Abstract
Objective:
To determine whether multiparametric MRI data can provide insight into the acute and long-lasting neuronal sequelae after a concussion in adolescent athletes.
Methods:
Players were recruited from Bantam hockey leagues in which body checking is first introduced (male, age 11–14 years). Clinical measures, diffusion metrics, resting-state network and region-to-region functional connectivity patterns, and magnetic resonance spectroscopy absolute metabolite concentrations were analyzed from an independent, age-matched control group of hockey players (n = 26) and longitudinally in concussed athletes within 24 to 72 hours (n = 17) and 3 months (n = 14) after a diagnosed concussion.
Results:
There were diffusion abnormalities within multiple white matter tracts, functional hyperconnectivity, and decreases in choline 3 months after concussion. Tract-specific spatial statistics revealed a large region along the superior longitudinal fasciculus with the largest decreases in diffusivity measures, which significantly correlated with clinical deficits. This region also spatially intersected with probabilistic tracts connecting cortical regions where we found acute functional connectivity changes. Hyperconnectivity patterns at 3 months after concussion were present only in players with relatively less severe clinical outcomes, higher choline concentrations, and diffusivity indicative of relatively less axonal disruption.
Conclusions:
Changes persisted well after players' clinical scores had returned to normal and they had been cleared to return to play. Ongoing white matter maturation may make adolescent athletes particularly vulnerable to brain injury, and they may require extended recovery periods. The consequences of early brain injury for ongoing brain development and risk of more serious conditions such as second impact syndrome or neural degenerative processes need to be elucidated.
Concussion remains a critical health problem, and the developing brain may be uniquely susceptible.1 In Canada, hockey-related concussions account for nearly half of all sports team–related concussions and disproportionately affect adolescents.2 The neuronal mechanisms that underlie concussion remain poorly understood. A complex cascade of structural, functional, and metabolic changes are secondary to rotational acceleration of the brain.3 MRI studies have reported neuronal vulnerabilities that persist after athletes have been cleared to return to play.4–7 Without full recovery, players may have increased susceptibility to injury and may be at risk of serious conditions such as second impact syndrome.8 Therefore, it is essential to understand the immediate effects of concussion on the adolescent brain and the nature of any long-lasting neuronal changes detectable with noninvasive multiparametric imaging.
In this study, concussed adolescent male hockey players were evaluated longitudinally after concussion and compared to independent, age-matched nonconcussed players as controls. While clinical measures in this population indicate recovery and prompt return to play, given previous imaging findings, we hypothesized that there would be persistent MRI-detectable neuronal changes that reflect long-lasting damage to white matter fibers and compensatory recovery mechanisms. Given this multiparametric MRI dataset, we were able to directly relate resting-state (RS-fMRI) changes with underlying structural abnormalities through diffusion tensor imaging (DTI),9 brain metabolic changes through magnetic resonance spectroscopy (MRS), and acute clinical deficits.
METHODS
Players diagnosed with a mild traumatic brain injury or concussion (age 13.3 ± 0.6 years) were assessed within 24 to 72 hours of their injury (n = 17) and again 3 months later (n = 14). An independent group of healthy, age-matched, nonconcussed male hockey players were assessed and acted as our controls (n = 26, age 13.0 ± 1 years). Portions of this study design have been described previously.10 Further clinical methods are described in appendix e-1 at Neurology.org. The numbers of included participants for each portion of the study are summarized in table e-1. MRI acquisition parameters are outlined in appendix e-2.
Standard protocol approvals, registrations, and patient consents.
Ethics approval was obtained through the University of Western Ontario's Health Sciences Research Ethics Board. All participants and parents/guardians provided informed written consent.
RS-fMRI analysis.
RS-fMRI data were analyzed with FSL (FMRIB Software Library) 5.0.6 (Oxford, England) with 2 major aims: to investigate RS network (RSN) reorganization and whole-brain region-to-region connectivity patterns. Standard preprocessing steps included brain extraction, a high-pass filter (0.01 Hz), spatial smoothing with a 5-mm full-width half-maximum gaussian filter, and motion correction using linear image registration relative to the middle volume. Data with excessive motion (>1-mm maximum displacement or >0.5-mm relative mean displacement) were excluded from further analyses. Preprocessed images were denoised with single-session independent component analysis and automatic dimensionality estimation. Components that were designated as noise (related voxels outside the brain, within the corticospinal fluid, primarily high-frequency profile, or motion artifacts) were regressed from the data. Each dataset was registered to standard space with a 2-stage affine registration technique that first registered the low-resolution functional data to the brain-extracted anatomic image and then to a 2-mm isotropic Montreal Neurologic Institute T1-weighted reference image using 12 df.
Multisession temporal concatenation–independent component analysis was used to create average RSN maps followed by dual regression to generate session-specific networks.11 In particular, the visual occipital pole, default mode (DMN), sensorimotor, executive control, and cerebellar networks were investigated. The FSL randomize permutation-testing tool was implemented to assess group differences according to a general linear model with threshold-free cluster enhancement and multiple-comparison Bonferroni-corrected p values (p < 0.001). In addition, a regional connectivity analysis was performed on the preprocessed data with the CONN toolbox12 because of the statistical advantages as it complements the network analysis and expands to look at whole-brain regional connectivity. Participant-specific CSF and white matter masks were used as regressors. Data were parcellated into 136 functionally relevant regions with the Harvard-Oxford cortical atlas and cerebellar regions with the Automated Anatomical Labeling atlas. Whole-brain region-to-region connectivity differences were assessed with false discovery rate correction.
Diffusion tensor and tractography analysis.
Raw diffusion datasets were corrected for eddy current distortions and relative head motion with the eddy command line tool. The non–diffusion-weighted (b = 0) volume was used to create a modestly extracted brain mask. A diffusion tensor was fit voxel-wise with the use of specific inputs of the gradient directions and b values (b = 1,000 s/mm2) directly from the scanner. Several diffusion metric spatial maps were created, including fractional anisotropy (FA) and mean (MD), axial (AD), and radial (RD) diffusivity. With the use of the John Hopkins University high-probability white matter tractography atlas,13 these DTI metrics were extracted and analyzed within the superior longitudinal fasciculus (SLF), cingulum, forceps major and minor of the corpus callosum, uncinate fasciculus, corticospinal tract (CST), anterior thalamic radiation, and inferior fronto-occipital and longitudinal fasciculus.
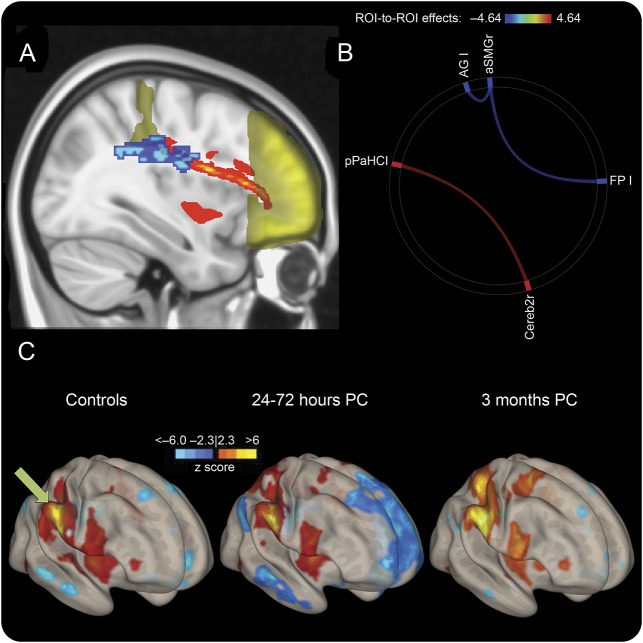
Once tracts with significant changes were identified with a multivariate analysis of variance, an iterative randomization tool within FSL was used to spatially quantify where the greatest changes occurred along those tracts while regressing for age. DTI metrics within the regions with the greatest changes were extracted and analyzed. These regions are referred to as DTImax and are described in Results. Probabilistic tractography with iterative Monte Carlo simulations of principal diffusion tensor vectors was used to quantify a probabilistic streamline between regions of interest (ROIs). Probabilistic structural connections were investigated between relevant parceled RS-fMRI regions to spatially relate structural and functional changes. To quantify these results, 2 identical spherical ROIs (5-mm radius) were placed before and after DTImax (that overlapped along the probabilistic tract connecting acute RS-fMRI ROIs that we identified, figure 1A). The probability distribution was displayed per participant and quantified the probability of a structural connection between the seed and waypoint masks (normalized by the total number of tracts modeled).
Figure 1. Acute connectivity changes spatially relate to axonal damage.
(A) Frontal pole (FP) and anterior supramarginal gyrus (aSMG) are shown in yellow, with the probabilistic tractography connecting them shown in red. DTImax region is overlaid in blue, showing the spatial relationship between structural abnormalities and resting-state fMRI connectivity. (B) Significant whole-brain region-to-region connectivity differences between controls and the 24- to 72-hour postconcussion (PC) group. Blue indicates a decrease and red indicates an increase in region-to-region connectivity. (C) Voxel-wise connectivity pattern using the right aSMG (green arrow) as a seed, displaying the enhanced anticorrelation (blue) with the FP and regions that mimic the default mode network. AG = angular gyrus; pPaHC = posterior parahippocampus; ROI = region of interest.
MRS analysis.
Spectra were postprocessed with in-house software to measure absolute N-acetylaspartate, choline, creatine, glutamate, glutamine, and myo-inositol as previously described.14–16 Briefly, analysis software created in our laboratory in IDL (version 5.4, Research Systems Inc, Boulder, CO) was used to correct spectral line shapes by combined QUALITY deconvolution and eddy current correction.17 Spectra were fitted in the time domain with the use of a Levenberg-Marquardt minimization routine14 with prior knowledge of metabolic line shapes. Prior knowledge was acquired from in vitro spectra obtained from aqueous solutions of metabolites before the study.14 We report absolute metabolite levels using unsuppressed water from the ROI as an internal standard as previously described in detail.14,16 In addition, absolute concentrations included a correction for tissue partial volume and T1 and T2 relaxation–related signal loss.
Statistical analysis.
Statistical analysis was performed with a multivariate analysis of variance with Tukey post hoc testing using GraphPad Prism (version 6.0). A hypothesis-driven 2-tailed Pearson correlation analysis was explored between data that had a significant effect for group (p < 0.05 after false discovery rate correction).
RESULTS
Clinical results are summarized in appendix e-3 and figure e-1.
RS-fMRI results.
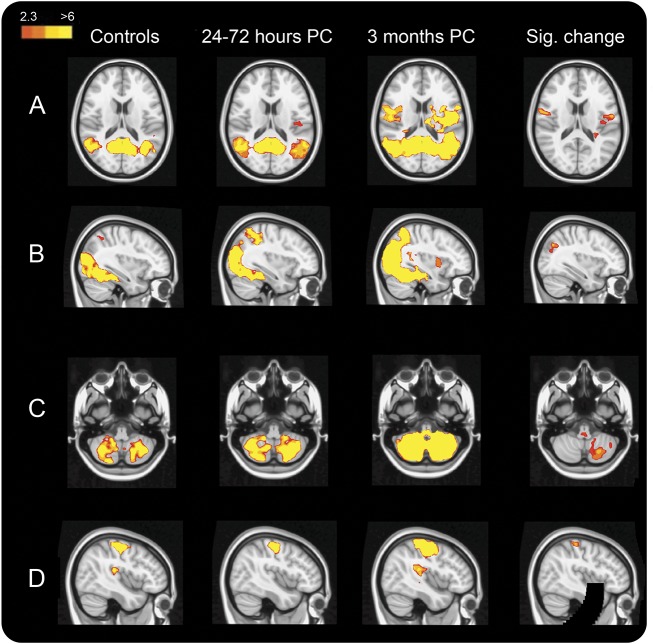
We found significant increases in connectivity at 3 months after concussion in several RSNs. In particular, there was significantly increased connectivity at 3 months after concussion compared to controls with the occipital pole visual network and cerebellar network (figure 2, B and C). Compared to 24 to 72 hours after concussion, there were significant increases in connectivity by 3 months with the sensorimotor network (figure 2D) and cerebellar network. There was increased DMN connectivity at 3 months compared to controls that did not survive statistical Bonferroni correction (p < 0.01, figure 2A), and no significant differences were found within the executive control network or between controls and the 24- to 72-hour postconcussion group in any network.
Figure 2. RSN hyperconnectivity at 3 months after concussion.
Each column represents the average resting-state network (RSN) for each of the 3 groups (scaled by z statistic), with areas that are significantly more highly correlated with that network at 3 months compared to (A–C) controls or (D) the 24- to 72-hour postconcussion (PC) group (p < 0.001 except for [A] where p < 0.01). Results are shown for the (A) default mode network, (B) occipital pole visual RSN, (C) cerebellar RSN, and (D) sensorimotor RSN.
There were a few subtle changes in region-to-region connectivity within 24 to 72 hours after concussion compared to controls (figure 1B); however, some were striking with a large effect size (>0.8) and prompted further analysis to directly relate with our DTI findings (figure 1C). Specifically, there were significant anticorrelations between the anterior supramarginal gyrus and areas that mimic the DMN, including the angular gyrus and frontal pole (p < 0.05, including portions of the medial prefrontal cortex, figure 1C). This was not found in our network analysis, possibly as a result of statistical power (voxel-level correction) or the dominance of the frontal pole. There was an increase in regional connectivity immediately after concussion compared to controls between the cerebellum and parahippocampal gyrus. The significant whole-brain region-to-region connectivity changes are shown in figure 3, and similar to the RSN results, there were increases in connectivity at 3 months compared to both the control and 24- to 72-hour postconcussion groups between regions that complimented our network-level connectivity changes.
Figure 3. Region-to-region hyperconnectivity at 3 months.
Significantly increased connectivity (red lines) is shown using (A) connectome rings with regions labeled around the perimeter and (B) 3-dimensional brain volumes in which the color of the spheres and the transparency of the connecting lines indicate the strength of the effect using a t statistic after false discovery rate correction (corrected p < 0.05). aSMG = anterior supramarginal gyrus; Cereb = cerebellum; CO = central opercular cortex; dmn.MPFC = default mode network medial prefrontal cortex; HG = Heschl gyrus; MedFC = medial frontal cortex; MidFG = middle frontal gyrus; OFusG = occipital fusiform gyrus; OP = occipital pole; PaCiG = paracingulate gyrus; pMTG = posterior middle temporal gyrus; PO = parietal operculum cortex; Pre/PostCG = precentral/postcentral gyri; sLOC = superior lateral occipital cortex; SPL = superior parietal lobule; toITG = temporoccipital inferior temporal gyrus; TP = temporal pole; Ver = vermis.
Diffusion results.
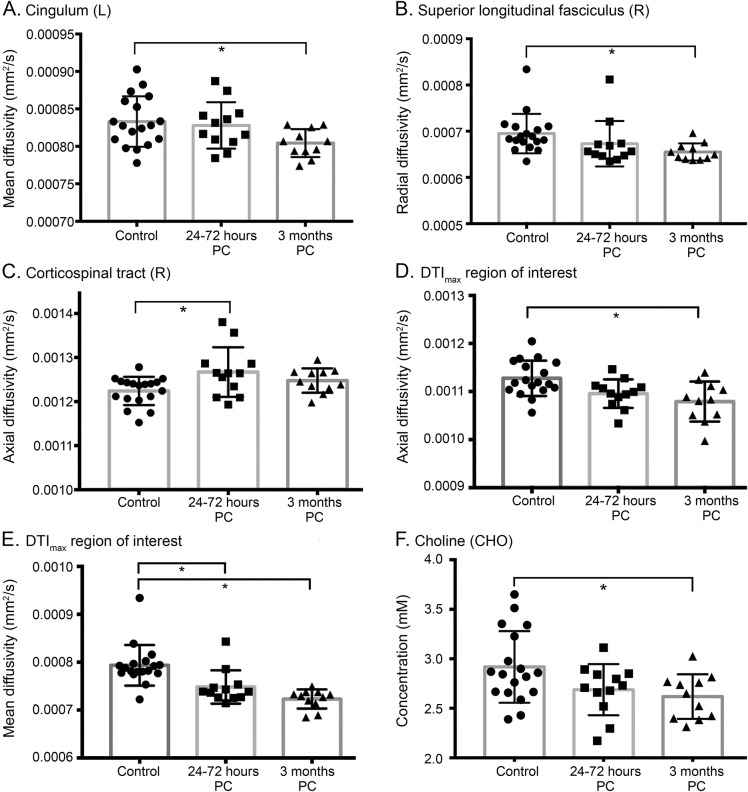
There was a significant main effect for group differences in the CST, cingulum, and SLF (F > 4.18, p < 0.05, figure 4). Along the CST, the greatest diffusion changes were located inferiorly near the brainstem (p < 0.1), and there were small, more scattered maximums along the cingulum (p > 0.1). A large region along the right SLF shown in figure 1 had significant MD, RD, and AD changes at 3 months after concussion (p < 0.01), and a mask was created over this localized hotspot of compromised neuronal integrity, DTImax. Within DTImax, the MD and RD values were significantly decreased at both 24 to 72 hours and 3 months after concussion compared to controls (F > 10.0, p < 0.01); furthermore, there were decreases in AD at both time points (F = 6.67, p = 0.060 and p = 0.003, respectively) with increases in FA by 3 months (F = 3.91, p = 0.025). Using the anterior supramarginal gyrus as a seed and the frontal pole as a waypoint, we confirmed that probabilistic tracts structurally connected regions where we found acute RS-fMRI differences (figure 1). The probability of a structural connection did not significantly decrease after concussion.
Figure 4. Diffusion and MRS results.
(A–E) Average diffusion metrics within standard atlas-derived white matter tracts and (F) absolute metabolite concentrations with the prefrontal white matter voxel. SDs are shown with error bars. *Significant multivariate analysis of variance–corrected post hoc differences (p < 0.05). DTI = diffusion tensor imaging; MRS = magnetic resonance spectroscopy; PC = postconcussion.
Spectroscopy results.
The spectroscopy voxel was placed in the prefrontal region with the following mean ± SD tissue content: gray matter 19% ± 7%, white matter 78% ± 8%, and CSF 3% ± 2%. The tissue composition of the voxel did not significantly change across participants or sessions.
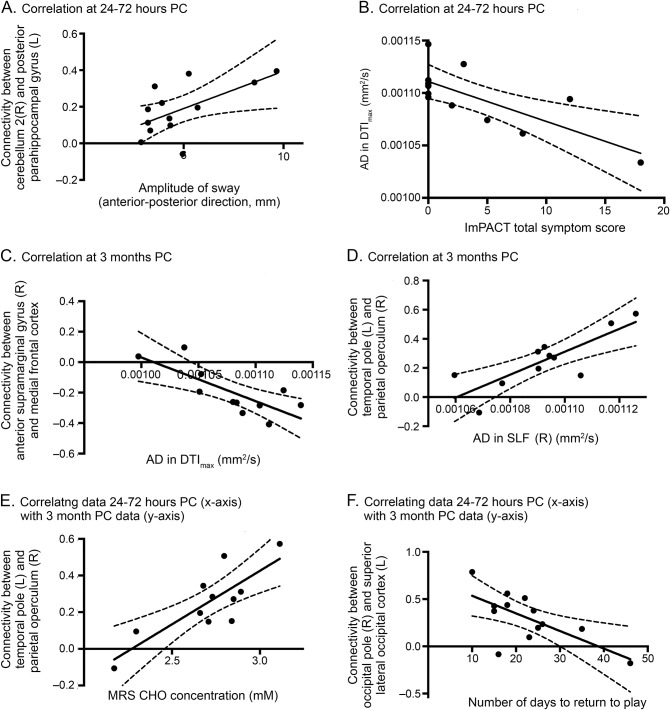
No changes in N-acetylaspartate were found between any of the groups. Choline was significantly reduced at 3 months relative to controls (F = 4.2, p = 0.02, figure 4), with a 10% decrease in the mean observed (p = 0.035). A reduction in glutamine levels was observed but was not significantly different from controls (F = 2.3, p = 0.09). Correlation results are summarized in detail in table e-2, and examples are shown in figure 5.
Figure 5. Examples of correlation results.
Relating data at (A and B) 24 to 72 hours postconcussion (PC), (C and D) 3 months PC, and (E and F) between 24 to 72 hours PC (x-axis) and 3 months PC (y-axis) data. Further details are provided in table e-2. The 95% confidence interval is shown using the dashed curves, and all relationships shown here have p < 0.05 after false discovery rate correction. AD = axial diffusivity; CHO = choline; DTI = diffusion tensor imaging; ImPACT = Immediate Post-Concussion Assessment and Cognitive Testing; MRS = magnetic resonance spectroscopy; SLF = superior longitudinal fasciculus.
DISCUSSION
In this study of adolescent hockey players from Bantam leagues in which body checking is first introduced, we found acute and longitudinal multiparametric MRI changes after concussion compared to controls. As expected, clinical composite scores elevated immediately after concussion, and within weeks of injury, they returned to control levels and players were cleared to return to play. However, we found persistent RS-fMRI connectivity changes, diffusion-related white matter abnormalities, and MRS metabolite decreases in the prefrontal white matter at 3 months after concussion.
The adolescent brain is still not fully developed during this age range (11–14 years), and as it continues to grow and mature, it may be more vulnerable to brain dysfunction and elongated periods of recovery after an acceleration-related injury. It has been shown that this heightened vulnerability may be due to both biomechanical (neck strength) and neurobiological (incomplete white matter myelination) characteristics.18 Axonal excitability and electrophysiologic recordings in a rat fluid percussion brain injury model have been used to assess differences in axonal vulnerability between myelinated and unmyelinated fibers during injury response and functional recovery.19 In this animal model, myelinated axons recovered within 7 days and were relatively protected compared to unmyelinated fibers, which were more severely injured and took longer to recover. It is critical to understand how the adolescent human brain reacts and recovers from concussion. A recent review of concussion-related MRI findings demonstrated the advantages of relating multiple MRI modalities,20 and here we aim to do just that to better understand the meaning of these changes.
We found diffuse changes in DTI metrics along 3 major white matter tracts. However, the tract-specific spatial changes were localized to a single, central region along the SLF (DTImax) where we found decreased AD, MD, and RD at both times after concussion. In vivo animal studies have been able to directly relate histology with MRI diffusion, specifically finding a direct relationship between AD and axonal injury through postmortem neurofilament immunostaining and confirmation of the presence of β-amyloid precursor protein.21 Diffuse axonal pathology has been observed in all traumatic brain injury severities, in which transport is disrupted and axonal bulb formations occur.22 The decreases in AD observed after concussion may reflect this axonal pathology because they relate directly to immediate symptoms. The AD within DTImax at 24 to 72 hours after concussion was significantly correlated with total symptom scores and the number of errors during balance testing, indicating that a more severe injury is associated with more severe axonal damage. This remained true at 3 months after concussion when the sustained decrease in AD within DTImax was associated with a higher symptom severity score.
The most common injury mechanism in our cohort was falling, affecting the back of the head. Investigations of the biomechanical deformations and shearing forces after even minimal acceleration of the human head have reported deformation of the human brain. Rapid deceleration directed to the back of the head results in anterior shortening and posterior elongation, as well as deformations in the axial plane.23 These results correspond to regions where we found diffusion changes indicative of structural damage to the long tracts running anterior to posterior. This acceleration injury and the consequent strain and shearing forces between brain tissues may damage axons themselves. The decrease in AD was concurrent with decreases in RD and MD and increases in FA within the same region. This could reflect different pathologies such as axonal disruption, cytotoxic edema, axonal swelling, or changes in myelin at the site of injury.24 These findings are similar to some previous DTI studies5,9,25–27 but not all.28,29 Cohorts of different ages, injury severity, and acquisition timing all may play a role in the direction of these changing diffusion metrics. Fluid-attenuated inversion recovery and turbo spin echo sequences were used to confirm a mild traumatic brain injury with no cerebral edema. We observed region-to-region RS-fMRI hyperconnectivity at 3 months after concussion compared to both controls and 24 to 72 hours after concussion. This was also true at the network level. Previous studies have variably found this pattern, and hyperconnectivity has been proposed to be involved in recovery and compensation for underlying white matter disruption.4,30–33
Here, we demonstrate the strength of multiparametric MRI by relating our connectivity findings directly to DTI and clinical data. Acute regional connectivity changes with the cerebellum correlated with all of the significant measures reflecting acute postural balance deficits and directly relate the role of the cerebellum in balance performance.34 The average AD along the SLF and within DTImax was correlated with changes in interregional connectivity in which, in general, more axonal injury (lower diffusivity) and a more severe injury as quantified by number and severity of symptoms or more days required to recover were associated with lower absolute values of connectivity. Only those players with a less severe injury as indicated by these acute clinical measures exhibited regional hyperconnectivity at 3 months after concussion, similar to previous work focused on the DMN in more severe and older patients with traumatic brain injury 6 months after their injury.35 This possible recovery mechanism may be compensating for the underlying damaged avenues of structural connections by recruiting regions of connectivity and enhancing both correlated and inhibitory communication. It is possible that some players have not yet reached or completed the recovery phase, and further longitudinal data are needed to understand the timing and potential long-term consequences of these mechanisms.
A 10% reduction in the MRS choline signal was observed at 3 months relative to controls. The choline signal is made up of different components, mainly phosphorylcholine (a precursor to membrane synthesis) and glycerophosphorylcholine (a breakdown product of the membrane).36 This change is particularly interesting because reductions in blood plasma glycerophospholipids at 24 to 72 hours were reported in a subset of these hockey players.10 Other studies have also reported reduced choline levels in the motor cortex after a month of high school football in nonconcussed players.37 This was suggested to be due to a decrease in membrane turnover as a result of repetitive subconcussive impacts. The correlations with the MRS data support similar interpretations. The choline concentration at both 24 to 72 hours and 3 months after concussion was significantly correlated with RS-fMRI connectivity; in particular, a lower choline concentration was associated with a lower absolute interregional connectivity value. Given the relationship between choline concentrations and the clinical, diffusion, and connectivity measures, we hypothesize that the decrease in choline is related to reduced membrane turnover rate.36
There are limitations in the present study that will motivate future MRI investigations of adolescent concussion. The average changes we report here represent a heterogeneous population whose individual injury mechanisms, severities, and recovery times vary. We followed up with players 3 months after their injury; however, the players had clinically recovered and were cleared to return to play at different times. It is possible that returning to play may affect the brain and any ongoing neuroreparative mechanisms. Given the long-lasting changes we found at 3 months, future longitudinal studies should continue to follow this demographic as they develop to understand exactly how long the recovery period lasts as a function of age and the consequences, if any, later in life, including increased vulnerability to future concussions and the development of neurodegenerative diseases such as chronic traumatic encephalopathy.38,39
The persistent changes we observed in this study provide evidence of prolonged axonal disruption in a localized area along the SLF. This result was spatially related to acute RS-fMRI connectivity changes and choline metabolite decreases and correlated significantly with clinical deficits. Diffuse hyperconnectivity patterns were present 3 months after concussion, well after clearance to return to play and symptomatic recovery. This adolescent population may be particularly vulnerable to injury while axons continue to myelinate and mature, and our results suggest that they may require longer recovery periods.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge Sandra Shaw for her efforts in organizing patient appointments. They also thank and commend the players and their parents/guardians for their willingness to participate in this study and the coaches and MRI technicians for their excellent support.
GLOSSARY
- AD
axial diffusivity
- CST
corticospinal tract
- DMN
default mode network
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- MD
mean diffusivity
- MRS
magnetic resonance spectroscopy
- RD
radial diffusivity
- ROI
region of interest
- RS
resting-state
- RSN
resting-state network
- SLF
superior longitudinal fasciculus
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
All authors approved the manuscript and provided critical edits. K.Y.M: manuscript preparation, analysis and interpretation of data, statistical analysis. A.S.: analysis and interpretation of data. R.B.: study concept and design. G.A.D: overall project design. C.B.: study coordinator. A.B.: overall project design. L.F.: overall project design, patient evaluation. K.A.: patient evaluation. T.J.D.: interpretation of data. D.D.F.: overall project design. J.H.: overall project design, clinical acquisition. R.S.M.: overall project and image acquisition design, interpretation of data.
STUDY FUNDING
Funding was provided by a grant from the Children's Health Foundation to Dr. D.D. Fraser and a grant from the Western University Schulich School of Medicine and Dentistry (co-principal investigators: Dr. D.D. Fraser and G.A. Dekaban).
DISCLOSURE
K. Manning and A. Schranz report no disclosures relevant to the manuscript. R. Bartha is funded by CIHR Project Grant 152927, Canadian Consortium on Neurodegeneration in Aging 003658, Natural Sciences and Engineering Research Council of Canada grant 250313-2013, Ontario Institute for Cancer Research Grant 00807 Smarter Imaging Program, Ontario Brain Institute grant ONDRI2013, and an Alzheimer Foundation of London and Middlesex Premier Research Grant. G. Dekaban is funded by CIHR project grant PJT 148651, is co–principal investigator on the London Health Sciences Centre Children's Health Foundation grant LHR F7806 R-13-116 CHRI (funded the work presented in this publication), is co–principal investigator or applicant on 3 additional CIHR grants, and receives funds from the NSE BioCanRx. C. Barreira, A. Brown, L. Fischer, and K. Asem report no disclosures relevant to the manuscript. T. Doherty receives research support from Allergan Canada. D. Fraser receives funding through the Children's Health Foundation. J. Holmes is funded by CIHR operating grant 326065 and an Alzheimer's Association International Research Grant. R. Menon is funded by CIHR Foundation Grant 353372, Natural Sciences and Engineering Research Council of Canada grant 261701-2012, and a Brain Canada Platform Support Grant; has sold a patent to Siemens Healthcare; and receives research support from Siemens Healthineers. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Benz B, Ritz A, Kiesow S. Influence of age-related factors on long-term outcome after traumatic brain injury (TBI) in children: a review of recent literature and some preliminary findings. Restor Neurol Neurosci 1999;14:135–141. [PubMed] [Google Scholar]
- 2.Cusimano MD, Cho N, Amin K, et al. Mechanisms of team-sport-related brain injuries in children 5 to 19 years old: opportunities for prevention. PLoS One 2013;8:e58868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bigler ED, Maxwell WL. Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging Behav 2012;6:108–136. [DOI] [PubMed] [Google Scholar]
- 4.Borich M, Babul AN, Yuan PH, Boyd L, Virji-Babul N. Alterations in resting-state brain networks in concussed adolescent athletes. J Neurotrauma 2015;32:265–271. [DOI] [PubMed] [Google Scholar]
- 5.Mayer AR, Ling JM, Yang Z, Pena A, Yeo RA, Klimaj S. Diffusion abnormalities in pediatric mild traumatic brain injury. J Neurosci 2012;32:17961–17969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maugans TA, Farley C, Altaye M, Leach J, Cecil KM. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics 2012;129:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westfall DR, West JD, Bailey JN, et al. Increased brain activation during working memory processing after pediatric mild traumatic brain injury (mTBI). J Pediatr Rehabil Med 2015;8:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkwood MW, Yeates KO, Wilson PE. Pediatric sport-related concussion: a review of the clinical management of an oft-neglected population. Pediatrics 2006;117:1359–1371. [DOI] [PubMed] [Google Scholar]
- 9.Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma 2007;24:1447–1459. [DOI] [PubMed] [Google Scholar]
- 10.Daley M, Dekaban G, Bartha R, et al. Metabolomics profiling of concussion in adolescent male hockey players: a novel diagnostic method. Metabolomics 2016;12:185. [Google Scholar]
- 11.Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA 2009;106:7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012;2:125–141. [DOI] [PubMed] [Google Scholar]
- 13.Mori S, Oishi K, Faria AV. White matter atlases based on diffusion tensor imaging. Curr Opin Neurol 2009;22:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartha R, Drost DJ, Williamson PC. Factors affecting the quantification of short echo in-vivo 1H MR spectra: prior knowledge, peak elimination, and filtering. NMR Biomed 1999;12:205–216. [DOI] [PubMed] [Google Scholar]
- 15.Kowalczyk I, Duggal N, Bartha R. Proton magnetic resonance spectroscopy of the motor cortex in cervical myelopathy. Brain 2012;135:461–468. [DOI] [PubMed] [Google Scholar]
- 16.Goncalves S, Stevens TK, Doyle-Pettypiece P, Bartha R, Duggal N. N-acetylaspartate in the motor and sensory cortices following functional recovery after surgery for cervical spondylotic myelopathy. J Neurosurg Spine 2016;25:436–443. [DOI] [PubMed] [Google Scholar]
- 17.Bartha R, Drost DJ, Menon RS, Williamson PC. Spectroscopic lineshape correction by QUECC: combined QUALITY deconvolution and eddy current correction. Magn Reson Med 2000;44:641–645. [DOI] [PubMed] [Google Scholar]
- 18.Toledo E, Lebel A, Becerra L, et al. The young brain and concussion: imaging as a biomarker for diagnosis and prognosis. Neurosci Biobehav Rev 2012;36:1510–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeves TM, Phillips LL, Povlishock JT. Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp Neurol 2005;196:126–137. [DOI] [PubMed] [Google Scholar]
- 20.Shin SS, Bales JW, Edward Dixon C, Hwang M. Structural imaging of mild traumatic brain injury may not be enough: overview of functional and metabolic imaging of mild traumatic brain injury. Brain Imaging Behav 2017;11:591–610. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol 2007;205:116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol 2014;246:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayly PV, Cohen TS, Leister EP, Ajo D, Leuthardt EC, Genin GM. Deformation of the human brain induced by mild acceleration. J Neurotrauma 2005;22:845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright AD, Jarrett M, Vavasour I, et al. Myelin water fraction is transiently reduced after a single mild traumatic brain injury: a prospective cohort study in collegiate hockey players. PLoS One 2016;11:e0150215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu Z, Wilde EA, Hunter JV, et al. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. Am J Neuroradiol 2010;31:340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lancaster MA, Olson DV, McCrea MA, Nelson LD, LaRoche AA, Muftuler LT. Acute white matter changes following sport-related concussion: a serial diffusion tensor and diffusion kurtosis tensor imaging study. Hum Brain Mapp 2016;37:3821–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilde EA, McCauley SR, Hunter JV, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 2008;70:948–955. [DOI] [PubMed] [Google Scholar]
- 28.Koerte IK, Kaufmann D, Hartl E, et al. A prospective study of physician-observed concussion during a varsity university hockey season: white matter integrity in ice hockey players, part 3 of 4. Neurosurg Focus 2012;33:E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goetz P, Blamire A, Rajagopalan B, Cadoux-Hudson T, Young D, Styles P. Increase in apparent diffusion coefficient in normal appearing white matter following human traumatic brain injury correlates with injury severity. J Neurotrauma 2004;21:645–654. [DOI] [PubMed] [Google Scholar]
- 30.Czerniak SM, Sikoglu EM, Liso Navarro AA, et al. A resting state functional magnetic resonance imaging study of concussion in collegiate athletes. Brain Imaging Behav 2014;9:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillary FG, Slocomb J, Hills EC, et al. Changes in resting connectivity during recovery from severe traumatic brain injury. Int J Psychophysiol 2011;82:115–123. [DOI] [PubMed] [Google Scholar]
- 32.Iraji A, Benson RR, Welch RD, et al. Resting state functional connectivity in mild traumatic brain injury at the acute stage: independent component and seed based analyses. J Neurotrauma 2014;1045:1–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang CY, Eaves E, Dams-O'Connor K, et al. Diffuse disconnectivity in tBi: a resting state fMri anD Dti study. Transl Neurosci 2012;3:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morton SM, Bastian AJ. Cerebellar control of balance and locomotion. Neurosci 2004;10:247–259. [DOI] [PubMed] [Google Scholar]
- 35.Sharp DJ, Beckmann CF, Greenwood R, et al. Default mode network functional and structural connectivity after traumatic brain injury. Brain 2011;134:2233–2247. [DOI] [PubMed] [Google Scholar]
- 36.Maddock RJ, Buonocore MH. MR spectroscopic studies of the brain in psychiatric disorders. Curr Top Behav Neurosci 2012;11:199–251. [DOI] [PubMed] [Google Scholar]
- 37.Poole VN, Breedlove EL, Shenk TE, et al. Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. Dev Neuropsychol 2015;40:12–17. [DOI] [PubMed] [Google Scholar]
- 38.Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics 2005;116:1374–1382. [DOI] [PubMed] [Google Scholar]
- 39.Daneshvar DH, Riley DO, Nowinski CJ, McKee AC, Stern RA, Cantu RC. Long-term consequences: effects on normal development profile after concussion. Phys Med Rehabil Clin N Am 2011;22:683–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.