher weight increase since 25 years of age (Table 1). Women with GSD had an earlier age at the first diagnosis and longer duration than men with GSD (P < 0.001).
Table 1.
Baseline characteristics of the 461,213 participants according to the presence of gallstone disease.
| Baseline characteristics | Men | Women | ||||
|---|---|---|---|---|---|---|
| With GSD | Without GSD | P Value | With GSD | Without GSD | P Value | |
| No. of participants | 6,854 | 182,300 | — | 19,353 | 252,706 | — |
| Age (year) | 53.5 | 51.5 | <0.001 | 52.9 | 49.9 | <0.001 |
| Urban area (%) | 54.5 | 41.0 | <0.001 | 44.8 | 42.6 | <0.001 |
| Currently married (%) | 94.5 | 92.9 | <0.001 | 90.2 | 89.7 | 0.033 |
| Middle school and above (%) | 62.2 | 57.4 | <0.001 | 47.0 | 43.5 | <0.001 |
| Current daily smoker (%) | 65.5 | 68.2 | <0.001 | 2.8 | 2.6 | 0.210 |
| Current daily drinker (%) | 15.7 | 21.3 | <0.001 | 0.7 | 1.0 | <0.001 |
| Physical activity (MET-hour/day) | 21.4 | 23.0 | <0.001 | 20.2 | 21.2 | <0.001 |
| Average weekly consumptiona | ||||||
| Red meat (day) | 4.0 | 4.0 | 0.228 | 3.5 | 3.5 | 0.056 |
| Fresh vegetables (day) | 6.9 | 6.8 | 0.105 | 6.84 | 6.83 | 0.032 |
| Fresh fruits (day) | 2.5 | 2.2 | <0.001 | 3.0 | 2.8 | <0.001 |
| BMI (kg/m2) | 23.8 | 23.3 | <0.001 | 24.1 | 23.6 | <0.001 |
| WC (cm) | 83.2 | 81.5 | <0.001 | 79.7 | 78.4 | <0.001 |
| Prevalence of | ||||||
| Hypertension (%) | 33.0 | 34.9 | <0.001 | 29.5 | 30.7 | <0.001 |
| Chronic hepatitis/cirrhosis (%) | 4.3 | 1.6 | <0.001 | 1.4 | 0.8 | <0.001 |
| Peptic ulcer (%) | 8.6 | 5.2 | <0.001 | 5.4 | 2.7 | <0.001 |
| Weight change since 25 years (kg)b | 5.7 | 4.5 | <0.001 | 5.7 | 4.7 | <0.001 |
| Weight change during the past 12 months (%)c | ||||||
| Same as before | 76.5 | 79.7 | — | 73.8 | 77.8 | — |
| Gain of ≥2.5 kg | 10.2 | 10.2 | 0.342 | 13.8 | 12.3 | <0.001 |
| Loss of ≥2.5 kg | 13.3 | 10.1 | <0.001 | 12.4 | 9.9 | <0.001 |
| Postmenopausal (%) | — | — | — | 50.1 | 49.1 | <0.001 |
| Family history of diabetes (%) | 7.9 | 5.8 | <0.001 | 8.0 | 6.4 | <0.001 |
| Characteristics of GSDd | ||||||
| Age at the first diagnosis (year) | 44.9 | — | — | 43.9 | — | <0.001e |
| Duration since the first diagnosis (year) | 8.1 | — | — | 9.2 | — | <0.001e |
| Still on treatment at baseline | 13.0 | — | — | 14.6 | — | 0.001e |
GSD indicates gallstone disease; MET, metabolic equivalent task; BMI, body mass index; and WC, waist circumference.
The results are presented as adjusted means or percentages. All variables are adjusted for age and survey sites, as appropriate.
aThe average weekly consumptions of red meat, fresh vegetables, and fruits were calculated by assigning participants the midpoint of their consumption category.
b74,458 participants with a missing value for self-reported weight at 25 years of age were excluded from this analysis.
cMultinomial logistic regression was used for testing, and the “same as before” was used as the reference category.
dThere were statistically significant differences in all three characteristics (P ≤ 0.001) between men and women.
eP value for comparison between men and women.
Association between GSD and incident type 2 diabetes
During a median follow-up of 9.1 years (interquartile range: 1.92 years; total person-years: 4,138,687), there were 4,735 incident cases of type 2 diabetes in men and 7,747 in women. In age- and sex-adjusted (model 1) and multivariable-adjusted analyses in the whole cohort (model 2), the presence of GSD was associated with increased risk of incident type 2 diabetes (Table 2). The association was moderately attenuated after further adjustment for BMI and WC (model 3). Compared with participants without GSD at baseline, the adjusted hazard ratio (HR) for type 2 diabetes (model 3) was 1.17 (95% confidence interval [CI]: 1.10−1.25; P < 0.001) for those with GSD in the whole cohort. There was no statistically significant heterogeneity between men (HR = 1.09; 95% CI: 0.96−1.24; P = 0.206) and women (HR = 1.21; 95% CI: 1.13−1.30; P < 0.001) in the aforementioned association (P = 0.347 for interaction with sex). These associations were not materially changed with additional adjustment for weight change since 25 years of age; or additional adjustment for significant weight change during the past 12 months; or additional adjustment for histories of chronic hepatitis/cirrhosis and peptic ulcer; or excluding participants with type 2 diabetes occurring during the first two years of follow-up (data not shown).
Table 2.
HRs (95% CIs) for the association between gallstone disease and incident type 2 diabetes.
| Person-years | Cases | Age- and sex-adjusted (model 1) | Multivariable-adjusteda (model 2) | Further adjustment for BMI and WC (model 3) | |
|---|---|---|---|---|---|
| Total | |||||
| Without GSD | 3,905,602 | 11,352 | 1.00 | 1.00 | 1.00 |
| With GSD | 233,084 | 1,130 | 1.33 (1.25−1.42) | 1.32 (1.24−1.40) | 1.17 (1.10−1.25) |
| Men | |||||
| Without GSD | 1,615,585 | 4,484 | 1.00 | 1.00 | 1.00 |
| With GSD | 59,953 | 251 | 1.29 (1.13−1.46) | 1.22 (1.08−1.39) | 1.09 (0.96−1.24) |
| Women | |||||
| Without GSD | 2,290,017 | 6,868 | 1.00 | 1.00 | 1.00 |
| With GSD | 173,131 | 879 | 1.33 (1.24−1.43) | 1.33 (1.24−1.43) | 1.21 (1.13−1.30) |
HR indicates hazard ratio; CI, confidence interval; GSD, gallstone disease; BMI, body mass index, and WC, waist circumference.
aAdjusted for age (years), sex (for the whole cohort), level of education (no formal school, primary school, middle school, high school, college, or university or above), marital status (married, widowed, divorced/separated, or never married), alcohol consumption (never, former, current weekly, current daily <15, 15–29, 30–59, or ≥60 g per day), smoking status (never, former, current daily <15, 15–24, or ≥25 cigarettes or equivalents per day; former smokers who stopped smoking for illness were included in the current smoker category to avoid misleadingly elevated risk), level of physical activity (MET-hours/day), intake frequencies of red meat, fresh fruits, and vegetables (daily, 4–6 days/week, 1–3 days/week, monthly, or rarely or never), prevalent hypertension (yes or no), family history of diabetes (yes or no), and menopausal status (premenopausal, perimenopausal, or postmenopausal; for women only).
Stratified analysis
We performed stratified analysis according to the combined categories of duration of GSD from the first diagnosis to the baseline and treatment status at baseline. The association of GSD with type 2 diabetes appeared to be strongest among those who reported more than five years of duration and were still on treatment at baseline, with HR of 1.48 (95% CI: 1.16−1.88; P = 0.001) in the whole cohort (Table 3).
Table 3.
HRs (95% CIs) for the association between gallstone disease and incident type 2 diabetes according to the combined categories of duration since the first diagnosis and treatment status at baseline.
| Person-years | Cases | HRs (95% CIs) | |
|---|---|---|---|
| Total | |||
| ≤5 years, no treatment | 95,516 | 504 | 1.25 (1.14−1.37) |
| ≤5 years, still on treatment | 20,326 | 82 | 1.21 (0.97−1.51) |
| >5 years, no treatment | 103,934 | 475 | 1.06 (0.96−1.16) |
| >5 years, still on treatment | 13,057 | 68 | 1.48 (1.16−1.88) |
| Men | |||
| ≤5 years, no treatment | 27,077 | 121 | 1.13 (0.94−1.35) |
| ≤5 years, still on treatment | 4,966 | 19 | 1.13 (0.72−1.78) |
| >5 years, no treatment | 25,307 | 98 | 0.99 (0.81−1.21) |
| >5 years, still on treatment | 2,584 | 13 | 1.40 (0.81−2.42) |
| Women | |||
| ≤5 years, no treatment | 68,439 | 383 | 1.31 (1.18−1.45) |
| ≤5 years, still on treatment | 15,360 | 63 | 1.25 (0.97−1.60) |
| >5 years, no treatment | 78,627 | 377 | 1.08 (0.97−1.20) |
| >5 years, still on treatment | 10,473 | 55 | 1.50 (1.15−1.96) |
HR indicates hazard ratio; and CI, confidence interval.
The reference group was participants without GSD at baseline. Multivariable model was adjusted for age (years), sex (for the whole cohort), level of education (no formal school, primary school, middle school, high school, college, or university or above), marital status (married, widowed, divorced/separated, or never married), alcohol consumption (never, former, current weekly, current daily <15, 15–29, 30–59, or ≥60 g per day), smoking status (never, former, current daily <15, 15–24, or ≥25 cigarettes or equivalents per day; former smokers who stopped smoking for illness were included in the current smoker category to avoid misleadingly elevated risk), level of physical activity (MET-hours/day), intake frequencies of red meat, fresh fruits, and vegetables (daily, 4–6 days/week, 1–3 days/week, monthly, or rarely or never), prevalent hypertension (yes or no), family history of diabetes (yes or no), menopausal status (premenopausal, perimenopausal, or postmenopausal; for women only), body-mass index (kg/m2), and waist circumference (cm).
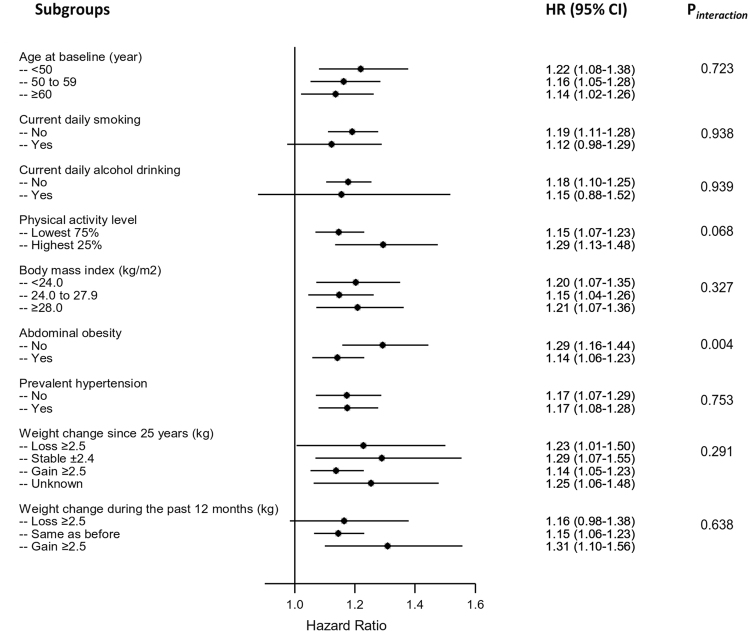
We also analyzed the association between GSD and type 2 diabetes according to other potential baseline risk factors. The positive associations were similar across subgroups stratified according to age, smoking status, alcohol consumption, level of physical activity, BMI, prevalent hypertension, weight change since 25 years of age, and weight change during the past 12 months in the whole cohort (Fig. 1) and in both men and women (data not shown) (all P values for interaction >0.05). Notably, statistically significant difference was observed across strata by the presence of abdominal obesity defined by WC in the whole cohort (P = 0.004 for interaction) and women (P = 0.018 for interaction), but not in men (P = 0.455 for interaction). The positive association between GSD and type 2 diabetes was stronger in non-abdominal obese (HR, 95% CI: 1.29, 1.16−1.44 for the whole cohort; 1.35, 1.19−1.54 for women) than abdominal obese participants (HR, 95% CI: 1.14, 1.06−1.23 for the whole cohort; 1.16, 1.06−1.27 for women). The corresponding HRs (95% CIs) for men was 1.13 (0.90−1.41) in non-abdominal obese participants and 1.09 (0.93−1.27) in abdominal obese participants.
Figure 1.
Subgroup analyses of the association between gallstone disease and type 2 diabetes according to potential baseline risk factors. Adjustments were made for age, sex, education, marital status, alcohol consumption, smoking status, physical activity, intakes of red meat, fresh fruits, and vegetables, prevalent hypertension, family history of diabetes, body-mass index, and waist circumference.
Discussion
In this large prospective study with close to ten years of follow-up, we observed that the presence of GSD was prospectively associated with increased risk of type 2 diabetes after adjustment for potential confounding from traditional risk factors of type 2 diabetes. Such association was strongest among participants who had a long history of GSD and were still on treatment at baseline. The association between GSD and type 2 diabetes was consistent in men and women, but the stronger association was observed in non-abdominal obese than in abdominal obese women.
To our knowledge, only one study has prospectively examined the association of GSD with type 2 diabetes in a European population (EPIC-Potsdam study) with a mean follow-up of 7.0 years11. It was consistent with our findings that persons with GSD had an increased risk of type 2 diabetes (HR = 1.42; 95% CI: 1.21–1.68) after adjustment for sex, age, WC, and lifestyle risk factors.
Several potential mechanisms may help explain the association between GSD and type 2 diabetes. Higher prevalence of GSD has been reported in persons with obesity13,14, hyperinsulinemia8,15, insulin resistance16, and metabolic syndrome17. The coexistence of these risk factors for type 2 diabetes might be the reason that participants with GSD had an increased diabetes risk. In the current study, the adjustment for BMI and WC moderately attenuated the association between GSD and type 2 diabetes, suggesting that obesity might only partly explain the higher risk of type 2 diabetes in patients with GSD. In addition, statistically significant associations remained after adjustment for risk factors such as hypertension and lifestyle factors, suggesting other mechanisms might also be involved. A similar change in the risk estimates with adjustment for potential confounders was also observed in the EPIC-Potsdam study11. In addition, we explored whether the association between GSD and type 2 diabetes was confounded by long- and short-term weight change, which has been related to both type 2 diabetes18,19 and gallstone formation3. Additional adjustment for weight change since 25 years of age or significant weight change in the past 12 months did not affect the association appreciably.
Recent studies in both human20 and experimental animals21 have linked gut microbiota dysbiosis with the formation of cholesterol gallstones. This relation is probably through distorted secretion of bile acids because the bile acids play a key role in regulating abundance or metabolism of gut microbiota22. Accumulating evidence implicates the involvement of gut microbiota in the development of type 2 diabetes and cardiovascular diseases23,24. The associations of GSD with type 2 diabetes and ischemic heart disease12 we observed in the same population suggest that the increased risks associated with GSD might be at least partly through affecting gut microbiota metabolism. Also, both of the associations were independent of each other because we excluded participants with a history of heart disease (or diabetes) at baseline for the analysis of diabetes (or ischemic heart disease12). Our findings would motivate further investigation of this hypothesis.
In the present study, the association between GSD and type 2 diabetes was dependent on the abdominal obese status in women. The stronger association was observed in non-abdominal obese than abdominal obese women. Weikert et al. reported a similar interaction between GSD and abdominal obesity on type 2 diabetes11. It is possible that obese women already had a high risk of diabetes, and GSD added only modestly deleterious effect on the relative scale. However, the absolute risk associated with abdominal obesity among women with GSD was much greater than those without abdominal obesity.
To our knowledge, this is one of the largest prospective studies that examined the association between GSD and type 2 diabetes. We carefully adjusted for potential confounders. We excluded participants with type 2 diabetes at baseline and those with type 2 diabetes occurring during the first two years of follow-up to minimize the potential bias arising from reverse causality.
However, several limitations warrant mention. First, the presence of GSD was self-reported. The possibility of including participants with asymptomatic GSD in the non-GSD group could lead to misclassification, which was more likely to be non-differential in a prospective study design. Second, the present study lacked information on the subtypes of gallstones. However, most gallstones in the Chinese population have been of the cholesterol type since the early 1990s25. We did not ask the severity of GSD, such as asymptomatic, symptomatic, or having undergone cholecystectomy, limiting our in-depth analysis. Third, residual confounding by other factors such as more detailed dietary factor, dyslipidemia, and hyperinsulinemia remains possible. However, Weikert et al. reported that the association between GSD and the risk of type 2 diabetes was not substantially attenuated after further adjustment for selected biomarkers including glucose, total cholesterol, and triglycerides11. Fourth, baseline identification of prevalent diabetes relied on the self-reported previous clinical diagnosis or on-site glucose testing, and identification of incident diabetes during follow-up relied mainly on the local disease and death registries and health insurance system. Missing some asymptomatic diabetes cases was inevitable. However, such misclassification was more likely to be nondifferential and might lead to attenuation of effect estimates.
Our findings showed an increased risk of incident type 2 diabetes associated with the presence of GSD. It highlights the importance of developing a novel prevention strategy to mitigate type 2 diabetes through improvement of gastrointestinal health (e.g., timely treatment of gastrointestinal diseases and maintaining healthy gut microbiota). More studies are warranted to confirm the association and to elucidate the potential biological mechanisms.
Methods
Study population
Details of the CKB study is available elsewhere26,27. Briefly, we enrolled 512,891 adults aged 30−79 years from 10 geographically diverse localities across China during 2004–08. All participants eligible for subsequent analysis had completed questionnaire, physical measurements, and a written informed consent form. The CKB study was approved by the Ethical Review Committee of the Chinese Center for Disease Control and Prevention (Beijing, China) and the Oxford Tropical Research Ethics Committee, University of Oxford (UK). All methods were performed in accordance with relevant guidelines and regulations.
For our analyses, we excluded 30,300 participants who had self-reported diabetes or screen-detected diabetes at baseline. Diabetes detected by on-site screening was defined as a fasting blood glucose ≥7.0 mmol/L or a random blood glucose ≥11.1 mmol/L28. We also excluded those who reported prior medical histories of cancer (n = 2,577), heart disease (n = 15,472), and stroke (n = 8,884), and those who had incomplete data of BMI (n = 2). We finally included 189,154 men and 272,059 women in the analysis.
Assessment of exposure
At baseline survey, trained staff administered a standardized questionnaire using a laptop-based data-entry system, with built-in functions to prevent logical errors and missing items. Participants reported whether they had ever been diagnosed with GSD (yes or no), with or without cholecystitis complication, by a doctor, the age of their first diagnosis, and whether they were still on treatment (yes or no).
Assessment of covariates
Trained staff collected socio-demographic characteristics (age, sex, education, and marital status), lifestyle behaviors (tobacco smoking, alcohol consumption, physical activity, and intakes of red meat, fresh fruits, and vegetables), personal health and medical history (hypertension, chronic hepatitis/cirrhosis, peptic ulcer, body weight at 25 years of age, and significant weight change during the past 12 months), women’s menopausal status, and family medical history. Questions about alcohol consumption used in the present analyses included drinking frequency, alcoholic beverage type, and volume of alcohol drunk on a typical drinking day in the past year. Questions about smoking included frequency, type, and amount of tobacco smoked per day for ever smokers, and the reason for quitting for former smokers. Questions about physical activity included type and duration of activities in occupational, commuting, domestic, and leisure-time related domains in the past 12 months. Habitual dietary intake in the past year was assessed by a qualitative food frequency questionnaire.
At baseline, body weight, height, WC, and blood pressure were measured by trained staff using calibrated instruments. Weight change since 25 years of age was calculated by subtracting recalled weight at 25 years from measured weight at baseline. BMI was calculated as weight in kilograms divided by height in meters squared.
Ascertainment of incident type 2 diabetes
Incident cases of type 2 diabetes were identified by linking to local disease and death registries, to the national health insurance system, and by active follow-up27. The 10th revision of the International Classification of Diseases (ICD-10) was used to code all incident cases of type 2 diabetes by trained staff members who were “blinded” to baseline information. In the present study, we included diabetes cases coded as E11 and E14. Other cases clearly defined as non-type 2 diabetes were excluded. Because most participants in the present study were aged over 40 years among whom the number of any non-type 2 diabetes was small, misclassification of other types of diabetes was minimal.
The validity of outcome ascertainment was verified in a subsample of 831 CKB participants who were identified with the incidence of nonfatal type 2 diabetes during 2004–08 and whose medical records were retrieved. All medical records were reviewed, and diagnoses were adjudicated by clinical research fellows in the Oxford International Coordinating Centre of the CKB in 2012. Of 831 cases, the diagnosis of type 2 diabetes was confirmed in 819 (98.6%).
Statistical analyses
We compared baseline characteristics between participants with and without GSD using analysis of covariance for continuous variables, and binary or multinomial logistic regression for categorical variables, with adjustment for age and survey sites. We calculated person-years at risk from the baseline recruitment date to the diagnosis of type 2 diabetes, death, loss to follow-up, or December 31, 2015, whichever came first. We used Cox proportional hazards model to estimate the HRs and the 95% CIs, with age as the underlying time scale, and stratified jointly by survey site and age at baseline in 5-year interval.
The multivariable model 1 was adjusted for age and sex. Model 2 additionally adjusted for education, marital status, smoking status, alcohol consumption, physical activity, intake frequencies of red meat, fresh fruits, and vegetables, prevalent hypertension at baseline, family history of diabetes, and menopausal status. Model 3 further included BMI and WC in the model 2. We performed four sensitivity analyses on the basis of model 3: (1) additionally adjusting for weight change since 25 years of age (loss of ≥5.0 kg, loss of 2.5−4.9 kg, stable ± 2.4 kg, gain of 2.5−4.9 kg, gain of 5.0−9.9 kg, gain of 10.0−14.9 kg, gain of ≥15.0 kg, or unknown due to missing information about weight at 25 years of age); (2) additionally adjusting for weight change during the past 12 months (same as before, gain of ≥2.5 kg, or loss of ≥2.5 kg); (3) additionally adjusting for the histories of digestive system diseases including chronic hepatitis/cirrhosis and peptic ulcer; and (4) excluding participants with type 2 diabetes occurring during the first two years of follow-up.
Subgroup analysis was conducted among combined categories of duration since their first diagnosis of GSD and treatment status at baseline, all compared with those without GSD at baseline. We also examined the association between GSD and type 2 diabetes across several baseline subgroups: age, smoking status, alcohol consumption, level of physical activity, BMI, abdominal obesity (defined as WC ≥85 cm in men and ≥80 cm in women), prevalent hypertension, weight change since 25 years of age, and weight change during the past 12 months. Interactions were tested using likelihood-ratio tests comparing models with and without cross-product terms between the baseline stratifying variable and GSD.
We performed all statistical analyses with Stata (version 14.2, StataCorp, College Station, TX, USA). All P values were two-sided, and statistical significance was defined as P < 0.05.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
The most important acknowledgment is to the participants in the study and the members of the survey teams in each of the 10 regional centres, as well as to the project development and management teams based at Beijing, Oxford and the 10 regional centres. The members of the China Kadoorie Biobank collaborative group include: International Steering Committee: Junshi Chen, Zhengming Chen (PI), Rory Collins, Liming Li (PI), Richard Peto. International Co-ordinating Centre, Oxford: Daniel Avery, Ruth Boxall, Derrick Bennett, Yumei Chang, Yiping Chen, Zhengming Chen, Robert Clarke, Huaidong Du, Simon Gilbert, Alex Hacker, Michael Holmes, Andri Iona, Christiana Kartsonaki; Rene Kerosi, Ling Kong, Om Kurmi, Garry Lancaster, Sarah Lewington, Kuang Lin, John McDonnell, Winnie Mei, Iona Millwood, Qunhua Nie, Jayakrishnan Radhakrishnan, Sajjad Rafiq, Paul Ryder, Sam Sansome, Dan Schmidt, Paul Sherliker, Rajani Sohoni, Iain Turnbull, Robin Walters, Jenny Wang, Lin Wang, Ling Yang, Xiaoming Yang. National Co-ordinating Centre, Beijing: Zheng Bian, Ge Chen, Yu Guo, Bingyang Han, Can Hou, Jun Lv, Pei Pei, Shuzhen Qu, Yunlong Tan, Canqing Yu, Huiyan Zhou. 10 Regional Co-ordinating Centres: Qingdao CDC: Zengchang Pang, Ruqin Gao, Shaojie Wang, Yongmei Liu, Ranran Du, Yajing Zang, Liang Cheng, Xiaocao Tian, Hua Zhang. Licang CDC: Silu Lv, Junzheng Wang, Wei Hou. Heilongjiang Provincial CDC: Jiyuan Yin, Ge Jiang, Xue Zhou. Nangang CDC: Liqiu Yang, Hui He, Bo Yu, Yanjie Li, Huaiyi Mu, Qinai Xu, Meiling Dou, Jiaojiao Ren. Hainan Provincial CDC: Shanqing Wang, Ximin Hu, Hongmei Wang, Jinyan Chen, Yan Fu, Zhenwang Fu, Xiaohuan Wang. Meilan CDC: Min Weng, Xiangyang Zheng, Yilei Li, Huimei Li,Yanjun Wang. Jiangsu Provincial CDC: Ming Wu, Jinyi Zhou, Ran Tao, Jie Yang. Suzhou CDC: Chuanming Ni, Jun Zhang, Yihe Hu, Yan Lu, Liangcai Ma, Aiyu Tang, Shuo Zhang, Jianrong Jin, Jingchao Liu. Guangxi Provincial CDC: Zhenzhu Tang, Naying Chen, Ying Huang. Liuzhou CDC: Mingqiang Li, Jinhuai Meng, Rong Pan, Qilian Jiang, Weiyuan Zhang, Yun Liu, Liuping Wei, Liyuan Zhou, Ningyu Chen, Hairong Guan. Sichuan Provincial CDC: Xianping Wu, Ningmei Zhang, Xiaofang Chen, Xuefeng Tang. Pengzhou CDC: Guojin Luo, Jianguo Li, Xiaofang Chen, Xunfu Zhong, Jiaqiu Liu, Qiang Sun. Gansu Provincial CDC: Pengfei Ge, Xiaolan Ren, Caixia Dong. Maiji CDC: Hui Zhang, Enke Mao, Xiaoping Wang, Tao Wang, Xi zhang. Henan Provincial CDC: Ding Zhang, Gang Zhou, Shixian Feng, Liang Chang, Lei Fan. Huixian CDC: Yulian Gao, Tianyou He, Huarong Sun, Pan He, Chen Hu, Qiannan Lv, Xukui Zhang. Zhejiang Provincial CDC: Min Yu, Ruying Hu, Hao Wang. Tongxiang CDC: Yijian Qian, Chunmei Wang, Kaixue Xie, Lingli Chen, Yidan Zhang, Dongxia Pan. Hunan Provincial CDC: Yuelong Huang, Biyun Chen, Li Yin, Donghui Jin, Huilin Liu, Zhongxi Fu, Qiaohua Xu. Liuyang CDC: Xin Xu, Hao Zhang, Youping Xiong, Huajun Long, Xianzhi Li, Libo Zhang, Zhe Qiu. This work was supported by grants (81390540, 81390544, 81390541) from the National Natural Science Foundation of China and grants (2016YFC0900500, 2016YFC0900501, 2016YFC0900504) from the National Key Research and Development Program of China. The CKB baseline survey and the first re-survey were supported by a grant from the Kadoorie Charitable Foundation in Hong Kong. The long-term follow-up is supported by grants from the UK Wellcome Trust (088158/Z/09/Z, 104085/Z/14/Z); by a grant from the Chinese Ministry of Science and Technology (2011BAI09B01). Dr Qi is supported by NIH grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024, HL132254), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, DK078616), the Boston Obesity Nutrition Research Center (DK46200), and United States – Israel Binational Science Foundation Grant 2011036. Dr Qi was a recipient of the American Heart Association Scientist Development Award (0730094 N). The funders had no role in the study design, data collection, data analysis and interpretation, writing of the report, or the decision to submit the article for publication.
Author Contributions
L.Q., J.L. and L.L. conceived and designed the paper. L.L., Z.C., and J.C., as the members of CKB steering committee, designed and supervised the conduct of the whole study, obtained funding, and together with Y.G., Z.B., L.Y., Y.C., S.L., Y.H., Y.F., P.H., and A.T. coordinated the data acquisition (for baseline, resurveys, and long-term follow-up) and standardization. J.L. and C.Y. analyzed the data. J.L. drafted the manuscript. L.Q. and L.L. contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript. All authors reviewed and approved the final manuscript. J.L. and L.L. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lu Qi, Email: lqi1@tulane.edu.
Liming Li, Email: lmlee@vip.163.com.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas, 6th edn. (International Diabetes Federation, Brussels, Belgium, 2013).
- 2.Xu Y, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 3.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. The Lancet. 2006;368:230–239. doi: 10.1016/S0140-6736(06)69044-2. [DOI] [PubMed] [Google Scholar]
- 4.Center for Health Statistics and Information, NHFPC. An analysis report of National Health Services Survey in China, 2013. (Peking Union Medical College Press, 2015).
- 5.Zhang ZW. Epidemiology and risk factors of gallstones (in Chinese) J Surg Concepts Pract. 2011;16:408–412. [Google Scholar]
- 6.Alberti KG, Zimmet P, Shaw J. International Diabetes Federation: a consensus on Type 2 diabetes prevention. Diabet Med. 2007;24:451–463. doi: 10.1111/j.1464-5491.2007.02157.x. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Y, et al. Gallstones and risk of coronary heart disease: Prospective analysis of 270 000 men and women from 3 US cohorts and meta-analysis. Arterioscler Thromb Vasc Biol. 2016;36:1997–2003. doi: 10.1161/ATVBAHA.116.307507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruhl CE, Everhart JE. Association of diabetes, serum insulin, and C-peptide with gallbladder disease. Hepatology. 2000;31:299–303. doi: 10.1002/hep.510310206. [DOI] [PubMed] [Google Scholar]
- 9.Chen CH, et al. Prevalence and risk factors of gallstone disease in an adult population of Taiwan: an epidemiological survey. J Gastroenterol Hepatol. 2006;21:1737–1743. doi: 10.1111/j.1440-1746.2006.04381.x. [DOI] [PubMed] [Google Scholar]
- 10.Sun H, et al. Gender and metabolic differences of gallstone diseases. World J Gastroenterol. 2009;15:1886–1891. doi: 10.3748/wjg.15.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weikert C, et al. Presence of gallstones or kidney stones and risk of type 2 diabetes. Am. J. Epidemiol. 2010;171:447–454. doi: 10.1093/aje/kwp411. [DOI] [PubMed] [Google Scholar]
- 12.Lv J, et al. Gallstone disease and the risk of ischemic heart disease. Arterioscler Thromb Vasc Biol. 2015;35:2232–2237. doi: 10.1161/ATVBAHA.115.306043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volzke H, et al. Independent risk factors for gallstone formation in a region with high cholelithiasis prevalence. Digestion. 2005;71:97–105. doi: 10.1159/000084525. [DOI] [PubMed] [Google Scholar]
- 14.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Prospective study of abdominal adiposity and gallstone disease in US men. Am. J. Clin. Nutr. 2004;80:38–44. doi: 10.1093/ajcn/80.1.38. [DOI] [PubMed] [Google Scholar]
- 15.Misciagna G, Guerra V, Di Leo A, Correale M, Trevisan M. Insulin and gall stones: a population case control study in southern Italy. Gut. 2000;47:144–147. doi: 10.1136/gut.47.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang Y, et al. Insulin resistance is associated with gallstones even in non-obese, non-diabetic Korean men. J Korean Med Sci. 2008;23:644–650. doi: 10.3346/jkms.2008.23.4.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen LY, et al. Metabolic syndrome and gallstone disease. World J Gastroenterol. 2012;18:4215–4220. doi: 10.3748/wjg.v18.i31.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Mutsert R, Sun Q, Willett WC, Hu FB, van Dam RM. Overweight in early adulthood, adult weight change, and risk of type 2 diabetes, cardiovascular diseases, and certain cancers in men: a cohort study. Am. J. Epidemiol. 2014;179:1353–1365. doi: 10.1093/aje/kwu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodama S, et al. Quantitative relationship between body weight gain in adulthood and incident type 2 diabetes: a meta-analysis. Obes Rev. 2014;15:202–214. doi: 10.1111/obr.12129. [DOI] [PubMed] [Google Scholar]
- 20.Wu T, et al. Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genomics. 2013;14:669. doi: 10.1186/1471-2164-14-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, et al. Alteration of gut microbiota in association with cholesterol gallstone formation in mice. BMC Gastroenterol. 2017;17:74. doi: 10.1186/s12876-017-0629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinje S, Stroes E, Nieuwdorp M, Hazen SL. The gut microbiome as novel cardio-metabolic target: the time has come! Eur Heart J. 2014;35:883–887. doi: 10.1093/eurheartj/eht467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JM, Hazen SL. The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu. Rev. Med. 2015;66:343–359. doi: 10.1146/annurev-med-060513-093205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu X, Zhang S, Huang Z. [The trend of the gallstone disease in China over the past decade] Zhonghua Wai Ke Za Zhi. 1995;33:652–658. [PubMed] [Google Scholar]
- 26.Chen Z, et al. Cohort profile: the Kadoorie Study of Chronic Disease in China (KSCDC) Int. J. Epidemiol. 2005;34:1243–1249. doi: 10.1093/ije/dyi174. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int. J. Epidemiol. 2011;40:1652–1666. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bragg F, et al. Associations of blood glucose and prevalent diabetes with risk of cardiovascular disease in 500 000 adult Chinese: the China Kadoorie Biobank. Diabet Med. 2014;31:540–551. doi: 10.1111/dme.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.