Abstract
Pathology specimen cross-contamination is a rare phenomenon in diagnostic pathology. Such “floaters” may result in delayed, missed or erroneous diagnoses. We describe the case of a patient with benign granuloma of the lung initially misdiagnosed as squamous cell carcinoma due to a “floater.”
Keywords: humans, pathology, surgical/standards, granuloma
Introduction
In diagnostic pathology, tissue fragments are encountered that are morphologically dissimilar to the main specimen. These contaminants or extraneous tissues are called “floaters.” This occurs as a result of carrying over tissue pieces from one case to another during specimen processing. The exact incidence of floaters in anatomic pathology is uncertain. However it has been estimated, depending on the study method, to be between 0.6% and 2.9%.1 The presence of floaters can have serious consequences, including delaying diagnosis or misdiagnosing patients. Herein we describe the case of a patient with a benign granuloma of the lung initially misdiagnosed as squamous cell carcinoma due to a “floater.”
Case Presentation
A 68-year-old man with a 50 pack-year smoking history and prior exposure to Agent Orange was referred to the pulmonary clinic for exertional dyspnea. CT Chest revealed a 1.1 cm spiculated right upper lobe nodule and mediastinal lymphadenopathy (Figures 1,2). PET-CT demonstrated abnormal uptake in the nodule and mediastinal lymph nodes (Figure 3). Endobronchial ultrasound-guided transbronchial needle aspiration biopsy of the right paratracheal lymph node was obtained. Cytopathology identified a small cluster of atypical cells with an immunohistochemical stain profile consistent with squamous cell carcinoma of the lung (Figures 4, 5), yielding an initial diagnosis of cT1aN2M0 (Stage IIIa) lung cancer (Table 1). Multidisciplinary review at tumor board raised concern that the cells were morphologically inconsistent with squamous cell carcinoma and N2 disease was incongruous with the putative primary lesion. Cervical mediastinoscopy was performed; all lymph nodes were benign. The nodule was resected by video assisted thoracic surgery wedge resection; final pathology indicated an old granuloma due to an endemic fungal pathogen (Figure 6).
Figure 1.
CT coronal cut, lung window, showing right upper lobe nodule
Figure 2.
CT axial cut, body window, showing 4R lymph node
Figure 3.
Fused PET CT, axial cut, showing 4R lymph node
Figure 4.
TBNA of right paratracheal lymph node, H&E, 400x, demonstrating predominantly lymphocytes, with rare admixed epithelioid cells
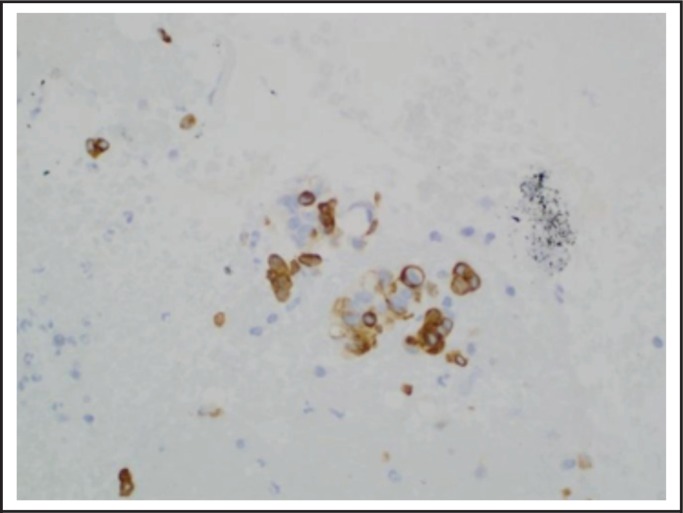
Figure 5.
TBNA of right paratracheal lymph node, CK5/6, 400x, demonstrating the rare epithelioid cells are reactive with CK5/6
Table 1.
Stage groups according to TNM descriptor and subgroups5
Figure 6.
Wedge resection of right upper lobe nodule; Gomori-methamine-silver stain; 600x; demonstrating numerous small (4–6 microns), mildly pleomorphic yeast forms with focal grooving and narrow based budding
Discussion
Floaters may be introduced during specimen grossing, embedding, sectioning, or histological staining. However there is no consensus as to which specific step of specimen processing is to blame for the introduction of extraneous tissue. Gephardt, et al, reported that their tissue contamination frequency was higher in paraffin blocks than it was in slides.2 In contrast, Layfield, et al, found that the histology laboratory was the origin of most contaminants either during the section cutting process or the staining process.3 Their water baths were also contaminated by minute fragments of tissue remaining from the cutting of prior specimens. Platt, et al, found a high number of contaminants in the staining baths, with approximately 26 tissue fragments per bath.4
When these floaters consist of cancerous tissue, it can result in a false positive diagnosis, but also incorrect cancer staging, whether higher or lower. Up to 30% of these contaminants consisted of either abnormal tissue or cancerous cells4 The recognition of a tissue sample as a floater is not always straightforward. It is easy to identify an error when the observed tissue is completely contrary to what one might expect eg, prostate tissue in an endometrial sample.3 However, interpretation becomes more difficult when the floater has the same tissue type or is a significant lesion. One of the most challenging contexts, when dealing with a possible floater occurs when the diagnostic tissue has neoplastic cells that cannot be ignored nor can it be declared as a malignancy with 100% confidence. When necessary, molecular testing can be used to establish identity of the tissue, but molecular testing is time-consuming and expensive, and repeat biopsy exposes the patient to additional procedure-related risks.
This data suggests that true diagnostic challenges due to slide contaminants are relatively rare. However, one must consider that in the era of minimally-invasive diagnostic techniques and small sample sizes, interpreting or identifying contamination has become even more challenging. Floaters are confounding even with surgical biopsies measured in centimeters. It is significantly more difficult to distinguish true sample from potential floater if the sample, in its totality, is small and hypo-cellular. As many specialists, including pulmonologists, continue to push the boundaries of minimally-invasive diagnostics, we must recognize the limitations of our techniques. In this case, it was the collective clinical intuition of thoracic specialists which questioned the sample results and prevented the inappropriate and potentially harmful administration of induction chemoradiation therapy.
Considering the consequences of evaluating contaminated slides and the risk of misdiagnosis, preventative measures should be taken in the pathology laboratory. Cleaning of the water bath and frequent changing of the water will prevent the transmission of contaminants to subsequent sections. Newer staining systems can also be used, that do not use shared baths. As far as contamination at the grossing station is concerned, having a clean technique and being organized seems to be the best approach to prevent errors. Finally, in addition to process improvement, clinicians should be aware of pathology specimen cross-contamination when interpreting biopsy results, especially with small samples in the era of minimally-invasive diagnostics. If results do not fit the clinical picture, a healthy dose of skepticism can protect patients from significant harm.
Conclusion
Clinicians should be aware of pathology specimen cross-contamination when interpreting biopsy results, especially with small samples in the era of minimally-invasive diagnostics. If results do not fit the clinical picture, a healthy dose of skepticism can protect patients from significant harm.
Acknowledgements
The authors extend their appreciation to Patrick Malafronte, MD of the Walter Reed National Military Medical Center Pathology Department and to William Londeree, MD of the Walter Reed National Military Medical Center Pulmonary Disease Service, for their assistance in the preparation of this case report.
Disclaimer
The views expressed in this abstract/manuscript are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
Conflict of Interest
None of the authors identify a conflict of interest.
References
- 1.Gephardt G. Extraneous tissue in surgical pathology: a College of American Pathologists Q-Probes study of 275 laboratories. Arch Pathol Lab Med. 1996;120(11):1009–1014. [PubMed] [Google Scholar]
- 2.Zarbo RJ, Gephardt GN. Extraneous tissue in surgical pathology: a College of American Pathologists Q-probes study of 275 laboratories. Arch Pathol Lab Med. 1996 Nov;120(11):1009–1014. [PubMed] [Google Scholar]
- 3.Layfield L. Extraneous tissue: a potential source for diagnostic error in surgical pathology. Am J Clin Pathol. 2011 Nov;136(5):767–772. doi: 10.1309/AJCP4FFSBPHAU8IU. [DOI] [PubMed] [Google Scholar]
- 4.Platt E. Tissue floaters and contaminants in the histology laboratory. Arch Pathol Lab Med. 2009 Jun;133(6):973–978. doi: 10.5858/133.6.973. [DOI] [PubMed] [Google Scholar]
- 5.Detterbeck F. The Stage Classification of Lung Cancer: Diagnosis and Management of Lung Cancer. CHEST. 2013 May;143(5):e191S–e210S. doi: 10.1378/chest.12-2354. [DOI] [PubMed] [Google Scholar]