Abstract
Objective:
In order to explore the mechanisms of cortical superficial siderosis (cSS) multifocality and its clinical implications for recurrent intracerebral hemorrhage (ICH) risk in patients with cerebral amyloid angiopathy (CAA), we used a new rating method that we developed specifically to evaluate cSS extent at spatially separated foci.
Methods:
Consecutive patients with CAA-related ICH according to Boston criteria from a single-center prospective cohort were analyzed. The new score that assesses cSS multifocality (total range 0–4) showed excellent interrater reliability (k = 0.87). The association of cSS with markers of CAA and acute ICH was investigated. Patients were followed prospectively for recurrent symptomatic ICH.
Results:
The cohort included 313 patients with CAA. Multifocal cSS prevalence was 21.1%. APOE ε2 allele prevalence was higher in patients with multifocal cSS. In probable/definite CAA, cSS multifocality was independently associated with neuroimaging markers of CAA severity, including lobar microbleeds, but not with acute ICH features, which conversely, were determinants of cSS in possible CAA. During a median follow-up of 2.6 years (interquartile range 0.9–5.1 years), the annual ICH recurrence rates per cSS scores (0–4) were 5%, 6.5%, 13.5%, 16.2%, and 26.9%, respectively. cSS multifocality (presence and spread) was the only independent predictor of increased symptomatic ICH risk (hazard ratio 3.19; 95% confidence interval 1.77–5.75; p < 0.0001).
Conclusions:
The multifocality of cSS correlates with disease severity in probable CAA; therefore cSS is likely to be caused by discrete hemorrhagic foci. The new cSS scoring system might be valuable for clinicians in determining annual risk of ICH recurrence.
Sporadic cerebral amyloid angiopathy (CAA) is a common age-related cerebral small vessel disease, characterized by progressive β-amyloid deposition in leptomeningeal and cortical small vessels.1 CAA is a common cause of spontaneous lobar intracerebral hemorrhage (ICH),1,2 especially in older patients. CAA-related lobar ICH is associated with a high risk of recurrence, contributing to the important morbidity and mortality of the disease.
In addition to lobar ICH and strictly lobar cerebral microbleeds (CMBs), cortical superficial siderosis (cSS) has recently been implicated as the third cardinal hemorrhagic neuroimaging signature of CAA and a potential marker for high ICH risk.3 However, the mechanisms of cSS are not clear.3 The prevailing hypothesis is that cSS reflects repeated episodes of hemorrhage into the subarachnoid space from brittle superficial CAA-laden vessels, but the possibility that cSS might represent transport of blood products from remote ICHs cannot be excluded. Experimental data for these hypotheses are missing; hence details on the pathophysiology of cSS remain highly speculative. Furthermore, whether cSS deposits originate from a single bleeding event vs multifocal in situ superficial bleeding events is unknown, but has important implications for future ICH risk stratification. Of note, the currently used scale does not take into account different extents of cSS in each hemisphere.4 Also, the initial validation study demonstrating cSS extent as a marker of ICH recurrence (n = 112 patients with CAA) did not show much of a risk gradient in terms of cSS extent but practically same ICH risk for the lower 2 categories and a very steep increase for disseminated cSS (4-year cumulative ICH risk for no cSS 25% vs focal 28.9% vs disseminated 74%).5
To evaluate the extent by which cSS occurs at spatially separated foci, i.e., cSS multifocality, we have designed a rating method that evaluates discrete regions with cSS within each cerebral hemisphere separately. We have used this new tool in a large cohort of consecutive patients with CAA to explore mechanisms of cSS multifocality and clinical implications for recurrent ICH risk.
METHODS
Study population.
We analyzed prospectively collected data from consecutive patients admitted to Massachusetts General Hospital (MGH) with spontaneous symptomatic lobar ICH (presumably due to small vessel disease6) who had MRI as described in previous publications.7–9 The patients were enrolled between January 2003 and February 2012. These patients are prospectively coded as having strictly lobar hemorrhages (including CMB and ICH) meeting original Boston criteria for definite, probable, or possible CAA,10 based on review of all neuroimaging (but not cSS) and clinical data.
Patient enrollment, clinical and genetic data definitions and collection, and MRI acquisition were performed as described previously.7–9,11 Patients without genetic data (n = 118) were older than patients with CAA with these data (median age 78.4 years, interquartile range [IQR] 69–83 vs 73.6, 66.1–79.9; p = 0.0082) but were not different in vascular risk factors, imaging markers (including cSS presence), or CAA diagnostic category (all p > 0.100; data not shown).
Standard protocol approvals, registrations, and patient consents.
This study was performed with approval and in accordance with the guidelines of the MGH institutional review board.
Neuroimaging acquisition and analysis.
Lobar ICH volumes on baseline acute CT were calculated by trained staff blinded to clinical or MRI data with the semiautomated planimetric method (Alice, PAREXEL International Corporation, Waltham, MA; and Analyze 10.0, Mayo Clinic, Rochester, MN). Presence of intraventricular blood at baseline acute CT was also rated.
MRI were obtained using a 1.5T scanner (GE Sigma; General Electric, Fairfield, CT) as previously detailed and included at least axial T2-weighted, T2*-weighted gradient-recalled echo (T2*-GRE), or susceptibility-weighted imaging (SWI, in 15 patients, of which 13 with follow-up data), fluid-attenuated inversion recovery (FLAIR), and T1-weighted sequences to allow analyses in line with Standards for Reporting Vascular Changes on Neuroimaging recommendations.6,7,9,12 Patients who underwent SWI instead of T2*-GRE were not different in any baseline demographic, clinical, or imaging data (data not shown).
Total white matter hyperintensity (WMH) volumes were quantitatively measured on FLAIR MRI in the ICH-free hemisphere.12 CMB presence and number were evaluated according to current consensus criteria.13 For statistical analyses, lobar CMB number was categorized using cutpoints (0, 1, 2–4, or ≥5). The presence, number, and hemisphere (right, left, or both) of macro ICHs (>5 mm in diameter on T2*-GRE) were also noted.
Enlarged perivascular spaces (EPVS) were assessed on axial T2-weighted MRI, in the basal ganglia and centrum semiovale (CSO), using a validated 4-point visual rating scale.14–16 We prespecified a dichotomized classification of EPVS degree as high (score >2) or low (score ≤2) in line with previous studies.14–16
cSS multifocality rating scale.
cSS was identified according to consensus recommended criteria3,17 as well-defined, homogeneous hypointense curvilinear signal loss (black) on T2*-GRE or SWI outlining the outer surface of cerebral cortex, within the adjacent subarachnoid space, or both.
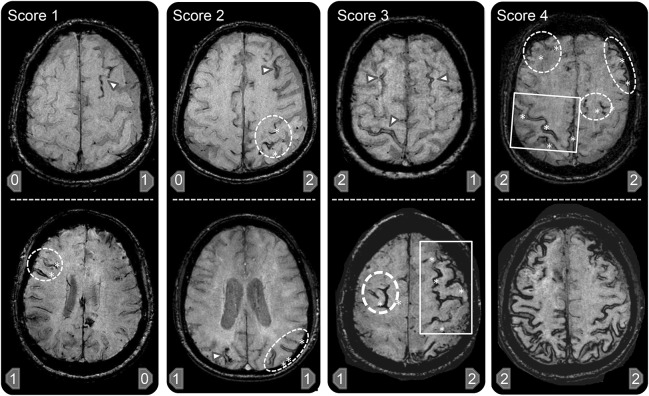
To assess cSS multifocality, potentially distinguishing cSS related to individual bleeding events originating from one focus vs multiple different foci, we designed a new visual scoring system (figure 1). This cSS multifocality rating tool was designed to further improve the advised standards for evaluating cSS in CAA.3 In this scheme, each hemisphere (right–left) is scored separately for cSS, as (1) 0–none; (2) 1–1 sulcus or up to 3 immediately adjacent sulci with cSS; or (3) 2–2 or more nonadjacent sulci or more than 3 adjacent sulci with cSS. Of note, >3 adjacent sulci are treated as multifocal cSS based on the underlying idea that a patient should not have that much involvement without multiple bleeding sources. The total cSS multifocality score is derived by adding the right and left hemisphere scores (range 0–4). Based on this total score, 0 denoted no cSS, 1 mild and unifocal cSS, ≥2 severe and multifocal cSS. cSS contiguous or potentially anatomically connected with any lobar ICH were not included in the aforementioned categories. We predefined that all areas of cSS should be separated from any lobar ICH by at least 3 unaffected sulci, or by at least 2 unaffected sulci (at multiple axial levels) if there is no potential communication path of the hematoma superficially along the cerebral convexities in 3D.
Figure 1. Representative examples of the cortical superficial siderosis (cSS) multifocality scale.
A score of 0 represents no cSS and is not exemplified here. Examples of T2* MRI scans representing different combinations that can total cSS multifocality scores of 1–4 are provided. The arrowhead represents single sulcus cSS whereas involvement of multiple sulci in one region is demonstrated by the oval (2 or 3 sulci) and rectangular (>3 sulci) shapes that also include asterisks denoting the discrete sulci with cSS within these shapes. The cSS scores for the right and left hemispheres are given respectively at the bottom of each individual picture. In brief, presence of 1–3 adjacent sulci with cSS counts as 1 point, whereas more than 3 adjacent sulci counts as 2 points for each hemisphere. Each hemisphere has a score range of 0–2, with a total cSS multifocality score range of 0–4 for the patient.
All scoring rules were formulated prior to the current analysis and without knowledge of other clinical or imaging data. Similarly, cSS scoring for the current study was performed blinded to all other data.
Follow-up data.
Individuals who consented to longitudinal follow-up among ICH patients who survived the first 30 days after their index event were studied for future recurrent lobar ICH as previously described.9,18,19 We collected information on clinically symptomatic lobar ICH, using all clinical, radiologic, and pathologic information available, and blinded to the presence of cSS at baseline MRI. All patients were followed from their date of enrollment until the occurrence of ICH, death, or the end of follow-up.
Statistics.
Separate logistic regression models were used to assess the relationship between APOE ε2 or ε4 allele presence and cSS multifocality presence (0–1 vs ≥2 score) (predetermined to adjust for age and sex). Multivariable logistic regression analyses were performed to look for independent associations for cSS mulifocality in the probable/definite and possible CAA groups separately. Two models were explored: (1) nominal logistic regression tested presence of cSS multifocality (0–1 vs ≥2) and (2) ordinal logistic regression tested 0 vs 1 vs ≥2 score. In all models, we predefined (i.e., before looking at the results) covariates to include age, key imaging markers of CAA (lobar CMBs, high degree of CSO-EPVS, and total WMH volume), and ICH measures (ICH volume and intraventricular hemorrhage [IVH] presence) in adjusted models. Similar ordinal logistic regression models for the full 0–4 cSS multifocality scale were performed as sensitivity analyses.
We determined the presence of cSS multifocality as a univariable predictor of recurrent ICH risk using the Kaplan-Meier plot with significance testing by the log-rank test. Survival time was calculated from date of baseline MRI scan until the date of ICH at follow-up or the last known date without the outcome event of interest. Prespecified adjusted Cox regression analyses were performed to calculate multivariable hazard ratio of presence of cSS multifocality (0–1 vs ≥2) and burden in relation to ICH recurrence. In these models we adjusted for all the known clinical predisposing factors of ICH recurrence found in previous CAA series18,19 including age, history of ICH (other than the baseline event), lobar CMB burden, and WMH. The proportional hazard assumption was tested using graphical checks and Schoenfeld residuals-based tests. The incidence rate ratio of recurrent ICH per cSS mutlifocality category was calculated based on Poisson distribution.
Multicollinearity was assessed in all models by the variance inflation factor. Significance level was set at 0.05. Stata software (version 11.2, StataCorp., College Station, TX) was used for all analyses. The manuscript was prepared with reference to Strengthening the Reporting of Observational Studies in Epidemiology guidelines.20
RESULTS
Our final cohort included 313 CAA-ICH patients (6 pathology-definite, 17 probable with supporting pathology, 172 probable CAA, and 118 possible CAA, i.e., patients with T2*-weighted MRI showing absence of microbleeds according to the Boston criteria10) (figure e-1 at Neurology.org). Characteristics of the study population according to possible vs probable CAA are presented in table e-1. The new cSS multifocality rating scale was reliable and quick to use (after training, rating typically takes less than 1 minute/scan). Interrater testing using representative MRI scans from 50 patients with CAA showed excellent agreement between 2 raters: weighted kappa was 0.91 for presence of cSS multifocality and 0.87 for individual multifocality degrees.
The overall prevalence of multifocal cSS in the whole cohort was 21.1%: 27.2% in probable/definite CAA vs 11.1% in patients with possible CAA (p = 0.001). The distribution of cSS multifocality according to the previous focal and disseminated cSS scale4 was 82% unifocal and 18% multifocal in those CAA classified as focal cSS and 3% unifocal and 97% multifocal in patients classified as having disseminated cSS. History of transient focal neurological episodes (TFNE) was more common in patients with multifocal cSS vs no or unifocal cSS (24.6% vs 8.1%; p < 0.0001) and was the most important clinical predictor for cSS multifocality presence and its severity after adjusting for age, hypertension, dementia, and CAA diagnostic category (odds ratio [OR] 3.88; 95% confidence interval [CI] 1.78–8.50; p = 0.001 in nominal logistic regression). In the cohort subset with genetic data available (n = 195), presence of APOE ε2 allele (but not ε4) had a higher prevalence in patients with multifocal cSS compared to those without (33% vs 16.1%; p = 0.012). Adjustment for age and sex did not alter the strength of this association (OR 2.27; 95% CI 1.04–4.94, p = 0.040).
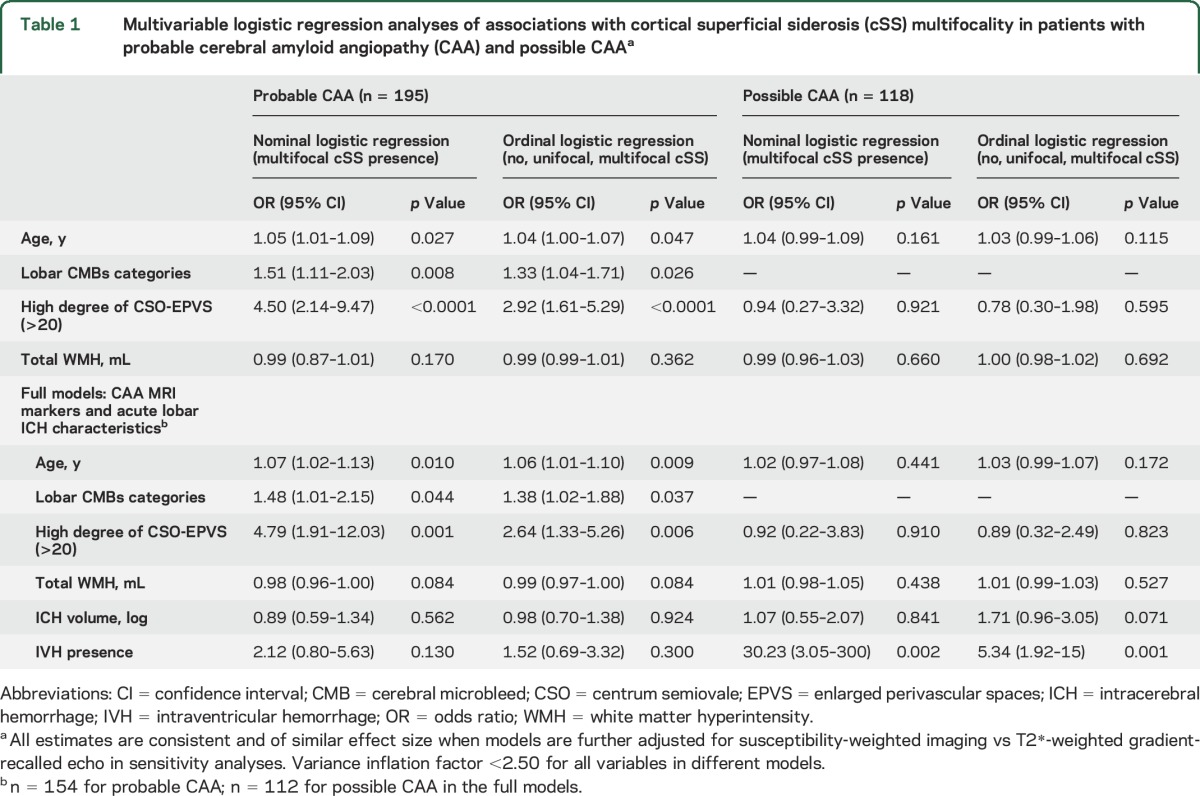
Within the group that only had unilateral ICH (n = 274), the ICH side did not correlate with the side with cSS or cSS multifocality (all p > 0.10 in univariable analyses). In multivariable logistic regression analyses in patients with definite/probable CAA, cSS multifocality was independently associated with neuroimaging markers of CAA severity, but was not related with acute ICH characteristics (table 1). By contrast, in patients with possible CAA, IVH presence and baseline ICH volume were the only independent predictors of cSS multifocality (table 1). These results remained of similar effect size in sensitivity analyses including multivariable ordinal logistic models of the full cSS multifocality score (0–4).
Table 1.
Multivariable logistic regression analyses of associations with cortical superficial siderosis (cSS) multifocality in patients with probable cerebral amyloid angiopathy (CAA) and possible CAAa
cSS multifocality and risk of recurrent ICH.
Follow-up data were available in 240 CAA-ICH survivors: 148 definite/probable CAA and 92 patients with possible CAA. Among the 73 patients not included in the survival analysis, 34 had an early death (within 1 month) after baseline ICH and 39 patients did not have follow-up information. Patients without follow-up information were not different from patients with CAA included in the longitudinal analysis in baseline clinical characteristics, imaging markers (including cSS multifocality), and CAA diagnostic category (all p > 0.05, data not shown).
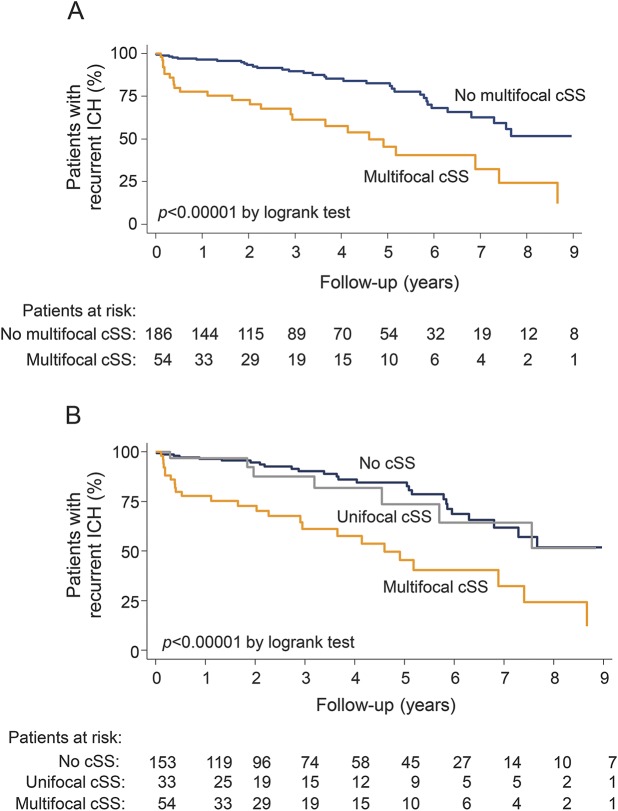
During a median follow-up of 2.6 years (IQR 0.9–5.1 years; 774.6 person-years), recurrent ICH occurred in 58 of 240 patients (24%). In Kaplan-Meier analysis, presence of multifocal cSS and cSS multifocality burden at baseline scans were predictors of time until recurrence (figure 2). The incidence rate of ICH recurrence was 5%/year (95% CI 3.4%–7.3%/year) for patients with CAA without cSS, 6.5%/year (95% CI 3.1%–13.6%/year) in patients with unifocal cSS, and 17.4%/year (95% CI 11.8%–25.6%/year) in multifocal cSS patients (hazard ratio 1.86; 95% CI 1.40–2.47; p < 0.0001). The recurrence rates corresponding to each value (0–4) of the new cSS scoring system are summarized in table 2.
Figure 2. Time to recurrent symptomatic lobar intracerebral hemorrhage (ICH) during follow-up.
Kaplan-Meier estimates of progression to symptomatic lobar ICH in the presence of (A) multifocal cortical superficial siderosis (cSS) and (B) unifocal or multifocal cSS in all patients with cerebral amyloid angiopathy. Testing of significance is by the log-rank test. Ellipse = single sulcus: 1 point. Dotted rectangle = up to 3 adjacent sulci: 1 point. Plain line rectangle = 4 or more adjacent sulci: 2 points.
Table 2.
Recurrent intracerebral hemorrhage (ICH) rate per 100 patient-years of follow-up according to each multifocality category in 240 cerebral amyloid angiopathy–ICH survivors

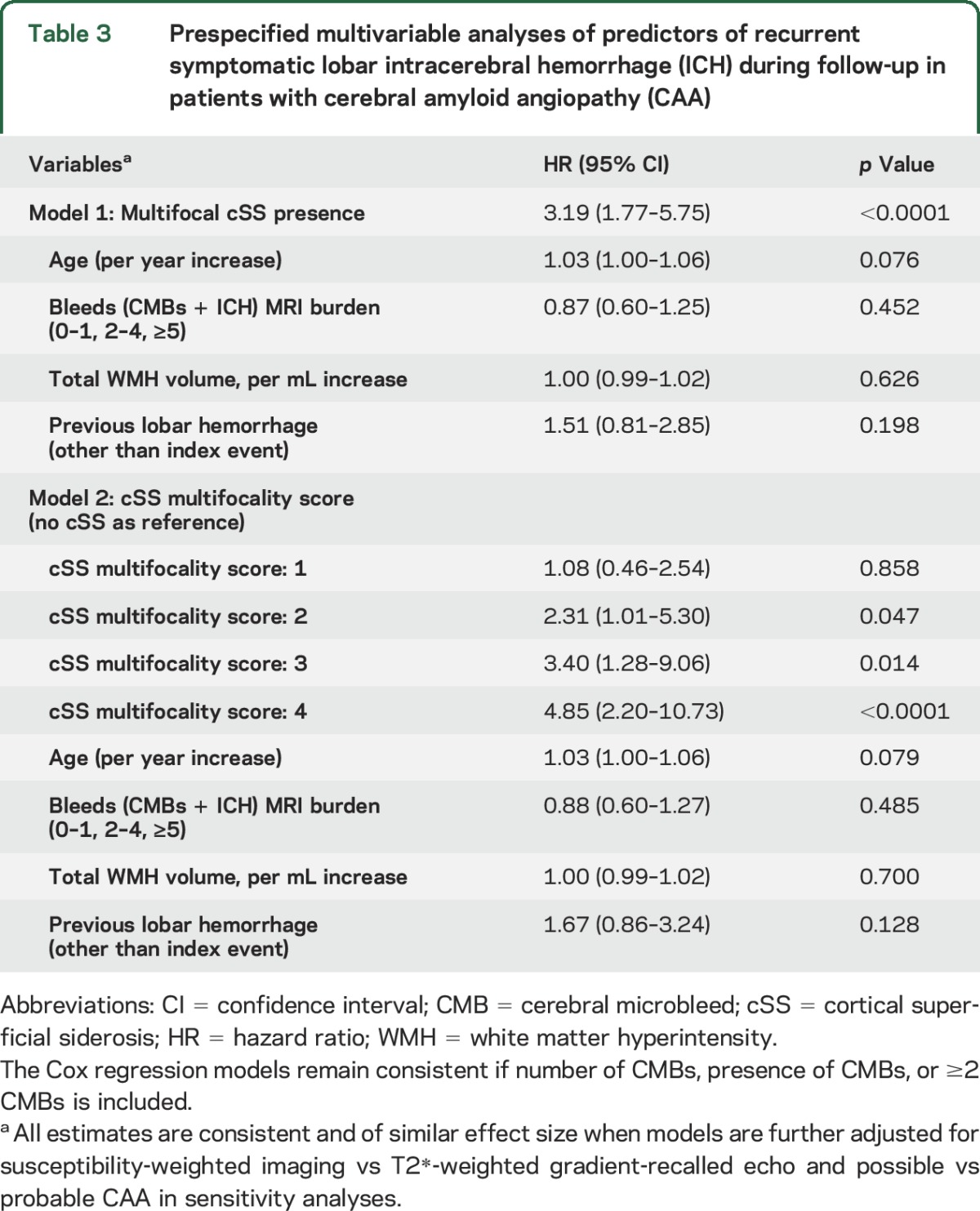
In prespecified multivariable Cox regression models adjusting for previously defined risk factors for recurrent ICH, cSS multifocality (presence and burden) was the only independent predictor of increased symptomatic ICH risk at follow-up (table 3). These results remained consistent in similar multivariable models additionally controlling CAA diagnostic category (definite/probable vs possible CAA). These associations did not change when aspirin use during follow-up was introduced into the model, and aspirin was not associated with increased ICH risk (p = 0.545).
Table 3.
Prespecified multivariable analyses of predictors of recurrent symptomatic lobar intracerebral hemorrhage (ICH) during follow-up in patients with cerebral amyloid angiopathy (CAA)
DISCUSSION
In this study, we have designed a scale that captures cSS multifocality. We applied this tool in consecutive CAA-ICH patients to provide further insights into cSS mechanisms and clinical significance. The main findings are that the extent and multifocality of cSS are associated with markers of vessel fragility and disease severity in probable CAA, whereas in patients with an isolated lobar ICH (possible CAA) it might reflect hematoma extension into the subarachnoid space. cSS multifocality is an independent strong predictor of ICH recurrence, and may help stratify future bleeding risk in CAA, with implications for prognosis and treatment decisions.
CAA might cause cSS through 2 mechanisms: (1) a primary in situ mechanism related to episodes of blood leaking into the subarachnoid space from brittle and fragile leptomeningeal or very superficial cortical CAA-affected vessels; and (2) a secondary mechanism due to blood leakage from, or expansion of, a lobar ICH, rather than an independent in situ bleeding event. The cSS multifocality tool might inform about the contribution of each of these potential mechanisms in cSS pathophysiology. In our study, cSS multifocality burden was not anatomically related to the brain hemisphere with ICH. These observations support a primary in situ mechanism for at least a substantial proportion of cSS, together with available evidence showing that repeated acute subarachnoid bleeding can cause cSS and TFNE and can occur in the absence of ICH.4,17,21,22 In patients with probable CAA, we have shown that cSS is related to lobar CMB burden, a hemorrhagic marker of disease severity, and severe CSO-EPVS, an MRI marker linked to perivascular drainage impairment.23 Combined with the lack of any association between cSS and ICH volume or IVH presence, these observations support the hypothesis that cSS in probable CAA mostly occurs as a result of in situ bleeding due to focal vessel fragility. In line with this, our study also raises the interesting possibility that APOE ε2 influences pathways causing cSS, likely by promoting vasculopathic changes that can lead to vessel rupture.24–26 For patients with possible CAA, our findings support a secondary mechanism for cSS, including ICH propagation.
cSS may be a marker of increased cortical and leptomeningeal small vessel fragility and high CAA disease activity, heralding a high risk for lobar ICH,27,28 as shown in a small study.5 However, the cSS scale4 used in this study did not take into account different cSS extent in each hemisphere. Another important point is that this initial article demonstrating cSS extent as a marker of ICH recurrence (n = 112 patients) did not show much of a gradient in terms of cSS extent to recurrence risk: no cSS had 25% 4-year cumulative ICH recurrence risk in CAA, unifocal cSS had 28.9%, and disseminated had 74%.5 Our findings provide external validity for these observations in a larger CAA cohort with longer follow-up and more outcome events. Our new scale (range 0–4) showed a much smoother gradient of differences in ICH recurrence risk and proved to be an important predictor of future ICH risk over its entire range. The annual ICH recurrence risks reported in table 2 thus can help clinicians better stratify such risk in patients with CAA, and guide decision-making on antithrombotic use when these medications are indicated for a different ischemic pathology.
The issue of starting or foregoing antithrombotics after an ICH and the timing of restarting are different issues that come up frequently in clinical practice, especially now that there are some alternatives to lifelong anticoagulation in nonvalvular atrial fibrillation such as left atrial appendage closure. Our study and another recent article from our group on cSS and early CAA-ICH recurrence risk29 provide key data to answer these 2 separate questions. Our work allows clinicians to stratify the ICH risk in patients with CAA, therefore helping clinicians to compare ischemic and hemorrhagic risks and deciding whether antithrombotics might be appropriate for the individual patient. If the individual risk decision analysis suggests benefit from antithrombotic use, detailed data on timing and risk of recurrence29 might guide the optimal timing of antithrombotic resumption.30 The current article has also identified a more clear dose–response relationship of cSS with ICH risk and clarified that the risk extends well beyond the first 6 months.
Notable strengths of our study include the systematic evaluation for a comprehensive range of small vessel disease imaging markers and the use of prespecified cSS mulifocality definitions, rating methods, and cutoffs. A limitation is the potential selection bias due to the requirement for routine clinical MRI, the lack of APOE data in a proportion of patients, and the lack of follow-up information in 12.5% of our cohort. Despite our prospective cohort being currently the largest in the field, the relatively low number of outcome events leads to relatively wide CIs around estimates for some analyses. This is particularly the case in identifying cSS risk factors in possible CAA—these models should be considered pilot and hypothesis-generating. Larger studies with longer follow-up are needed to further explore the association between cSS extent and ICH recurrence risks in probable vs possible CAA, categories known to carry different diagnostic certainty for the disease.10
Taken together, our observations further support the notion that cSS is a central component of hemorrhagic small vessel disease and can potentially identify new mechanisms and distinct CAA phenotypes.31–33 cSS multifocality seems to consistently increase the risk of future lobar ICH recurrence with important clinical implications for patients with CAA and future disease-modifying treatments. More work is needed into potential pathophysiologic mechanisms of cSS; the multifocality scale suggested here might facilitate such future studies.
Supplementary Material
GLOSSARY
- CAA
cerebral amyloid angiopathy
- CI
confidence interval
- CMB
cerebral microbleed
- CSO
centrum semiovale
- cSS
cortical superficial siderosis
- EPVS
enlarged perivascular spaces
- FLAIR
fluid-attenuated inversion recovery
- ICH
intracerebral hemorrhage
- IQR
interquartile range
- IVH
intraventricular hemorrhage
- MGH
Massachusetts General Hospital
- OR
odds ratio
- SWI
susceptibility-weighted imaging
- T2*-GRE
T2*-weighted gradient-recalled echo
- TFNE
transient focal neurological episodes
- WMH
white matter hyperintensity
Footnotes
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Drs. Charidimou and Gurol. A. Charidimou: project concept and design, imaging analysis, data analysis, writeup. G. Boulouis: project design, imaging analysis, critical revisions. D. Roongpiboonsopit: data collection, imaging analysis, critical revisions. M. Pasi: imaging analysis, critical revisions. E. Auriel: imaging analysis, critical revisions. E.S. van Etten: imaging analysis, critical revisions. K. Haley: imaging analysis, critical revisions. A. Ayres: data collection and management. A. Vashkevich: imaging analysis, data collection and management. K.M. Schwab: data collection and management. S. Martinez-Ramirez: imaging analysis, critical revisions. J. Rosand: data collection, critical revisions. J.N. Goldstein: data collection, critical revisions. A. Viswanathan: data collection, critical revisions. S.M. Greenberg: funding, data collection, critical revisions. M.E. Gurol: project concept and design, data collection, imaging analysis, data analysis, writeup, funding.
STUDY FUNDING
This study was supported by the following NIH grants: 5K23NS083711 (M.E.G.), R01 NS070834, and 2R01 AG26484 (S.M.G.).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry 2012;83:124–137. [DOI] [PubMed] [Google Scholar]
- 2.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol 2011;70:871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charidimou A, Linn J, Vernooij MW, et al. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain 2015;138:2126–2139. [DOI] [PubMed] [Google Scholar]
- 4.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010;74:1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charidimou A, Peeters AP, Jager R, et al. Cortical superficial siderosis and intracerebral hemorrhage risk in cerebral amyloid angiopathy. Neurology 2013;81:1666–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auriel E, Gurol ME, Ayres A, et al. Characteristic distributions of intracerebral hemorrhage-associated diffusion-weighted lesions. Neurology 2012;79:2335–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Donnell HC, Rosand J, Knudsen KA, et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med 2000;342:240–245. [DOI] [PubMed] [Google Scholar]
- 9.van Etten ES, Auriel E, Haley KE, et al. Incidence of symptomatic hemorrhage in patients with lobar microbleeds. Stroke 2014;45:2280–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;56:537–539. [DOI] [PubMed] [Google Scholar]
- 11.Brouwers HB, Biffi A, McNamara KA, et al. Apolipoprotein E genotype is associated with CT angiography spot sign in lobar intracerebral hemorrhage. Stroke 2012;43:2120–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurol ME, Irizarry MC, Smith EE, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology 2006;66:23–29. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009;8:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke 2010;41:2483–2490. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Ramirez S, Pontes-Neto OM, Dumas AP, et al. Topography of dilated perivascular spaces in subjects from a memory clinic cohort. Neurology 2013;80:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charidimou A, Jaunmuktane Z, Baron JC, et al. White matter perivascular spaces: an MRI marker in pathology-proven cerebral amyloid angiopathy? Neurology 2014;82:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charidimou A, Jager RH, Fox Z, et al. Prevalence and mechanisms of cortical superficial siderosis in cerebral amyloid angiopathy. Neurology 2013;81:626–632. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke 2004;35:1415–1420. [DOI] [PubMed] [Google Scholar]
- 19.Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology 2010;75:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 21.Calviere L, Cuvinciuc V, Raposo N, et al. Acute convexity subarachnoid hemorrhage related to cerebral amyloid angiopathy: clinicoradiological features and outcome. J Stroke Cerebrovasc Dis 2016;25:1009–1016. [DOI] [PubMed] [Google Scholar]
- 22.Ni J, Auriel E, Jindal J, et al. The characteristics of superficial siderosis and convexity subarachnoid hemorrhage and clinical relevance in suspected cerebral amyloid angiopathy. Cerebrovasc Dis 2015;39:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charidimou A, Jager RH, Peeters A, et al. White matter perivascular spaces are related to cortical superficial siderosis in cerebral amyloid angiopathy. Stroke 2014;45:2930–2935. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg SM, Vonsattel JP, Segal AZ, et al. Association of apolipoprotein E epsilon2 and vasculopathy in cerebral amyloid angiopathy. Neurology 1998;50:961–965. [DOI] [PubMed] [Google Scholar]
- 25.Charidimou A, Martinez-Ramirez S, Shoamanesh A, et al. Cerebral amyloid angiopathy with and without hemorrhage: evidence for different disease phenotypes. Neurology 2015;84:1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shoamanesh A, Martinez-Ramirez S, Oliveira-Filho J, et al. Interrelationship of superficial siderosis and microbleeds in cerebral amyloid angiopathy. Neurology 2014;83:1838–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda S, Hinokuma K, Yamazaki K, et al. The hemorrhage caused by sporadic-type cerebral amyloid angiopathy occurs primarily in the cerebral sulci. Neuropathology 2012;32:38–43. [DOI] [PubMed] [Google Scholar]
- 28.Takeda S, Yamazaki K, Miyakawa T, et al. Subcortical hematoma caused by cerebral amyloid angiopathy: does the first evidence of hemorrhage occur in the subarachnoid space? Neuropathology 2003;23:254–261. [DOI] [PubMed] [Google Scholar]
- 29.Roongpiboonsopit D, Charidimou A, William CM, et al. Cortical superficial siderosis predicts early recurrent lobar hemorrhage. Neurology 2016;87:1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pennlert J, Overholser R, Asplund K, et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke 2017;48:314–320. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg SM, Vonsattel JP, Stakes JW, Gruber M, Finklestein SP. The clinical spectrum of cerebral amyloid angiopathy: presentations without lobar hemorrhage. Neurology 1993;43:2073–2079. [DOI] [PubMed] [Google Scholar]
- 32.Maia LF, Mackenzie IR, Feldman HH. Clinical phenotypes of cerebral amyloid angiopathy. J Neurol Sci 2007;257:23–30. [DOI] [PubMed] [Google Scholar]
- 33.Charidimou A. Convexity subarachnoid hemorrhage in cerebral amyloid angiopathy: the saga continues. J Cereb Blood Flow Metab 2015;35:707–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.