The skin fragility disorder recessive dystrophic epidermolysis bullosa (RDEB) results from mutations in COL7A1 leading to reduced or absent type VII collagen (C7) and defective anchoring fibrils at the dermal-epidermal junction (DEJ) (Fine et al., 2014). Currently, there is no cure and most individuals develop life-shortening squamous cell carcinomas (Fine and Mellerio, 2009). RDEB also has a major health economic burden; wound dressings for a 10-year old child can cost $680 per day (Kirkorian et al., 2014), which equates to >$250,000 annually.
Reported clinical trials of cell-based therapies for RDEB comprise intradermal allogeneic fibroblasts (Petrof et al., 2013; Venugopal et al., 2013), and bone marrow transplantation (Wagner et al., 2010), as well as intradermal bone marrow-derived mesenchymal stromal cells (BM-MSCs) (Conget et al., 2010), and intravenous BM-MSCs in RDEB adults (El-Darouti et al., 2013; abstract only). Ex vivo COL7A1 keratinocyte gene therapy is also being evaluated (Siprashvili et al., 2014).
MSCs are heterogeneous cells that undergo self-renewal or differentiate into mesenchymal lineages (Caplan, 1991). MSCs also have non-progenitor functions in immune regulation, cell-growth, and tissue repair (Phinney and Prockop, 2007; Chen et al., 2008). Nevertheless, healing is not associated with large numbers of therapeutic MSCs in injured tissues, suggesting paracrine benefits may modulate inflammatory and immune responses (Baraniak and McDevitt, 2010). Our interest focuses on the potential of intravenous allogeneic BM-MSCs to help people living with RDEB.
Following statutory approvals (see Supplementary Methods online), 10 children were included in the clinical trial and are detailed in Table S1 online, with the trial protocol shown in Figure S1 online. Inclusion/exclusion criteria are presented in Table S2 online and study interventions are listed in Table S3 online. Details of the BM-MSCs are provided in Table S4 online. Each participant received three intravenous infusions of BM-MSCs (Day 0, 7 and 28; each dose 1–3 x 106 cells/kg) with no HLA-matching or pre-conditioning.
With regard to safety, there were 163 adverse events (AEs; Tables S5, S6 and S7 online). Initially two serious AEs (SAEs), esophageal dilatation and skin infection, were reported but were subsequently downgraded (protocol version 4.0, 1st August 2014) as they were considered to be complications of RDEB and not the cells. Indeed, 127/163 (78%) of AEs were either unlikely or not related to the BM-MSCs.
Concerning the severity of MSC-related AEs, there were two severe events of DMSO odor, although odor was noted following 28/30 infusions (lasting up to 48 hours). Mild nausea occurred during two infusions, and abdominal pain and bradycardia were observed during two other infusions; all resolved within 15 minutes without treatment or hemodynamic compromise. No AEs resulted in discontinuation of MSCs.
Laboratory assessments did not reveal any adverse impact of the BM-MSCs on renal, liver or bone marrow function. Anti-C7 antibodies were detected by ELISA at baseline in 9/10 participants but no sera bound to the DEJ by indirect immunofluorescence microscopy (IIF). Following MSCs, there were no changes in ELISA or IIF data (Table S8 online).
Collectively, the tolerance data appear encouraging, although it should be noted that a zero event rate for a SAE in just 10 patients is compatible with an upper 95% confidence interval of over 30%.
With regard to secondary outcome measures, the data are summarized in Table S9 online. Skin biopsies revealed no increase in C7 and no new anchoring fibrils at Day 60. Fluorescence in situ hybridization (FISH) analysis did not show donor cell chimerism.
Birmingham epidermolysis bullosa severity score (BEBSS) and global severity score (GSS) questionnaires were completed for all 10 participants (Figures S2 and S3 online). Mean parent-reported pain score was lower at 60 days than at baseline (difference in means: −5.5 points; 95% CI −16.3, 5.3); similar changes were seen at day 180 (difference in means −3.0 (− 14.7, 8.7) (Figure S4 online). Change in mean disease severity (total BEBSS) was −5.2 points (95% CI −10.7, 0.3) and change in mean BEBSS total body surface area (TBSA%) was −5.9 points from baseline to Day 60 (−15.3, 3.5); similar changes were seen to 180 days for both BEBSS measures (Figure S5 online). Mean global severity score was 7.0 at baseline and 4.6 at Day 60 (mean difference: −2.4 (95% CI: −3.4, −1.4). Corresponding mean change at Day 180 was −1.6 (−3.0, −0.24). Mean quality of life score (higher is worse) reported by parents was 41.9 at baseline and 37.5 at Day 60 (difference: −4.4; 95% CI: −8.1, −0.7) and 39.0 at Day 180 (difference: −2.9; 95% CI: −7.5, 1.8) (Figure S6 online).
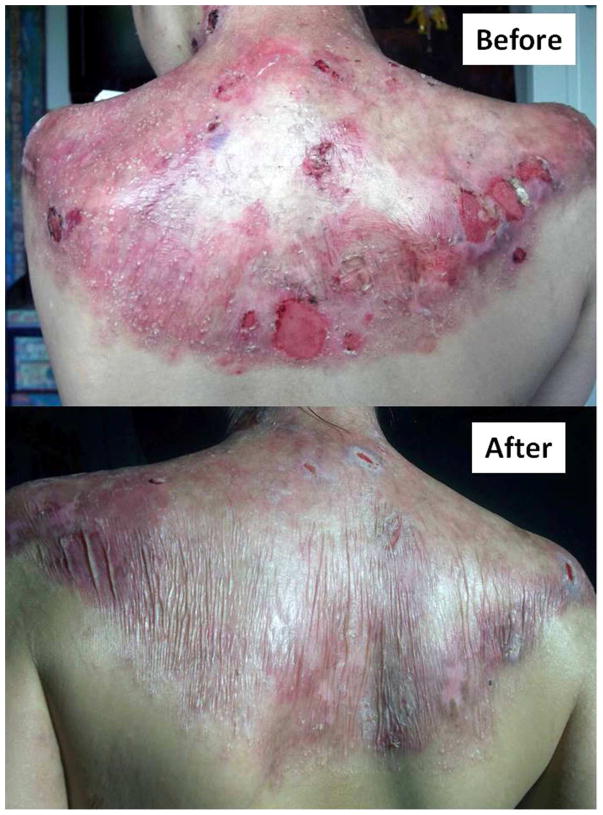
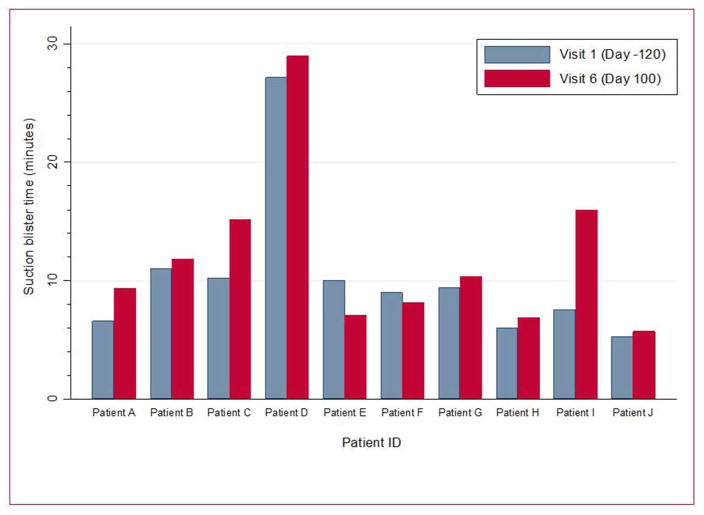
Qualitative data (telephone interviews at 9 months) revealed impressions for better wound healing in all 10 subjects and for less skin redness in 9/10 with clinical benefits lasting for ~4–6 months (Figure 1 and Figures S7 and S8 online). These data are presented in Tables S10 and S11 online. Median blister counts at baseline, Day 60 and 180 were 5.5, 3.5, and 3.5, respectively (Figure S9 online). Mean suction blister times were limited to just two time-points: 10.2 at baseline and 11.9 at day 100 (difference: 1.7; 95% CI: −0.5, 3.9); individual data are shown in Figure 2.
Figure 1.
Improved wound healing and reduced skin erythema 8 weeks after the third infusion of BM-MSCs in Subject I.
Figure 2.
Suction blister times for each subject at baseline (Day -120) and 100 days after the infusions of BM-MSCs.
Overall, the changes in efficacy outcomes were promising although it should be remembered that this is an unblinded study of participants who are keen to help, thus giving a potential for positive information bias.
Many of the subjective verbatim interviews, however, disclosed perceived benefits, such as better sleep (child and parents), a parent being able to return to work part-time because of reduced caring needs, and a family being able to plan their first vacation together etc. Thus although further studies of BM-MSCs in RDEB are needed to demonstrate efficacy, address mechanisms of action, and determine optimal cell dosage, our current findings indicate some benefits for daily life.
Practically, MSCs can be given as a bolus over 10 minutes without sedation and the child can resume normal activities within one hour. If MSCs were given in clinic at the doses used in this trial, repeated every 6 months, we estimate that costs would be similar to current biologics licensed to treat patients with chronic inflammatory diseases, but with the potential for cost-neutrality or savings based on reduced dressing needs and shortened carer time.
Supplementary Material
Acknowledgments
The Sohana Research Fund (SRF; with support from Goldman Sachs Gives) and the Dystrophic Epidermolysis Bullosa Research Association (DEBRA, UK) funded the study. The study is also supported by the UK National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London.
References
- Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–43. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem cells. J Orthopaed Res. 1991;9:641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Chen L, Tredget EE, Wu PY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conget P, Rodriguez F, Kramer S, et al. Replenishment of type VII collagen and re-epithelialization of chronically ulcerated skin after intradermal administration of allogeneic mesenchymal stromal cells in two patients with recessive dystrophic epidermolysis bullosa. Cytotherapy. 2010;12:429–31. doi: 10.3109/14653241003587637. [DOI] [PubMed] [Google Scholar]
- El-Darouti M, Amin FM, Abdel Hay R, et al. Allogeneic Bone marrow derived mesenchymal stem cells for the treatment of dystrophic epidermlysis bullosa. The European Society for Gene and Cell Therapy and the Spanish Society for Gene and Cell Therapy. Hum Gene Ther. 2013;24 abstr54. [Google Scholar]
- Fine JD, Mellerio JE. Extracutaneous manifestations and complications of inherited epidermolysis bullosa: part II. Other organs. J Am Acad Dermatol. 2009a;61:387–402. doi: 10.1016/j.jaad.2009.03.053. [DOI] [PubMed] [Google Scholar]
- Fine JD, Bruckner-Tuderman L, Eady RA, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103–26. doi: 10.1016/j.jaad.2014.01.903. [DOI] [PubMed] [Google Scholar]
- Kirkorian AY, Weitz NA, Tlougan B, Morel KD. Evaluation of wound care options in patients with recessive dystrophic epidermolysis bullosa: a costly necessity. Pediatr Dermatol. 2014;31:33–37. doi: 10.1111/pde.12243. [DOI] [PubMed] [Google Scholar]
- Petrof G, Martinez-Queipo M, Mellerio JE, et al. Fibroblast cell therapy enhances initial healing in recessive dystrophic epidermolysis bullosa wounds: results of a randomized, vehicle-controlled trial. Br J Dermatol. 2013;169:1025–33. doi: 10.1111/bjd.12599. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Prockop DJ. Mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- Siprashvili NN, Gorell E, Khuu P, et al. Phase I clinical trial of genetically corrected autologous epidermal keratinocytes for recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2014;134 abstr 75. [Google Scholar]
- Venugopal SS, Yan W, Frew JW, et al. A phase II randomized vehicle-controlled trial of intradermal allogeneic fibroblasts for recessive dystrophic epidermolysis bullosa. J Am Acad Dermatol. 2013;69:898–908. doi: 10.1016/j.jaad.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Wagner JE, Ishida-Yamamoto A, McGrath JA, et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N Engl J Med. 2010;363:629–39. doi: 10.1056/NEJMoa0910501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.