Abstract
Objective:
To evaluate whether frontal-lobe magnetic resonance spectroscopy measures of γ-aminobutyric acid (GABA) would be altered in a sample of adolescents scanned after sport concussion because mild traumatic brain injury is often associated with working memory problems.
Methods:
Eleven adolescents (age 14–17 years) who had sustained a first-time sport concussion were studied with MRI/magnetic resonance spectroscopy within 23 to 44 days after injury (mean 30.4 ± 6.1 days). Age- and sex-matched healthy controls, being seen for sports-related injuries not involving the head and with no history of concussion, were also examined. GABA/creatine + phosphocreatine (Cre) was measured in left-sided frontal lobe and central posterior cingulate regions. The frontal voxel was positioned to overlap with patient-specific activation on a 1-back working memory task.
Results:
Increased GABA/Cre was shown in the frontal lobe for the concussed group. A decreased relationship was observed in the parietal region. High correlations between GABA/Cre and task activation were observed for the control group in the frontal lobe, a relationship not shown in the concussed participants.
Conclusions:
GABA/Cre appears increased in a region colocalized with working memory task activation after sport concussion. Further work extending these results in larger samples and at time points across the injury episode will aid in refining the clinical significance of these observations.
Traumatic brain injury has been the subject of extensive MRI study over the past 20 years. While much of this work has focused on moderate and severe injury as defined by Glasgow Coma Scale score on admission, a growing literature focuses on milder traumatic brain injury defined as Glasgow Coma Scale score >13 to 15 with no loss of consciousness caused by sport mechanisms.1 Indeed, many new studies now focus on the most mild spectrum of injury, often called exposure.2 In sport terms, this is defined as the potential, counted, or measured head blows (e.g., football participation, soccer heading, sensor triggering) without necessitating a diagnosis of concussion.3 Although this injury category of risk represents the goal state for defining injury at its subtlest manifestation, the potential for outliers driving generalizations4 and whether head injury rotational mechanics can be adequately quantified via sensor technologies5 remain work for further optimization.
Commonly used imaging techniques of brain injury focus on structural or connectivity measures6 and task-based fMRI used to look at known vulnerable systems such as working memory.7 A growing body of literature outside traumatic injury aims to integrate fMRI and neurotransmitter results, with a focus on γ-aminobutyric acid (GABA) because of its measurement specificity. To evaluate the utility of this measure in children diagnosed with concussion and having persistent symptoms at clinic visit, we enrolled a pilot cohort of children with concussion compared with age- and sex-matched healthy controls.
METHODS
Participants were recruited from patients seen at the Seattle Children's Hospital Sports Concussion Program and diagnosed according to the 2012 Zurich consensus statement.8 Healthy controls were recruited from the Seattle Children's Hospital Sports Medicine program.
Recruitment.
One hundred eighty children were screened at clinic visits for inclusion in this study. Twenty-three were enrolled, with MRI data collected on 11 concussed children with persisting symptoms (5 male, 6 female, all right-handed, age 15.2 ± 1.2 years) and 11 healthy controls matched for age and sex who had sport-related injuries not involving the head and no history of concussion (age 15.2 ± 1.2 years, 2 left-handed). The sport activities related to injury for those participants enrolled in the concussion group were as follows: physical education (2), weight lifting (1), football (1), soccer (4), lacrosse (1), ultimate Frisbee (1), and skateboarding (1). Symptoms were assessed at the clinic visit with the Sport Concussion Assessment Tool 3 http://bjsm.bmj.com/content/47/5/259 with chart notes also reviewed. Neuropsychological testing at the time of MRI (Wechsler Intelligence Scale for Children, Processing Speed, Digit Span, Delis-Kaplan Executive Function System, Symbol Digit Modalities Test) was an optional component of this single-time point study with 6 concussed and 9 control children volunteering for testing.
Standard protocol approvals, registrations, and patient consents.
Informed consent for all participants and parents was obtained in accordance with the protocol approved by the Seattle Children's Hospital Institutional Review Board.
MRI study.
Participants underwent a single imaging session on a Siemens 3T PRISMA scanner (Siemens, Erlangen, Germany) using a 64-channel head coil. Structural imaging included 3-plane localizers, a high-resolution sagittal T1 magnetization-prepared rapid gradient echo (512 × 512 matrix, echo time [TE] 3.5 milliseconds, repetition time [TR] 1,650 milliseconds, isotropic 1-mm resolution, 160 slices), axial susceptibility-weighted imaging (672 × 768, TE 20 milliseconds, TR 28 milliseconds, 1.6-mm thickness yielding phase and magnitude maps), field map (64 × 64 matrix, TE 5.2/7.7 milliseconds, TR 488 milliseconds, 3-mm thickness), and axial diffusion tensor imaging (DTI) scan (b value 1000, 10 directions, field of view 230, slice thickness 3 mm, 44 slices; matrix = 128 × 128, TE = 65 milliseconds, TR = 4,600 milliseconds). Next, an axial fMRI acquisition (64 × 64 matrix, TE = 30 milliseconds, TR = 3,000 milliseconds, 45 interleaved slices, 80 acquisitions) was performed with a working memory 1-back task with items (upper and lower case letters) presented at a rate of 2 seconds on a liquid-crystal display screen that was visible via a mirror mounted on a head coil. Left frontal lobe activation was identified from activation vs rest blocks (30 seconds) with maps generated in real time. Participants responded to the task via button press with the right hand denoting correct or incorrect response. These responses and latencies were written to a text file for offline analyses.
Two spectroscopy acquisitions followed fMRI: one localized in left frontal lobe as guided by the activation map generated from the working memory task and displayed on the scanner, and another placed in the central posterior cingulate cortex. Both acquisitions used MEGA-PRESS (Siemens Medical Systems, Malvern, PA) with a 3 × 3 × 3-cm voxel, TE of 68 milliseconds (with and without frequency selection), TR of 2,000 milliseconds, and 128 averages. Lastly, resting-state fMRI with eyes open was collected at slice positions similar to the task-based scan and slightly varying parameters (TE 27 milliseconds, TR 2,000 milliseconds, 33 interleaved slices, 192 acquisitions).
Analyses.
MRIs were reviewed clinically by the study radiologist (D.W.S.). Automated processing of MEGA-PRESS data was done by Gannet.9 Magnetic resonance spectroscopy (MRS) data with off-resonance editing were processed in LCModel10 to generate non-GABA metabolite estimates. To measure the gray/white ratio underlying each measurement, T1 images were segmented in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/; UCL, London, UK) and masked by the spectral voxel location extracted from the raw spectral file with MATLAB (Mathworks, Natick, MA). Automated summation of pixels for each tissue class yielded gray/white ratio data. These masks were also multiplied by fMRI working memory activation maps and summarized to yield measures of median activation. DTI data were analyzed in batch in FSL (http://fsl.fmrib.ox.ac.uk/), and resting-state FMRI data were analyzed with CONN (https://www.nitrc.org/projects/conn/) as per our prior work.11,12
Statistics.
Paired parametric statistics were compared for GABA/creatine + phosphocreatine (Cre), gray/white fraction, and task performance. Regression analyses were performed to compare the association between GABA and median voxel fMRI task activation. Output maps from FSL (DTI) and CONN (resting fMRI) were examined for significant effects at p < 0.05 corrected for multiple comparisons without a priori masking. Exploratory analyses were performed for other MRS metabolites and neuropsychological testing data.
RESULTS
One control had missing data because of claustrophobia, precluding MRI scanning, and 1 had missing latency performance data as a result of computer malfunction. One concussed patient’s data were excluded because of motion artifact on MRI data and MRS failure. This yielded 10 (5 male, 5 female) participants per group. The clinic visit occurred 12 ± 8 days (minimum = 2, maximum = 28) after injury, with Sport Concussion Assessment Tool 3 symptoms ranging from 10 to 88 (mean 48 ± 25, maximum = 132). Symptoms described in the chart notes for the concussed group were headaches (10 of 10), feeling in a fog (6 of 10), difficulty concentrating (9 of 10), confusion (6 of 10), and fatigue (8 of 10). MRI examination occurred at a mean of 18 days (SD 7, minimum 8, maximum 29 days) after the clinic visit. Measured from the date of injury, MRIs occurred for the group at a group recovery time point of ≈1 month (mean 30.4 ± 6.1, minimum = 23, maximum = 44 days).
MRI study.
No structural abnormalities, including any suggestive of traumatic brain injury (e.g., diffusion alterations, punctate hemorrhage, gliosis), were observed on the structural imaging scans.
During the fMRI working memory scan, task percent correct and latency of response did not differ between groups (table). For spectroscopy data, no statistical differences were observed for Gannet fit error or gray/white voxel fractions by anatomic location. Analyses of GABA/Cre in the frontal lobe demonstrated increases for the concussed group compared to healthy controls (table and figure 1). In contrast, the parietal cingulate cortex voxel showed decreased values in the concussion sample but not below the threshold of p < 0.05. Frontal lobe regression comparing group × GABA/Cre and fMRI median activation measures demonstrated an interaction (β = 4.96, t = 2.74, p = 0.015), with concordance between the control data (t = 3.35, p = 0.010) but not the concussed patients (t = −0.28, p = ns). Plots with correlation coefficients are shown in figure 2.
Table.
Means of GABA/Cre measures, gray/white fractions, and percent correct/latency of the 1-back task
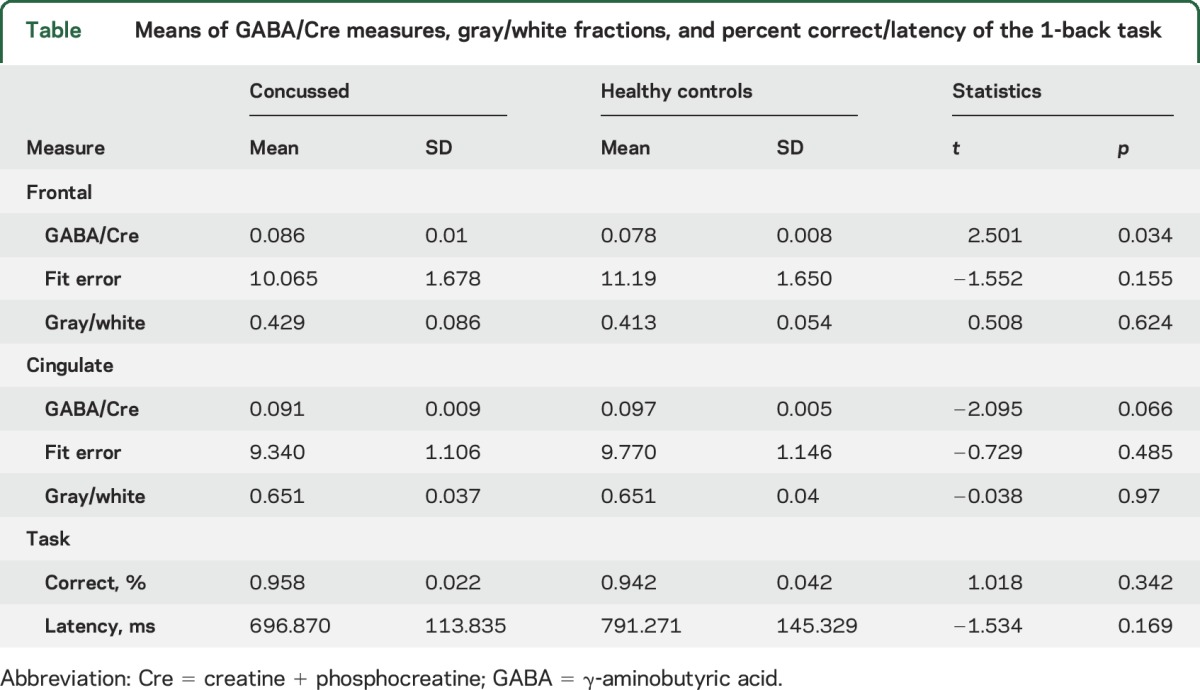
Figure 1. GABA in frontal lobe.
(A) Gannet output showing edited spectrum and (B) fitted GABA peak. (C) Concussed participants demonstrated elevated GABA/Cre ratio in frontal lobe as shown on the right. Cre = creatine + phosphocreatine; GABA = γ-aminobutyric acid.
Figure 2. GABA vs fMRI.
Plot comparing GABA/Cre and median activation in the frontal voxel for healthy controls (open circles) and concussed participants (solid circles) with linear terms plotted. High concordance is shown between measures in the healthy controls but not for the concussed group. Descriptive examination of concussed individuals who seem to have become shifted off the control regression line did not reveal clear descriptive differences in injury type, location, or severity that would explain the results. Cre = creatine + phosphocreatine; GABA = γ-aminobutyric acid.
Exploratory analyses of choline-containing compounds, N-acetylaspartate, or glutamate referenced to Cre in the off-resonance spectrum demonstrated no differences for any metabolite by group (all t < 1.18 and >−1.4, p > 0.2). A similar lack of group differences was shown for the DTI data (measures of fractional anisotropy and mean diffusivity) by tract-based spatial statistics in FSL, resting-state fMRI connectivity matrices in CONN, and neuropsychological test scores in the subgroups receiving testing (all p > 0.05 corrected for multiple comparisons).
DISCUSSION
In this pilot sample of teenagers with first-time concussions and persisting symptoms, frontal lobe GABA levels were elevated 1 month after injury. Supportive of this finding, the normally consistent relationship between GABA and working memory fMRI activation seen in the control group was disrupted. In contrast, decreased GABA was seen in medial parietal areas for the concussed group, a system that has broad functional implications for connectivity. Ascribing a theoretical basis to the direction of change is challenging. What we are seeing at this single postacute time point could be directly related to injury, recovery (a responsive change), or a mixture thereof, none of which can be discriminated without further longitudinal data. In terms of clinical relevance, GABA/Cre alterations after concussion could help to measure the arc of injury and recovery. Evaluating such measures in the context of “return to learn,” cognitive rest, or symptom resolution will be exciting avenues to explore in future work.
GABA is the major inhibitory neurotransmitter and is an important factor in both normal cognitive function and the sequelae of traumatic brain injury.13 While spectroscopy has now been widely applied in traumatic brain injury, primarily to look for markers of neuronal loss before the development of DTI14 (for review, see reference 15), to the best of our knowledge, no study to date has evaluated changes in GABA. Relative to structural markers of injury (e.g., N-acetylaspartate and choline-containing compounds16), GABA is unique in that levels have been shown to be associated with cognitive functioning. For example, correlation of magnetoencephalographymeasures of gamma band activity (likely mediated by GABAa17), fMRI, and GABA was demonstrated in the visual cortex in response to a visual discrimination tasks.18,19 Similar findings have been found for motor control and GABA in the supplementary motor area,20 tactile discrimination and GABA in the sensorimotor cortex,21 and gaze shifting and GABA levels in the frontal eye fields.22 In 2 of these studies, control regions, chosen to assess cortical GABA levels remote from the site of interest, demonstrated no concordance to performance.21,22 Although GABA level changes do no incorporate measurement of other factors (e.g., receptor density changes, enzyme alterations), the concordance between GABA and discrete task performance supports the need to evaluate this biomarker in conditions in which structural changes may be difficult to characterize.
Evidence on a cellular level also supports that GABA may be a particularly important marker of injury and recovery in concussion. Alterations in GABA, especially GABA transport, have been seen in trauma models, both fluid percussion injury23 and shear injury to the developing brain.24 GABA-mediated inhibition can increase after injury such as stroke in response to transport and receptor dysregulation, and the corresponding increase in tonic inhibition may be deleterious to recovery from injury.25 Intervention, by pharmacologic inhibition of specific GABA subunits, improved motor function recovery in an animal model.25 This is a proposed mechanism for impaired recovery after cortical injury, with pharmacologic interventions potentially available to reverse this process.26 GABA changes may alter postconcussive motor plasticity.27 However, excessive impairment in GABA can also lead to deleterious effects such as seizures, and the understanding of the complexities of GABA after injury is critical to guiding future interventions.28 It is plausible that our finding of elevated GABA is imaging evidence of changes known to occur with cellular injury.23,24,28 The selective change in the frontal lobe would be consistent with the increased vulnerability of this region to traumatic injury.
The potential that GABA provides a protective effect or corresponds to the postconcussive cognitive symptoms or impairment often observed remains unresolved from this work. While no child in the study was experiencing headaches that would be classified as migraines, literature supporting GABA elevations in this condition provides interesting fodder for consideration.29 If elevated GABA occurs as a byproduct of injury, it could be envisioned to influence local activity. In general, elevated GABA is associated with improved tuning and performance, suggesting that GABA elevation could be the result of recovery attempts or possibly increased cognitive effort to perform the task as well as healthy controls. Somewhat concordant with this idea, increased activation in the frontal lobe has been described after injury or in impaired states.30 The correlation between GABA and fMRI activation has been studied,21,31–33 with how well abnormal tissue fits these associations not known. Our GABA voxels included areas outside the strongest activation, but we did find a GABA/fMRI ratio in the frontal lobe that was consistent across the healthy control sample and much higher, and varied, in the concussion group. Elevated GABA was not seen in the other area studied, the medial parietal lobe. Thus, there is some selectivity to the GABA elevation, suggesting that it is related to the function of this brain region or perhaps the task load being applied. This seems possible but, without differences in behavioral performance between groups, premature to conclude. Whether the GABA elevation is causal or only indirectly associated with postconcussive symptoms, it may provide an objective measure for following disease progression and offering a target for modulation.
GLOSSARY
- Cre
creatine + phosphocreatine
- DTI
diffusion tensor imaging
- GABA
γ-aminobutyric acid
- MRS
magnetic resonance spectroscopy
- TE
echo time
- TR
repetition time
AUTHOR CONTRIBUITONS
Seth D. Friedman: designed and conceptualized the study, performed data collection and analyses, wrote the manuscript. Andrew V. Poliakov: set up the fMRI task, oversaw data collection, analyzed DTI and fMRI data, participated in writing the manuscript. Christopher Budech: oversaw patient evaluation by MRI/MRS and neuropsychological testing, contributed to manuscript editing. Dennis W.W. Shaw: evaluated images for trauma features, participated in manuscript editing. David Breiger: oversaw neuropsychological evaluation and related analyses integrated into manuscript. Thomas Jinguji: oversaw enrollment, criteria for inclusion, manuscript drafting. Brian Krabak: participated in study enrollment, revision of manuscript. David Coppel: oversaw neuropsychological evaluation and related analyses, helped to revise final manuscript. Tressa Mattioli Lewis: responsible for patient enrollment, inclusion criteria revision, paper editing. Samuel Browd: participated in clinical oversight and general oversight of study activities. Jeffrey G. Ojemann: designed and conceptualized the study, performed critical interpretation of data, and wrote the manuscript.
STUDY FUNDING
This work was conducted with a grant from the NIH (5R03NS08650-02) and work-in-progress MEGA-PRESS software package from Siemens Medical Systems.
DISCLOSURE
The authors reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med 2013;47:15–26. [DOI] [PubMed] [Google Scholar]
- 2.Daniel RW, Rowson S, Duma SM. Head impact exposure in youth football. Ann Biomed Eng 2012;40:976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chrisman SP, Mac Donald CL, Friedman S, et al. Head impact exposure during a weekend youth soccer tournament. J Child Neurol 2016;31:971–978. [DOI] [PubMed] [Google Scholar]
- 4.Bahrami N, Sharma D, Rosenthal S, et al. Subconcussive head impact exposure and white matter tract changes over a single season of youth football. Radiology 2016;281:919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegmund GP, Guskiewicz KM, Marshall SW, DeMarco AL, Bonin SJ. Laboratory validation of two wearable sensor systems for measuring head impact severity in football players. Ann Biomed Eng 2016;44:1257–1274. [DOI] [PubMed] [Google Scholar]
- 6.Hannawi Y, Stevens RD. Mapping the connectome following traumatic brain injury. Curr Neurol Neurosci Rep 2016;16:44. [DOI] [PubMed] [Google Scholar]
- 7.Dunning DL, Westgate B, Adlam AL. A meta-analysis of working memory impairments in survivors of moderate-to-severe traumatic brain injury. Neuropsychology 2016;30:811–819. [DOI] [PubMed] [Google Scholar]
- 8.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport, Zurich, November 2012. J Athl Train 2012;48:554–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging 2014;40:1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672–679. [DOI] [PubMed] [Google Scholar]
- 11.Ishak GE, Poliakov AV, Poliachik SL, et al. Tract-based spatial statistical analysis of diffusion tensor imaging in pediatric patients with mitochondrial disease: widespread reduction in fractional anisotropy of white matter tracts. AJNR Am J Neuroradiol 2012;33:1726–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poliachik SL, Friedman SD, Poliakov AV, et al. Corpus callosum diffusion and connectivity features in high functioning subjects with pyridoxine-dependent epilepsy. Pediatr Neurol 2016;54:43–48. [DOI] [PubMed] [Google Scholar]
- 13.Guerriero RM, Giza CC, Rotenberg A. Glutamate and GABA imbalance following traumatic brain injury. Curr Neurol Neurosci Rep 2015;15:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman SD, Brooks WM, Jung RE, Hart BL, Yeo RA. Proton MR spectroscopic findings correspond to neuropsychological function in traumatic brain injury. AJNR Am J Neuroradiol 1998;19:1879–1885. [PMC free article] [PubMed] [Google Scholar]
- 15.Croall I, Smith FE, Blamire AM. Magnetic resonance spectroscopy for traumatic brain injury. Top Magn Reson Imaging 2015;24:267–274. [DOI] [PubMed] [Google Scholar]
- 16.Brooks WM, Stidley CA, Petropoulos H, et al. Metabolic and cognitive response to human traumatic brain injury: a quantitative proton magnetic resonance study. J Neurotrauma 2000;17:629–640. [DOI] [PubMed] [Google Scholar]
- 17.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 2007;8:45–56. [DOI] [PubMed] [Google Scholar]
- 18.Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci 2009;29:15721–15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muthukumaraswamy SD, Evans CJ, Edden RA, Wise RG, Singh KD. Individual variability in the shape and amplitude of the BOLD-HRF correlates with endogenous GABAergic inhibition. Hum Brain Mapp 2012;33:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boy F, Evans CJ, Edden RA, Singh KD, Husain M, Sumner P. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr Biol 2010;20:1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puts NA, Edden RA, Evans CJ, McGlone F, McGonigle DJ. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J Neurosci 2011;31:16556–16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sumner P, Edden RA, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat Neurosci 2010;13:825–827. [DOI] [PubMed] [Google Scholar]
- 23.Raible DJ, Frey LC, Cruz Del Angel Y, Russek SJ, Brooks-Kayal AR. GABA(A) receptor regulation after experimental traumatic brain injury. J Neurotrauma 2012;29:2548–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dzhala V, Valeeva G, Glykys J, Khazipov R, Staley K. Traumatic alterations in GABA signaling disrupt hippocampal network activity in the developing brain. J Neurosci 2012;32:4017–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature 2010;468:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pangilinan PH, Giacoletti-Argento A, Shellhaas R, Hurvitz EA, Hornyak JE. Neuropharmacology in pediatric brain injury: a review. PM R 2010;2:1127–1140. [DOI] [PubMed] [Google Scholar]
- 27.De Beaumont L, Tremblay S, Poirier J, Lassonde M, Theoret H. Altered bidirectional plasticity and reduced implicit motor learning in concussed athletes. Cereb Cortex 2012;22:112–121. [DOI] [PubMed] [Google Scholar]
- 28.Imbrosci B, Mittmann T. Functional consequences of the disturbances in the GABA-mediated inhibition induced by injuries in the cerebral cortex. Neural Plast 2011;2011:614329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aguila ME, Lagopoulos J, Leaver AM, et al. Elevated levels of GABA+ in migraine detected using (1) H-MRS. NMR Biomed 2015;28:890–897. [DOI] [PubMed] [Google Scholar]
- 30.Maccotta L, Buckner RL, Gilliam FG, Ojemann JG. Changing frontal contributions to memory before and after medial temporal lobectomy. Cereb Cortex 2007;17:443–456. [DOI] [PubMed] [Google Scholar]
- 31.Balz J, Keil J, Roa Romero Y, et al. GABA concentration in superior temporal sulcus predicts gamma power and perception in the sound-induced flash illusion. Neuroimage 2016;125:724–730. [DOI] [PubMed] [Google Scholar]
- 32.Harris AD, Puts NA, Anderson BA, et al. Multi-regional investigation of the relationship between functional MRI blood oxygenation level dependent (BOLD) activation and GABA concentration. PLoS One 2015;10:e0117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang GY, van Eijk J, Demirakca T, et al. ACC GABA levels are associated with functional activation and connectivity in the fronto-striatal network during interference inhibition in patients with borderline personality disorder. Neuroimage 2016;147:164–174. [DOI] [PubMed] [Google Scholar]




