Abstract
The motor symptoms of Parkinson’s disease (PD) result from striatal dopamine (DA) deficiency due to a progressive degeneration of nigral dopaminergic cells. Although DA replacement therapy is the mainstay to treat parkinsonian symptoms, a long-term daily administration of levodopa often develops levodopa-induced dyskinesia (LID). LID is closely linked to the dysregulation of cyclic adenosine monophosphate (cAMP) signaling cascades in the medium spiny neurons (MSNs), the principal neurons of the striatum, which are roughly halved with striatonigral MSNs by striatopallidal MSNs. The olfactory type G-protein α subunit (Gαolf) represents an important regulator of the cAMP signal activities in the striatum, where it positively couples with D1-type dopamine receptor (D1R) and adenosine A2A receptor (A2AR) to increase cAMP production in the MSNs. Notably, D1Rs are primarily expressed in striatonigral MSNs, whereas D2Rs and A2ARs are expressed in striatopallidal MSNs. Based on the evidence obtained from parkinsonian mice, we hypothesized that in the DA-denervated striatum with D1R hypersensitivity, a repeated and pulsatile exposure to levodopa might cause a usage-induced degradation of Gαolf proteins in striatal MSNs, resulting in increased and decreased levels of Gαolf protein in the striatonigral and striatopallidal MSNs, respectively. As a principal cause for generating LID, this might lead to an increased responsiveness to levodopa exposure in both striatonigral and striatopallidal MSNs. Our hypothesis reinforces the long-standing concept that LID might result from the reduced activity of the striatopallidal pathway and has important clinical implications.
Keywords: olfactory type G-protein α subunit, levodopa-induced dyskinesia, Parkinson’s disease, dopamine, striatum
Introduction
By transducing extracellular signals carried by neuromodulators, the cyclic adenosine monophosphate (cAMP) signaling plays a crucial role in the regulation of neuronal activities in the brain. Multiple guanine nucleotide-binding protein (G-protein)-coupled receptor (GPCR) cascades regulate the intracellular levels of cAMP, which activates its key effector protein kinase A. Seven-transmembrane domain receptors can transmit extracellular signals to the intracellular signaling cascades through the activation of heterotrimeric G-proteins, which are composed of the guanine nucleotide-binding Gα subunit and the dimeric βγ subunits (Pierce et al., 2002). Gαs is the predominant stimulatory G-protein subunit in the brain. However, in the striatum, Gαs is replaced by the olfactory type G protein α subunit (Gαolf), which is encoded by the GNAL gene (Jones and Reed, 1989). Cellular Gαolf/cAMP signaling pathway represents a principal regulator for the striatal functions in normal physiological processes and pathological conditions (Hervé, 2011). It is worth noting that mutations in the GNAL gene have been identified as a cause for generating dystonia (Fuchs et al., 2013; Pelosi et al., 2017), suggesting that the Gαolf function might participate in the brain circuit involving motor control.
The motor symptoms of Parkinson’s disease (PD) are caused by striatal dopamine (DA) deficiency, predominantly in the putamen, resulting from a progressive degeneration of nigrostriatal DA-producing cells (Kish et al., 1988; Goto et al., 1989). Although the DA replacement therapy remains the mainstay to treat PD symptoms, long-term exposure to dopaminergic drugs, particularly to the DA precursor levodopa, eventually causes adverse effects such as motor fluctuations and levodopa-induced dyskinesia (LID; Jenner, 2008; Calabresi et al., 2010; Bastide et al., 2015). LID is a major cause of disability in patients with PD, and occurs in approximately 80% of patients after 5 years of treatment with a daily administration of levodopa (Obeso et al., 1989; Luquin et al., 1992; Rascol et al., 2000). Importantly, once LID has been primed (or established), its severity progressively increases despite even when the used dosage of dopaminergic drugs is not increased (Brotchie, 2005). LID is known to be closely linked to the altered function of the DA signaling pathways in the striatum (Brotchie, 2005; Jenner, 2008; Bastide et al., 2015; Calabresi et al., 2016). It has also been suggested that LID is associated with the hypersensitivity of striatal MSNs to DA receptor stimulation and with ongoing deregulation of corticostriatal inputs, which activate striatal glutamate receptors, such as N-methyl-D-aspartate (NMDA) receptors (Brotchie, 2005; Jenner, 2008; Bastide et al., 2015; Calabresi et al., 2016). In this hypothesis article, we primarily considered the levodopa-induced changes in cellular Gαolf protein levels in the DA-denervated striatum as the key mechanism to increase the striatal responsiveness to DA receptor stimulation in LID.
Gαolf Regulates The Agonist-Induced cAMP Production in Striatal Cells
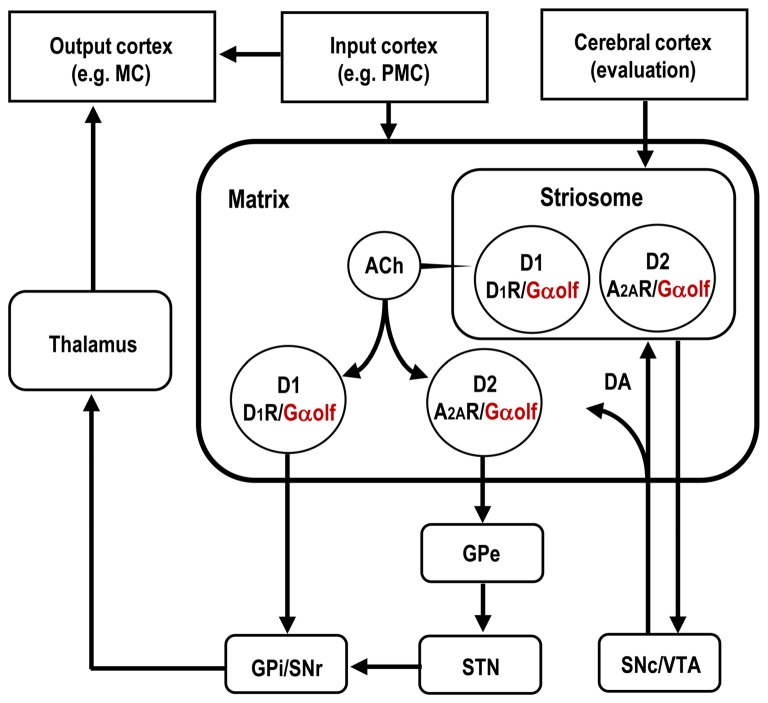
As being innervated by massive dopaminergic afferents originating from the midbrain, the striatum is highly enriched in DA receptors, which belong to a superfamily of GPCRs and are classified into two subtypes, D1- and D2-type receptors. Through their specific targeting of G proteins, the D1-type receptors (D1Rs) elicit the adenylyl cyclase type (AC) to increase the cAMP production, whereas the D2-type dopamine receptors (D2Rs) inhibit the cAMP production (Kebabian and Calne, 1979; Missale et al., 1998). Medium spiny neurons (MSNs) constitute more than 90% of the neuronal types in the striatum (Graybiel, 2008; Kreitzer, 2009; Gerfen and Surmeier, 2011). Anatomically, they are roughly halved with the MSN group to form the “direct” striatonigral pathway by the MSN group to from the “indirect” striatopallidal pathway (Crittenden and Graybiel, 2011; Gerfen and Surmeier, 2011; Calabresi et al., 2014). The striatonigral and striatopallidal MSNs express D1Rs and D2Rs, respectively. Moreover, the striatopallidal MSNs, but not the striatonigral MSNs, are enriched in adenosine A2A receptors (A2ARs), which are prototypical Gs-coupled receptors that elicit AC to increase cAMP production (Svenningsson et al., 1999; Schwarzschild et al., 2006; Fuxe et al., 2007). Figure 1 depicts the cell-type specific localization of Gαolf, D1R and A2AR among the striatal MSNs that constitute the basic circuits of the basal ganglia.
Figure 1.
Distributional pattern of Gαolf proteins in striatal medium spiny neurons (MSNs) that form the basal ganglia circuit. Gαolf proteins are colocalized with DA D1 receptors (D1Rs) in the striatonigral MSNs (D1-cells), and with adenosine A2A receptors (A2ARs) in striatopallidal MSNs expressing DA D2 receptors (D2Rs; D2-cells). The striatonigral and striatopallidal pathways arising from the striosome are omitted in this scheme. ACh, acetylcholine; DA, dopamine; GPe, globus pallidus externa; GPi, globus pallidus interna; MC, motor cortex; PMC, premotor cortex; SNr, substantia nigra pars reticulata; SNc, substantia nigra pars compacta; STN, subthalamic nucleus; VTA, ventral tegmental area.
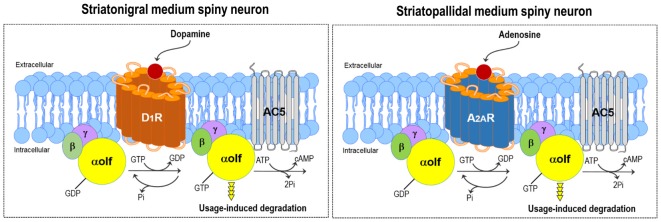
Gαolf is highly expressed in all striatal MSNs including those expressing the D1Rs and A2ARs (Kull et al., 2000; Hervé, 2011; Morigaki et al., 2017; see Figure 2). As Gαolf positively couples with D1R and A2AR to activate the AC type 5 (AC5) and, thereby, increase the intracellular cAMP levels, it serves as the rate-limiting factor for both the D1R- and A2AR-dependent cAMP production in striatal MSNs (Kull et al., 2000; Corvol et al., 2001). The Gαolf protein level plays a key role in regulating the D1R/cAMP- and A2AR/cAMP-signal activities of striatonigral and striatopallidal MSNs, respectively. The D1R/Gαolf-mediated increases in the cAMP levels cause the activation of the striatonigral MSNs (Hervé, 2011). On one hand, as D2R activation inhibits AC5 through Gi/o proteins but A2AR activation elicits AC5 through Gs/olf proteins (Kull et al., 2000), the A2AR/Gαolf-signal stimulation functionally opposes the actions of D2Rs on the striatopallidal MSNs (Schwarzschild et al., 2006; Fuxe et al., 2007).
Figure 2.
DA and adenosine-induced cAMP production in striatal MSNs. Gαolf positively couples with the DA D1 receptor (D1R) and adenosine A2A receptor (A2AR) to activate adenylyl cyclase type 5 (AC5) and subsequently increase cAMP production in striatonigral (left) and striatopallidal (right) MSNs, respectively. Thus, the DA-induced activation of D1R or adenosine-induced activation of A2AR leads to the degradation of the Gαolf protein through the usage-dependent mechanism in striatonigral or striatopallidal MSN, respectively.
Subdivisional and Compartmental Localization of Gαolf in The Striatum
Quantitative immunohistochemistry (IHC) has shown that the Gαolf protein is unevenly distributed within the mouse striatum, where it is highly concentrated in the dorsolateral striatum (Morigaki et al., 2017). Since the dorsolateral portion of the mouse striatum corresponds to the motor territory in rodents and is analogous to the putamen in primates (Graybiel, 2008), this strategic expression of Gαolf protein indicates that Gαolf may function as the stimulatory G protein that has a tight link to the basal ganglia “motor” circuit (Alexander and Crutcher, 1990) at the striatal level. With respect to the striatal compartments, there was a differential localization of Gαolf with higher densities of Gαolf proteins in the striosomes relative to the matrix compartment (Sako et al., 2010; Ruiz-DeDiego et al., 2015; Morigaki et al., 2017). This suggests that Gαolf may be a key molecule that determines differential responses between the striosome and matrix compartments to the D1R or A2AR activation in the striatum at maturity.
Homeostatic Regulation of The Cellular Gαolf Protein Levels in The Striatum
Rodent animal models for PD (Iderberg et al., 2012; Francardo and Cenci, 2014) have so far been used to elucidate the regulatory mechanism for the striatal expression of Gαolf. In line with the evidence that there is a significant increase in Gαolf protein levels in the putamen of patients with PD (Corvol et al., 2004), a dramatic increase in Gαolf protein levels has been identified in the DA-depleted striatum of rats (Hervé et al., 1993; Marcotte et al., 1994; Penit-Soria et al., 1997; Corvol et al., 2004; Rangel-Barajas et al., 2011) and mice (Alcacer et al., 2012; Ruiz-DeDiego et al., 2015; Morigaki et al., 2017) with nigrostriatal 6-hydroxydopamine lesions. However, this upregulation of the Gαolf protein levels is not associated with a parallel increase of the Gαolf mRNA expression. Accordingly, the homeostatic regulation of Gαolf protein levels is thought to occur through post-translational mechanisms in the striatum, where the altered expression of the Gαolf protein depends directly on its usage rate (Hervé, 2011). The persistent lack in the use of D1R and Gαolf could lower the Gαolf degradation rate and thereby result in the accumulation of Gαolf protein in the DA-denervated striatum of PD models. In agreement with this hypothesis, a total lack of D1Rs by D1R gene targeting induces a significant increase of the Gαolf protein levels without any changed expression of Gαolf mRNAs in the striatum of mutant mice (Hervé et al., 2001). In contrast, the decreased levels of striatal Gαolf proteins were found in mutant mice lacking the DA transporter (Hervé et al., 2001), which exhibit a marked increase in the extracellular DA levels leading to persistent activation of D1Rs in the striatum (Giros et al., 1996). Importantly, the lack of A2ARs in homozygous A2AR knock-out mice (Ledent et al., 1997) also results in an upregulation of Gαolf proteins with no obvious changes in the levels of Gαolf transcripts (Hervé et al., 2001). Collectively, the agonist-induced activation of D1Rs (Hervé et al., 2001; Corvol et al., 2004, 2007; Alcacer et al., 2012; Ruiz-DeDiego et al., 2015) or A2ARs (Hervé et al., 2001) might lead to the degradation of Gαolf proteins in striatal MSNs through posttranslational usage-dependent mechanism (see Figure 2).
Gαolf Protein Levels in Striatonigral and Striatopallidal MSNs in LID
On the hypothesis that the upregulation of the Gαolf protein levels results from the disuse of the D1Rs in the DA-depleted striatum in rodent models for PD, several studies with IHC and western blot analyses revealed that the Gαolf could be returned to normal levels by DA replacement with a daily exposure to levodopa in rodent models for PD with LID (Corvol et al., 2004; Rangel-Barajas et al., 2011; Ruiz-DeDiego et al., 2015; Morigaki et al., 2017). With respect to the striosome-matrix system, IHC studies revealed that the Gαolf levels were normally found in both the striosome and matrix compartments in PD with LID, although they were markedly increased in the matrix compartment, but not or only mildly increased in the striosome compartment, in PD (Ruiz-DeDiego et al., 2015; Morigaki et al., 2017). This novel finding indicates that there is a difference in the dopaminergic regulation of the Gαolf expression between the striosome and matrix compartments.
In situ proximity ligation assay (PLA) for dual-antigen recognition disclosed cell-type specific changes in the Gαolf levels in the DA-depleted striatum of mice with and without LID (Morigaki et al., 2017). The in situ PLA technique can indicate the presence of the Gαolf protein in close proximity to the D1R protein (D1R-Gαolf) or A2AR protein (A2AR-Gαolf). Quantitative in situ PLA showed that DA depletion caused a marked (~90%) increase in the striatal levels of D1R-Gαolf PLA signals, which were downregulated by a daily administration of levodopa. However, there remained a significant (~50%) increase in the striatal D1R-Gαolf PLA signals in mice with LID when compared with normal controls. On one hand, quantitative in situ PLA also disclosed that a daily exposure to levodopa, but not DA depletion per se, caused a significant (~40%) decrease in the striatal A2AR-Gαolf PLA signals in the DA-depleted striatum of mice with LID. These findings indicate that, in the DA-depleted striatum, DA replacement could induce the downregulation of the Gαolf protein levels not only in the striatonigral MSNs but also in the striatopallidal MSNs.
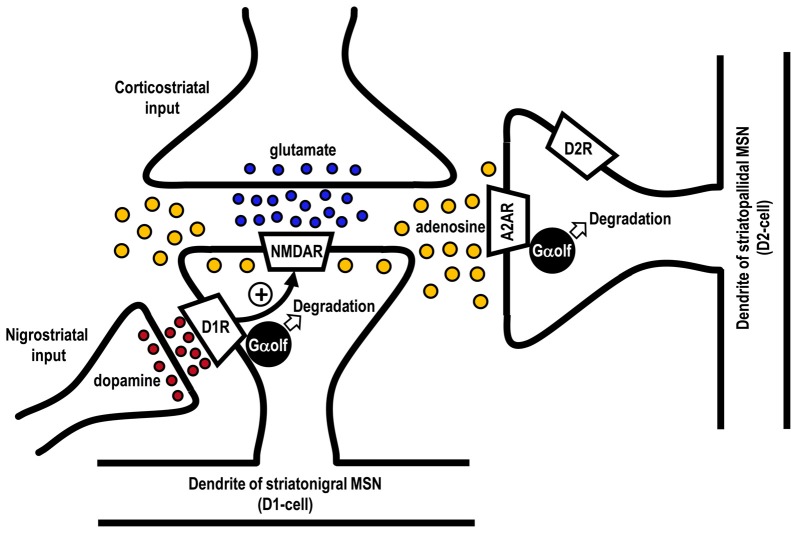
An intriguing question is how the Gαolf protein levels are decreased in the striatopallidal MSNs in LID. In animal models with nigrostriatal 6-OHDA-lesions, persistent (chronic) DA depletion per se has been shown to cause no apparent changes (Ballarin et al., 1987; Herrera-Marschitz et al., 1994; Nomoto et al., 2000) or mild decrease (Pinna et al., 2002) in the extracellular levels of adenosine in the DA-denervated striatum. However, evidence shows that the striatal adenosine levels are elevated by the activation of NMDA receptors (Delaney and Geiger, 1998; Delaney et al., 1998), which can be enhanced by D1R activation (Cepeda and Levine, 2012; Morigaki and Goto, 2015; see Figure 3). Interestingly, a pulsatile exposure to the D1R agonist reportedly facilitated the NMDA receptor-evoked increase in the extracellular adenosine release in the rat striatum (Harvey and Lacey, 1997). This evidence suggests that, in the DA-depleted striatum with D1R hypersensitivity, a repeated administration of levodopa may exert a pulsatile activation of D1Rs, which subsequently facilitates the NMDA receptor-evoked increase in the extracellular adenosine levels. Moreover, in the DA-depleted striatum, the activation of NMDA receptor could lead to a marked increase in the extracellular adenosine levels and, then, indirectly activate A2ARs (Nash and Brotchie, 2000). Thus, it is likely that the downregulation of the Gαolf levels in striatopallidal MSNs in LID might result from an increased usage of Gαolf proteins through the A2AR activation subsequent to the daily pulsatile activation of striatal D1Rs. This notion also suggests that the striatal D1R signals might play a critical role in the regulation of the Gαolf protein levels not only in the striatonigral MSNs, but also in the striatopallidal MSNs in the DA-denervated striatum. This consideration may corroborate the general concept that increased activities of striatal D1Rs are requisite for the genesis of LID (Westin et al., 2007; Darmopil et al., 2009; Alcacer et al., 2012).
Figure 3.
Possible mechanism for agonist-induced degradation of Gαolf proteins in striatonigral and striatopallidal MSNs. Glutamate released from the corticostriatal afferents could activate postsynaptic N-methyl-D-aspartate (NMDA) receptors (NMDARs) to increase the extracellular adenosine levels in the striatum. Repeated exposure to levodopa might cause a pulsatile release of DA from the nigrostriatal afferents to activate DA D1 receptors (D1Rs) in striatonigral MSNs (D1-cells). This might facilitate the NMDAR-evoked increase in extracellular adenosine release and, thereby, indirectly activate the adenosine A2A receptors (A2ARs) in striatopallidal MSNs expressing DA D2 receptors (D2Rs; D2-cells). Thus, a usage-induced downregulation of Gαolf protein levels could occur not only in the striatonigral MSNs but also in striatopallidal MSNs.
Striatal Gαolf/cAMP Signal-Dependent Mechanism for Generating LID
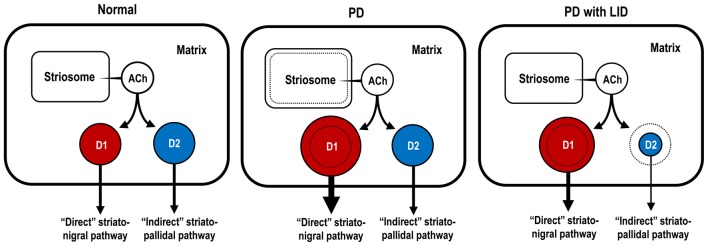
Figure 4 shows the hypothetical representation of the Gαolf protein levels in striatonigral and striatopallidal MSNs in the DA-denervated striatum under the conditions of both PD with and without LID. In PD, there is a dramatic increase in the Gαolf protein levels in the striatonigral MSNs, but not in the striatopallidal MSNs. Because of no apparent changes in the striatal D1R levels (Shinotoh et al., 1993; Turjanski et al., 1997; Hurley et al., 2001) and other principal mediators of the D1R signaling cascades (Girault et al., 1989; Nishino et al., 1993) in patients with PD, the marked increase in the Gαolf protein levels in the striatonigral MSNs may be a principal cause for generating striatal D1R hypersensitivity to levodopa exposure in PD. This notion corroborates the evidence that there is a marked increase in the responsiveness of the striatonigral MSNs to D1R activation in PD, as determined by the fos induction experiments (Engber et al., 1989; Asin et al., 1995; Kashihara et al., 2000; Xu et al., 2003; Morigaki et al., 2017).
Figure 4.
Hypothetical diagram for dopaminergic regulation of Gαolf protein levels in striatonigral and striatopallidal MSNs. The sizes of the circles, colored in red and blue, indicate the abundance of Gαolf proteins in striatonigral MSNs expressing dopamine D1 receptors (D1Rs; D1-cells; red) and in striatopallidal MSNs expressing dopamine D2 receptors (D2Rs; D2-cells; blue), respectively. In the conditions of Parkinson’s disease (PD), D1-cells, but not D2-cells, might exhibit a DA D1 hypersensitivity caused by a dramatic increase in their Gαolf levels. In the conditions of PD with levodopa-induced dyskinesia (LID), D1-cells might show an increase in their Gαolf levels, while D2-cells might show a decrease in their Gαolf levels, which might result in an enhanced responsiveness to D2R activation. ACh, acetylcholine; D1-cell, striatonigral medium spiny neuron expressing DA D1 receptor; D2-cell, striatopallidal medium spiny neuron expressing DA D2 receptor; PD, Parkinson’s disease; PD with LID, Parkinson’s disease with levodopa-induced dyskinesia.
In PD with LID, there is an important decrease in the Gαolf protein levels in the striatopallidal MSNs after a prolonged and pulsatile administration of levodopa. This leads to the facilitation of the effects of DA on striatopallidal MSNs by reducing the A2AR/Gαolf signal-mediated cAMP production and subsequently to the increase in the responsiveness of striatopallidal MSNs to D2R activation. Indeed, it was importantly noted that, during the increasing phase of dyskinesias, an abnormal lowering of intracellular cAMP levels transiently occurred in the DA-denervated striatum in rat model of LID (Sancesario et al., 2014). These novel findings parallel the evidence that a repeated exposure to levodopa results in a significant increase in the responsiveness of striatopallidal MSNs to dopaminergic stimulation, as determined by fos induction experiments (Engber et al., 1989; Asin et al., 1995; Kashihara et al., 2000; Xu et al., 2003; Morigaki et al., 2017). In addition, there is a significant increase in the Gαolf protein levels in striatonigral MSNs in PD with LID as compared to normal controls. Because Gαolf is the regulator of cAMP signal-dependent activities in the striatum, an increase in the responsiveness of both striatonigral and striatopallidal MSNs to levodopa exposure, which depends on the Gαolf protein levels, serves as a principal cause for generating LID.
Concluding Remarks
Since the intracellular cAMP signaling cascades serve as a determinant of striatal cell activities (Girault, 2012), maladaptive change in Gαolf protein levels is thought to be closely linked to the pathophysiology of PD (Hervé, 2011). Here, we hypothesized that DA depletion might cause a marked upregulation of the Gαolf protein levels in striatonigral MSNs, which results in a crucial hypersensitivity of the striatum to D1R stimulation in PD. A prolonged and pulsatile exposure to levodopa might lead to a usage-dependent decrease in the Gαolf protein levels not only in the nigrostriatal MSNs but also in the striatopallidal MSNs in PD with LID. This levodopa-induced decrease in Gαolf protein levels, which might be due to a pulsatile activation of postsynaptic D1Rs and NMDA receptors, could result in reduced A2AR/Gαolf/cAMP signal levels in striatopallidal MSNs. This might cause an increase in the responsiveness of striatopallidal MSNs to D2R activation, and thereby develop LID in PD. Our hypothesis corroborates the long-lasting concept that LIDs are associated with a decreased activity of the “indirect” striatopallidal pathway (Crossman, 1990; DeLong, 1990; Brotchie, 2005).
As an important cellular mechanism to regulate the activities of striatal MSNs, the recurrent collateral connections between the MSNs have also been identified (Bolam et al., 1983; Yung et al., 1996). The activities of striatopallidal MSNs can be inhibited by the GABAergic collateral axon branches from neighboring MSNs (Taverna et al., 2008; Lalchandani et al., 2013; Dobbs et al., 2016; Wei et al., 2017). Thus striatal D1 hypersensitivity could lead to an increased responsiveness of striatopallidal MSNs to D2R activation in the conditions of PD with and without LID, although only a small population of the striatonigral MSNs has been found to form collateral axon connections with striatopallidal MSNs in the mouse striatum (Taverna et al., 2008). However, this notion per se could not explain the progressive increase in the severity of LID, which occurs in the PD patients treated with unaltered dosages of given dopaminergic drugs (Brotchie, 2005), because there is an ongoing decline in striatal responsiveness to D1R activation along a repeated exposure to levodopa under the conditions of PD, as determined by fos induction experiments (Saka et al., 1999; Kashihara et al., 2000; Xu et al., 2003; Morigaki et al., 2017).
Finally, we suggest that the pharmacological concomitant therapy to increase Gαolf protein levels in the striatum might be useful in the management of LID and motor fluctuations in patients with PD treated with DA replacement therapy. The normalization of the decreased Gαolf protein levels in the striatopallidal MSNs might suppress LID. On one hand, the elevation of the Gαolf protein levels in the striatonigral MSNs could increase the striatal responsiveness to D1R activation and, thereby, facilitate the therapeutic efficacy of dopaminergic drugs. In considering the possible involvement of the activated NMDA receptors in lowering striatal Gαolf levels in LID, NMDA receptor antagonists (e.g., amantadine or memantine) might attenuate LID, as already shown in clinical practice (Rascol et al., 2015). Because A2AR activation, which could reduce the Gαolf protein levels in the striatopallidal MSNs leading to LID, might be required for the “priming” of LID (Brotchie, 2005; Xiao et al., 2006), it is suggested that A2AR antagonists (e.g., istradefylline) might be effective in dampening the “priming” of LID. However, after the establishment of LID, the adjunct use of A2AR antagonists might exacerbate the dyskinetic symptoms as shown in clinical practice (Kondo and Mizuno, 2015).
Author Contributions
SG wrote the manuscript.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The author wishes to thank Dr. Ryoma Morigaki and Dr. Shinya Okita for their experimental supports. This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grants-in-aid for Scientific Research no. 24390223, 26461272, 26430054 and 16k10788), Japan Agency for Medical Research and Development (AMED; No. 16ek0109182h0001) and the Research Cluster of Tokushima University (No. 1702004).
References
- Alcacer C., Santini E., Valjent E., Gaven F., Girault J.-A., Hervé D. (2012). Gαolf mutation allows parsing the role of cAMP-dependent and extracellular signal-regulated kinase-dependent signaling in L-3,4,-dihydroxyphenylalanine-induced dyskinesia. J. Neurosci. 32, 5900–5910. 10.1523/JNEUROSCI.0837-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander G. E., Crutcher M. D. (1990). Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13, 266–271. 10.1016/0166-2236(90)90107-l [DOI] [PubMed] [Google Scholar]
- Asin K. E., Bednarz L., Nikkel A., Perner R. (1995). Rotation and striatal c-fos expression after repeated, daily treatment with selective dopamine receptor agonists and levodopa. J. Pharmacol. Exp. Ther. 273, 1483–1490. [PubMed] [Google Scholar]
- Ballarin M., Herrera-Marschitz M., Casas M., Ungerstedt U. (1987). Striatal adenosine levels measured ‘in vivo’ by microdialysis in rats with unilateral dopamine denervation. Neurosci. Lett. 83, 338–344. 10.1016/0304-3940(87)90111-x [DOI] [PubMed] [Google Scholar]
- Bastide M. F., Meissner W. G., Picconi B., Fasano S., Fernagut P. O., Feyder M., et al. (2015). Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog. Neurobiol. 132, 96–168. 10.1016/j.pneurobio.2015.07.002 [DOI] [PubMed] [Google Scholar]
- Bolam J. P., Somogyi P., Takagi H., Fodor I., Smith A. D. (1983). Localization of substance P-like immunoreactivity in neurons and nerve terminals in the neostriatum of the rat: a correlated light and electron microscopic study. J. Neurocytol. 12, 325–344. 10.1007/bf01148468 [DOI] [PubMed] [Google Scholar]
- Brotchie J. M. (2005). Nondopaminergic mechanisms in levodopa-induced dyskinesia. Mov. Disord. 20, 919–931. 10.1002/mds.20612 [DOI] [PubMed] [Google Scholar]
- Calabresi P., Di Filippo M., Ghiglieri V., Tambasco N., Picconi B. (2010). Levodopa-induced dyskinesias in patients with Parkinson’s disease: filling the bench-to-bedside gap. Lancet Neurol. 9, 1106–1117. 10.1016/S1474-4422(10)70218-0 [DOI] [PubMed] [Google Scholar]
- Calabresi P., Picconi B., Tozzi A., Ghiglieri V., Di Filippo M. (2014). Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat. Neurosci. 17, 1022–1030. 10.1038/nn.3743 [DOI] [PubMed] [Google Scholar]
- Calabresi P., Pisani A., Rothwell J., Ghiglieri V., Obeso J. A., Picconi B. (2016). Hyperkinetic disorders and loss of synaptic downscaling. Nat. Neurosci. 19, 868–875. 10.1038/nn.4306 [DOI] [PubMed] [Google Scholar]
- Cepeda C., Levine M. S. (2012). Dopamine-NMDA receptor interations: twenty years later. Dev. Neurosci. 34, 2–4. 10.1159/000338590 [DOI] [PubMed] [Google Scholar]
- Corvol J. C., Muriel M.-P., Valjent E., Féger J., Hanoun N., Girault J.-A., et al. (2004). Persistent increase in olfactory type G-protein α subunit levels may underlie D1 receptor functional hypersensitivity in Parkinson’s disease. J. Neurosci. 24, 7007–7014. 10.1523/jneurosci.0676-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvol J. C., Studler J. M., Schonn J. S., Girault J. A., Hervé D. (2001). Gαolf is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J. Neurochem. 76, 1585–1588. 10.1046/j.1471-4159.2001.00201.x [DOI] [PubMed] [Google Scholar]
- Corvol J. C., Valjent E., Pascoli V., Robin A., Stipanovich A., Luedtke R. R., et al. (2007). Quantitative changes in Gαolf protein levels, but not D1 receptor, alter specifically acute responses to psychostimulants. Neuropsychopharmacology 32, 1109–1121. 10.1038/sj.npp.1301230 [DOI] [PubMed] [Google Scholar]
- Crittenden J. R., Graybiel A. M. (2011). Basal ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front. Neuroanat. 5:59. 10.3389/fnana.2011.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman A. R. (1990). A hypothesis on the pathophysiological mechanisms that underlie levodopa- or dopamine agonist-induced dyskinesia in Parkinson’s disease: implications for future strategies in treatment. Mov. Disord. 5, 100–108. 10.1002/mds.870050203 [DOI] [PubMed] [Google Scholar]
- Darmopil S., Martín A. B., De Diego I. R., Ares S., Moratalla R. (2009). Genetic inactivation of dopamine D1 but not D2 receptors inhibits L-DOPA-induced dyskinesia and histone activation. Biol. Psychiatry 66, 603–613. 10.1016/j.biopsych.2009.04.025 [DOI] [PubMed] [Google Scholar]
- Delaney S. M., Geiger J. D. (1998). Levels of endogenous adenosine in rat striatum. II. Regulation of basal and N-methyl-D-aspartate-induced levels by inhibitors of adenosine transport and metabolism. J. Pharmacol. Exp. Ther. 285, 568–572. [PubMed] [Google Scholar]
- Delaney S. M., Shepel P. N., Geiger J. D. (1998). Levels of endogenous adenosine in rat striatum I. Regulation by ionotropic glutamate receptors, nitric oxide and free radicals. J. Pharmacol. Exp. Ther. 285, 561–567. [PubMed] [Google Scholar]
- DeLong M. R. (1990). Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–285. 10.1016/0166-2236(90)90110-v [DOI] [PubMed] [Google Scholar]
- Dobbs L. K., Kaplan A. R., Lemos J. C., Matsui A., Rubinstein M., Alvarez V. A. (2016). Dopamine regulation of lateral inhibition between striatal neurons gates the stimulant actions of cocaine. Neuron 90, 1100–1113. 10.1016/j.neuron.2016.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engber T. M., Susel Z., Juncos J. L., Chase T. N. (1989). Continuous and intermittent levodopa differentially affect rotation induced by D-1 and D-2 dopamine agonists. Eur. J. Pharmacol. 168, 291–298. 10.1016/0014-2999(89)90790-5 [DOI] [PubMed] [Google Scholar]
- Francardo V., Cenci M. A. (2014). Investigating the molecular mechanisms of L-DOPA-induced dyskinesia in the mouse. Parkinsonism Relat. Disord. 20, S20–S22. 10.1016/s1353-8020(13)70008-7 [DOI] [PubMed] [Google Scholar]
- Fuchs T., Saunders-Pullman R., Masuho I., Luciano M. S., Raymond D., Factor S., et al. (2013). Mutations in GNAL cause primary torsion dystonia. Nat. Genet. 45, 88–92. 10.1038/ng.2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K., Marcellino D., Genedani S., Agnati L. (2007). Adenosine A2A receptors, dopamine D2 receptors and their interactions in Parkinson’s disease. Mov. Disord. 22, 1990–2017. 10.1002/mds.21440 [DOI] [PubMed] [Google Scholar]
- Gerfen C. R., Surmeier D. J. (2011). Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 34, 441–466. 10.1146/annurev-neuro-061010-113641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault J. A. (2012). Integrating neurotransmission in striatal medium spiny neurons. Adv. Exp. Med. Biol. 970, 407–429. 10.1007/978-3-7091-0932-8_18 [DOI] [PubMed] [Google Scholar]
- Girault J. A., Raisman-Vozari R., Agid Y., Greengard P. (1989). Striatal phosphoproteins in Parkinson disease and progressive supranuclear palsy. Proc. Natl. Acad. Sci. U S A 86, 2493–2497. 10.1073/pnas.86.7.2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B., Jaber M., Jones S. R., Wightman R. M., Caron M. G. (1996). Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379, 606–612. 10.1038/379606a0 [DOI] [PubMed] [Google Scholar]
- Goto S., Hirano A., Matsumoto S. (1989). Subdivisional involvement of nigrostriatal loop in idiopathic Parkinson’s disease and striatonigral degeneration. Ann. Neurol. 26, 766–770. 10.1002/ana.410260613 [DOI] [PubMed] [Google Scholar]
- Graybiel A. M. (2008). Habits, rituals, and the evaluative brain. Annu. Rev. Neurosci. 31, 359–387. 10.1146/annurev.neuro.29.051605.112851 [DOI] [PubMed] [Google Scholar]
- Harvey J., Lacey M. G. (1997). A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J. Neurosci. 17, 5271–5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Marschitz M., Luthman J., Ferré S. (1994). Unilateral neonatal intracerebroventricular 6-hydroxydopamine administration in rats: II. Effects on extracellular monoamine, acetylcholine and adenosine levels monitored with in vivo microdialysis. Psychopharmacology 116, 451–456. 10.1007/bf02247477 [DOI] [PubMed] [Google Scholar]
- Hervé D. (2011). Identification of a specific assembly of the G protein Golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Front. Neuroanat. 5:48. 10.3389/fnana.2011.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé D., Le Moine C., Corvol J. C., Belluscio L., Ledent C., Fienberg A. A., et al. (2001). Gα(olf) levels are regulated by receptor usage and control dopamine and adenosine action in the striatum. J. Neurosci. 21, 4390–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé D., Lévi-Strauss M., Marey-Semper I., Verney C., Tassin J. P., Glowinski J., et al. (1993). Golf and Gs in rat basal ganglia: possible involvement of Golf in the coupling of dopamine D1 receptor with adenylyl cyclase. J. Neurosci. 13, 2237–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley M. J., Mash D. C., Jenner P. (2001). Dopamine D1 receptor expression in human basal ganglia and changes in Parkinson’s disease. Mol. Brain Res. 87, 271–279. 10.1016/s0169-328x(01)00022-5 [DOI] [PubMed] [Google Scholar]
- Iderberg H., Francardo V., Pioli E. Y. (2012). Animal models of L-DOPA-induced dyskinesia: an update on the current options. Neuroscience 211, 13–27. 10.1016/j.neuroscience.2012.03.023 [DOI] [PubMed] [Google Scholar]
- Jenner P. (2008). Molecular mechanisms of L-DOPA-induced dyskinesia. Nat. Rev. Neurosci. 9, 665–677. 10.1038/nrn2471 [DOI] [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. (1989). Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science 244, 790–795. 10.1126/science.2499043 [DOI] [PubMed] [Google Scholar]
- Kashihara K., Manabe Y., Shiro Y., Warita H., Abe K. (2000). Effects of repeated methyl levodopa administration on apomorphine sensitivity of rotational behavior and striatal Fos expression of rats with unilateral 6-OHDA lesions. Neurosci. Res. 38, 273–279. 10.1016/s0168-0102(00)00167-x [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. (1979). Multiple receptors for dopamine. Nature 277, 93–96. 10.1038/277093a0 [DOI] [PubMed] [Google Scholar]
- Kish S. J., Shannak K., Hornykiewicz O. (1988). Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. N. Engl. J. Med. 318, 876–880. 10.1056/nejm198804073181402 [DOI] [PubMed] [Google Scholar]
- Kondo T., Mizuno Y., Japanese Istradefylline Study Group . (2015). A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clin. Neuropharmacol. 38, 41–46. 10.1097/WNF.0000000000000073 [DOI] [PubMed] [Google Scholar]
- Kreitzer A. C. (2009). Physiology and pharmacology of striatal neurons. Annu. Rev. Neurosci. 32, 127–147. 10.1146/annurev.neuro.051508.135422 [DOI] [PubMed] [Google Scholar]
- Kull B., Svenningsson P., Fredholm B. B. (2000). Adenosine A2A receptors are colocalized with and activate Golf in rat striatum. Mol. Pharmacol. 58, 771–777. 10.1124/mol.58.4.771 [DOI] [PubMed] [Google Scholar]
- Lalchandani R. R., van der Goes M. S., Partridge J. G., Vicini S. (2013). Dopamine D2 receptors regulate collateral inhibition between striatal medium spiny neurons. J. Neurosci. 33, 14075–14086. 10.1523/JNEUROSCI.0692-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C., Vaugeois J. M., Schiffmann S. N., Pedrazzi T., El Yacoubi M., Vanderhaeghen J. J., et al. (1997). Agressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388, 674–678. 10.1038/41771 [DOI] [PubMed] [Google Scholar]
- Luquin M. R., Scipioni O., Vaamonde J., Gershanik O., Obeso J. A. (1992). Levodopa-induced dyskinesias in Parkinson’s disease: clinical and pharmacological classification. Mov. Disord. 7, 117–124. 10.1002/mds.870070204 [DOI] [PubMed] [Google Scholar]
- Marcotte E. R., Sullivan R. M., Mishra R. K. (1994). Striatal G-proteins: effects of unilateral 6-hydroxydopamine lesions. Neurosci. Lett. 169, 195–198. 10.1016/0304-3940(94)90390-5 [DOI] [PubMed] [Google Scholar]
- Missale C., Nash S. R., Robinson S. W., Jaber M., Caron M. G. (1998). Dopamine receptors: from structure to function. Physiol. Rev. 78, 189–225. [DOI] [PubMed] [Google Scholar]
- Morigaki R., Goto S. (2015). Postsynaptic density protein 95 in the striosome and matrix compartments of the human neostriatum. Front. Neuroanat. 9:154. 10.3389/fnana.2015.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morigaki R., Okita S., Goto S. (2017). Dopamine-induced changes in Gαolf protein levels in striatonigral and striatopallidal medium spiny neurons underlie the genesis of L-DOPA-induced dyskinesia in parkinsonian mice. Front. Cell. Neurosci. 11:26. 10.3389/fncel.2017.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash J. E., Brotchie J. M. (2000). A common signaling pathway for striatal NMDA and adenosine A2a receptors: implications for the treatment of Parkinson’s disease. J. Neurosci. 20, 7782–7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino N., Kitamura N., Hashimoto T., Tanaka C. (1993). Transmembrane signaling systems in the brain of patients with Parkinson’s disease. Rev. Neurosci. 4, 213–222. 10.1515/REVNEURO.1993.4.2.213 [DOI] [PubMed] [Google Scholar]
- Nomoto M., Kaseda S., Iwata S., Shimizu T., Fukuda T., Nakagawa S. (2000). The metabolic rate and vulnerability of dopaminergic neurons and adenosine dynamics in the cerebral cortex, nucleus accumbens, caudate nucleus, and putamen of the common marmoset. J. Neurol. 247, V16–V22. 10.1007/pl00007779 [DOI] [PubMed] [Google Scholar]
- Obeso J. A., Grandas F., Vaamonde J., Luquin M. R., Artieda J., Lera G., et al. (1989). Motor complications associated with chronic levodopa therapy in Parkinson’s disease. Neurology 39, 11–19. [PubMed] [Google Scholar]
- Pelosi A., Menardy F., Popa D., Girault J. A., Hervé D. (2017). Heterozygous GNAL mice are a novel animal model with which to study dystonia pathophysiology. J. Neurosci. 37, 6253–6267. 10.1523/JNEUROSCI.1529-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penit-Soria J., Durand C., Besson M. J., Hervé D. (1997). Levels of stimulatory G protein are increased in the rat striatum after neonatal lesion of dopamine neurons. Neuroreport 8, 829–833. 10.1097/00001756-199703030-00005 [DOI] [PubMed] [Google Scholar]
- Pierce K. L., Premont R. T., Lefkowitz R. J. (2002). Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3, 639–650. 10.1038/nrm908 [DOI] [PubMed] [Google Scholar]
- Pinna A., Corsi C., Carta A. R., Valentini V., Pedata F., Morelli M. (2002). Modification of adenosine extracellular levels and adenosine A2A receptor mRNA by dopamine denervation. Eur. J. Pharmacol. 446, 75–82. 10.1016/s0014-2999(02)01818-6 [DOI] [PubMed] [Google Scholar]
- Rangel-Barajas C., Silva I., Lopéz-Santiago L. M., Aceves J., Erlij D., Florán B. (2011). L-DOPA-induced dyskinesia in hemiparkinsonian rats is associated with up-regulation of adenylyl cyclase type V/VI and increased GABA release in the substantia nigra reticulata. Neurobiol. Dis. 41, 51–61. 10.1016/j.nbd.2010.08.018 [DOI] [PubMed] [Google Scholar]
- Rascol O., Brooks D. J., Korczyn A. D., De Deyn P. P., Clarke C. E., Lang A. E. (2000). A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. Mov. Disord. 342, 1484–1491. 10.1056/NEJM200005183422004 [DOI] [PubMed] [Google Scholar]
- Rascol O., Perez-Lloret S., Ferreira J. J. (2015). New treatments for levodopa-induced motor complications. Mov. Disord. 30, 1451–1460. 10.1002/mds.26362 [DOI] [PubMed] [Google Scholar]
- Ruiz-DeDiego I., Naranjo J. R., Hervé D., Moratalla R. (2015). Dopaminergic regulation of olfactory type G-protein α subunit expression in the striatum. Mov. Disord. 30, 1039–1049. 10.1002/mds.26197 [DOI] [PubMed] [Google Scholar]
- Saka E., Elibol B., Erdem S., Dalkara T. (1999). Compartmental changes in expression of c-Fos and FosB proteins in intact and dopamine-depleted striatum after chronic apomorphine treatment. Brain Res. 825, 104–114. 10.1016/s0006-8993(99)01231-7 [DOI] [PubMed] [Google Scholar]
- Sako W., Morigaki R., Nagahiro S., Kaji R., Goto S. (2010). Olfactory type G-protein α subunit in striosome-matrix dopamine systems in adult mice. Neuroscience 170, 497–502. 10.1016/j.neuroscience.2010.06.072 [DOI] [PubMed] [Google Scholar]
- Sancesario G., Morrone L. A., D’Angelo V., Castelli V., Ferrazzoli D., Sica F., et al. (2014). Levodopa-induced dyskinesias are associated with transient down-regulation of cAMP and cGMP in the caudate-putamen of hemiparkinsonian rats: reduced synthesis or increased catabolism? Neurochem. Int. 79, 44–56. 10.1016/j.neuint.2014.10.004 [DOI] [PubMed] [Google Scholar]
- Schwarzschild M. A., Agnati L., Fuxe K., Chen J. F., Morelli M. (2006). Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 29, 647–654. 10.1016/j.tins.2006.09.004 [DOI] [PubMed] [Google Scholar]
- Shinotoh H., Inoue O., Hirayama K., Aotsuka A., Asahina M., Suhara T., et al. (1993). Dopamine D1 receptors in Parkinson’s disease and striatonigral degeneration: a positron emission tomography study. J. Neurol. Neurosurg. Psychiatry 56, 467–472. 10.1136/jnnp.56.5.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P., Le Moine C., Fisone G., Fredholm B. B. (1999). Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog. Neurobiol. 59, 355–396. 10.1016/s0301-0082(99)00011-8 [DOI] [PubMed] [Google Scholar]
- Taverna S., Ilijic E., Surmeier D. J. (2008). Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson’s disease. J. Neurosci. 28, 5504–5512. 10.1523/jneurosci.5493-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turjanski N., Lees A. J., Brooks D. J. (1997). In vivo studies on striatal dopamine D1 and D2 site binding in L-dopa-treated Parkinson’s disease patients with and without dyskinesias. Neurology 49, 717–723. 10.1212/wnl.49.3.717 [DOI] [PubMed] [Google Scholar]
- Wei W., Ding S., Zhou F. M. (2017). Dopaminergic treatment weakens medium spiny neuron collateral inhibition in the parkinsonian striatum. J. Neurophysiol. 117, 987–999. 10.1152/jn.00683.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin J. E., Vercammen L., Strome E. M., Konradi C., Cenci M. A. (2007). Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of L-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol. Psychiatry 62, 800–810. 10.1016/j.biopsych.2006.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D., Bastia E., Xu Y. H., Benn C. L., Cha J. H., Peterson T. S., et al. (2006). Forebrain adenosine A2A receptors contribute to L-3,4-dihydroxyphenylalanine-induced dyskinesia in hemiparkinsonian mice. J. Neurosci. 26, 13548–13555. 10.1523/jneurosci.3554-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Sun S., Cao X. (2003). Effect of levodopa chronic administration on behavioral changes and fos expression in basal ganglia in rat model of PD. J. Huazhong Univ. Sci. Technolog. Med. Sci. 23, 258–262. 10.1007/bf02829507 [DOI] [PubMed] [Google Scholar]
- Yung K. K., Smith A. D., Levey A. I., Bolam J. P. (1996). Synaptic connections between spiny neurons of the direct and indirect pathways in the neostriatum of the rat: evidence from dopamine receptor and neuropeptide immunostaining. Eur. J. Neurosci. 8, 861–869. 10.1111/j.1460-9568.1996.tb01573.x [DOI] [PubMed] [Google Scholar]