Abstract
Objective:
In patients with acute ischemic stroke, we aimed to investigate the relation between preexisting small vessel disease (SVD) and the amount of blood–brain barrier (BBB) leakage in ischemic and nonischemic area before IV thrombolysis.
Methods:
We retrospectively accessed anonymous patient-level data from the Stroke Imaging Repository and the Virtual International Stroke Trials Archive resources and included patients treated with IV thrombolysis with pretreatment MRI. We rated SVD features using validated qualitative magnetic resonance (MR) scales. Leakage of BBB was assessed with postprocessing of perfusion-weighted images. We evaluated associations between SVD features (individually and summed in a global SVD score) and BBB leakage using linear regression analysis, adjusting for major clinical confounders.
Results:
A total of 212 patients, mean age (±SD) 69.5 years (±16.1), 102 (48%) male, had available MR before IV thrombolysis. Evidence of BBB leakage was present in 175 (80%) and 205 (94%) patients in the ischemic and nonischemic area, respectively. Lacunar infarcts (β = 0.17, p = 0.042) were associated with BBB leakage in the ischemic area, and brain atrophy was associated with BBB leakage in both ischemic (β = 0.20, p = 0.026) and nonischemic (β = 0.27, p = 0.001) areas. Increasing SVD grade was independently associated with BBB leakage in both ischemic (β = 0.26, p = 0.007) and nonischemic (β = 0.27, p = 0.003) area.
Conclusions:
Global SVD burden is associated with increased BBB leakage in both acutely ischemic and nonischemic area. Our results support that SVD score has construct validity, and confirm a relation between SVD and BBB disruption also in patients with acute stroke.
Small vessel disease (SVD) refers to a wide range of pathologic processes that affect microcirculation in the brain.1 Effects of SVD on brain parenchyma can be detected in vivo with either CT or magnetic resonance (MR) scan. Imaging phenotypes of SVD include white matter changes, lacunar strokes, cerebral microbleeds, enlarged perivascular spaces, and brain atrophy.2 Moreover, postmortem studies have identified microscopic pathologic features, such as cortical microinfarcts and changes in the normal-appearing white matter, which are undetectable with conventional imaging, suggesting that the SVD seen on imaging studies is only part of a larger spectrum.3 Recently, the concept that a global estimate of SVD may provide a better overview of the effect of the pathology on the brain than one single SVD feature resulted in the development of a combined score,4 which has been validated in regards to cognitive status.5
SVD has been associated with dysfunction of the blood–brain barrier (BBB).6 Animal models using chronic cerebral hypoperfusion to produce SVD7 have identified deficits of BBB integrity, and it has been suggested that SVD may lead to BBB damage through endothelial dysfunction8 and subsequent inflammatory process.9 Conversely, some authors have hypothesized that BBB disruption itself may play a pathogenic role in SVD. In fact, BBB leakage is present in normal-appearing white matter in patients with leukoaraiosis,10 and areas of normal-appearing white matter with BBB leakage seems to predate development of white matter changes.11 However, SVD is a composite product of various imaging features, and it is not known whether combining different rather than single SVD features could provide information on the amount of BBB disruption. In this cross-sectional study of patients with acute ischemic stroke suitable for treatment with recombinant tissue plasminogen activator (rtPA), we sought to investigate the associations between increasing grade of individual and summed imaging markers consistent with SVD and levels of BBB leakage within the following:
The acutely ischemic region
The regions remote from acute ischemia
METHODS
Patients.
We accessed patient-level data from the Stroke Imaging Repository/Virtual International Stroke Trials Archive resource to perform a retrospective analysis. We included patients with acute ischemic stroke with available MRI before IV thrombolytic treatment with rtPA. All patients included in the present study received rtPA after MR scanning.
Clinical variables of interest included age, sex, and baseline (before rtPA treatment) variables, such as stroke severity assessed with NIH Stroke Scale (NIHSS), blood glucose, blood pressure, and time from stroke symptoms onset to rtPA administration (onset-to-treatment time [OTT]), as a surrogate of time from symptom onset to brain imaging. We included anamnestic relevant cardiovascular risk factors such as hypertension, diabetes, atrial fibrillation, hypercholesterolemia, and history of smoking (current and past).
SVD assessment.
A stroke neurologist (F.A.) trained in MR assessment for SVD and blinded to clinical and BBB leakage data rated all the available scans (T1, T2, fluid-attenuated inversion recovery [FLAIR] sequences) for presence and severity of SVD features, according to Standards For Reporting Vascular Changes on Neuroimaging recommendations.2 An expert stroke physician (D.I.) cross-checked the ratings. Although previous attempts of SVD score also included microbleeds and enlarged perivascular spaces,4 to allow transferability of methods to CT, we only considered MR surrogates of SVD detectable with CT scan. We therefore rated preexisting lacunes, white matter hyperintensities, and brain atrophy. We defined lacunes as round CSF isointense lesions measuring ≤20 mm in diameter on axial section in the white matter, basal ganglia, or brainstem on T1, T2, or FLAIR sequences. We graded white matter hyperintensities as 0–2 according to the Van Swieten Scale (VSS) in anterior and posterior periventricular white matter to obtain a 5-point ordinal scale (0–4).12 Brain atrophy was defined as deep and cortical, and rated as none, mild-moderate and severe against a reference MR brain template, then we combined the deep and cortical scores into a 5 point ordinal scale (0–4).13
For assessment of total SVD burden, we built a combined aggregate SVD score as follows: we summed the scores of white matter changes, lacunes, and brain atrophy, assigning 1 point for each of the following: severe white matter changes (VSS ≥ 3), lacunes (≥2), and severe brain atrophy (≥3). The resulting 4-point ordinal score evaluated the global burden of SVD from 0 (no imaging features of severe SVD) to 3 (imaging features of SVD scored as severe for each imaging variable). The aforementioned SVD score has been previously evaluated on clinical outcomes14 and white matter perfusion.15
BBB leakage assessment.
BBB leakage was calculated by one author (R.L.) blinded to demographic and clinical data. The analysis was performed on the dynamic susceptibility contrast source images that are acquired in stroke patients for the purpose of generating perfusion-weighted images. We obtained a relative measure of BBB leakage comparing the signal change in regions of intact BBB with the signal change in regions with BBB disruption. Normal tissue was identified using an automated process that excludes regions of BBB disruption based on signal characteristics. We separately assessed BBB leakage in regions of hypoperfusion, representing the ischemic brain area, and regions with normal perfusion, representing the background brain status. Selection of the gadolinium injection time was done by visual inspection. All other aspects of the analysis were automated (see e-Methods at Neurology.org).
BBB disruption calculations were performed in these 2 regions of interest (ROIs) that were defined by time-to-peak (TTP) maps. The acute ROI, identifying the ischemic area in the affected hemisphere, was defined by region with a TTP delay of greater than 4 seconds, with all other regions falling into the nonacutely ischemic ROI. Although cerebral blood flow is different in gray vs white matter, TTP maps are relatively insensitive to these differences, and thus, when using a threshold of 4 seconds, the resulting ROI represents acute perfusion deficit of the involved vascular territory (figure 1). Thus the region remote from the acute ischemia comprises the rest of the brain (outside of the TTP lesion). We defined mean permeability derangement (MPD) as the mean value of all the voxels greater than the derangement threshold within the ROI. MPD is used to identify regions of focal high BBB derangement (figure 1). The BBB quantification was performed with an automated script in MATLAB software package, thus there was no user dependence or variability between repeated calculations. All BBB leakage calculations were submitted prior to unblinding.
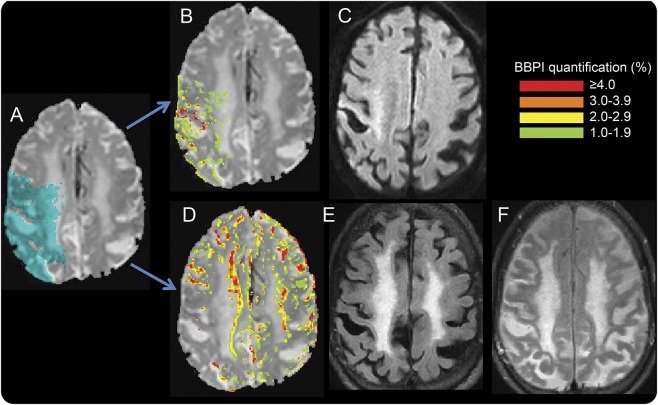
Figure 1. An example of blood–brain barrier (BBB) leakage detected with MRI.
(A) Source image from the dynamic susceptibility contrast scan with the acute ischemic region of interest (ROI) shaded in blue. (B) BBB leakage map within the acute ROI. Regions of BBB leakage are overlain on the dynamic susceptibility contrast source image according to the color code in the legend with red representing the most severe areas of BBB disruption. (C) Corresponding diffusion-weighted image (DWI), which was not used in the analysis but is shown to demonstrate the pattern of elevated (red) BBB leakage in the region that is bright (acutely ischemic) on the DWI. (D) BBB leakage map for regions remote from the acute ischemia. (E) The corresponding fluid-attenuated inversion recovery (FLAIR) image and small vessel disease (SVD). The SVD composite score for this patient is 3. Note the pattern of increased BBB leakage at the junction between the white matter hyperintensities and the normal-appearing white matter. (F) FLAIR image from a follow-up MRI in which the previously administered gadolinium has leaked through the BBB into the CSF, causing a lack of CSF suppression.
Standard protocol approvals, registrations, and patient consents.
Ethical approval was not required as the dataset was anonymized. The NIH Office of Human Subjects Research Protections determined that the BBB analysis, which was conducted at the NIH, did not require institutional review board approval.
Statistical analysis.
We described general characteristics of the population with summary statistics, and used analysis of variance (ANOVA), Kruskal-Wallis, and Pearson χ2, as appropriate, to test differences among groups. For the purposes of the present study, we performed a normal logarithmic transformation of BBB leakage values to obtain normally distributed data. We therefore evaluated associations between single SVD features, SVD score, and BBB leakage with ANOVA. To investigate independent associations, we built multivariable linear regression models with SVD features and SVD score as explanatory variables and BBB leakage as dependent variable. For BBB leakage within the ischemic area, we adjusted the analysis for relevant confounders in acute stroke physiopathology, such as age, sex, stroke severity, baseline glucose, and OTT. For BBB leakage in the nonischemic area, we adjusted the analysis for relevant confounders in SVD such as age, sex, hypertension, diabetes, and smoke exposure. In a further multivariable model, we adjusted the analysis for all the aforementioned variables in the same analysis. We considered a p value <0.05 statistically significant. Statistical analysis was carried out using SPSS for Windows (version 22.0; SPSS, IBM Corp., Armonk, NY).
RESULTS
From a database of 285 patients, 219 (77%) had both clinical data and baseline MR scans of interest for the purposes of the present study. In 7 patients, MR assessment was not possible for technical reasons (poor scan quality or incomplete sequences). This left 212 (74% of the original dataset) patients with pretreatment MR scans available for the analysis.
Demographic and clinical characteristics of patients included in the study are listed in table 1. Mean age (±SD) was 69.5 ± 16.1 years; 102 (45%) patients were male. Median baseline NIHSS was 10; 75% of patients had a NIHSS >5; hypertension was the most frequent risk factor (71%).
Table 1.
Baseline characteristics of study patients
Distribution of single SVD features is shown in figure e-1. Seventy-eight (37%) patients had evidence of severe white matter changes, 42 (20%) had 2 or more lacunes, 86 (41%) had brain atrophy graded as severe. The combined SVD score showed 95 (45%) patients with absence of SVD signs (score 0), 48 (23%) patients with mild SVD (score 1), 49 (23%) patients with moderate SVD (score 2), and 20 (9%) patients with severe SVD (score 3) burden. As expected, hypertension (p = 0.001) and atrial fibrillation (p = 0.002) prevalence increased with SVD burden.
Acute ischemic region and BBB leakage.
Before IV rtPA administration, 175 (80%) patients showed some degree of BBB leakage within the ischemic area, with a mean (±SD) derangement of 3.02 (±0.59). Univariate analysis showed that permeability increased with severity of white matter changes (p = 0.006), grade of brain atrophy (p = 0.005), and number of lacunes (p = 0.008) (figure e-2). The combined SVD score was strongly associated with BBB leakage (mean permeability 2.84 for SVD = 0; 3.00 for SVD = 1; 3.21 for SVD = 2; 3.39 for SVD = 3; p < 0.001; figure 2). Multivariable linear regression analysis among single SVD features showed that severe brain atrophy (β = 0.20; p = 0.026) and preexisting lacunar infarcts (per lacunar infarct increase, β = 0.17; p = 0.042) were associated with BBB leakage, whereas both brain atrophy (as ordinal scale) and white matter changes were not. Compared to single SVD features and to its single components, the combined SVD score displayed a stronger association with BBB leakage (β = 0.26; p = 0.007, β = 0.25; p = 0.014 in 2 multivariable models; table 2).
Figure 2. Blood–brain barrier leakage (mean and 95% confidence interval [CI]) within the ischemic area across small vessel disease (SVD) score.
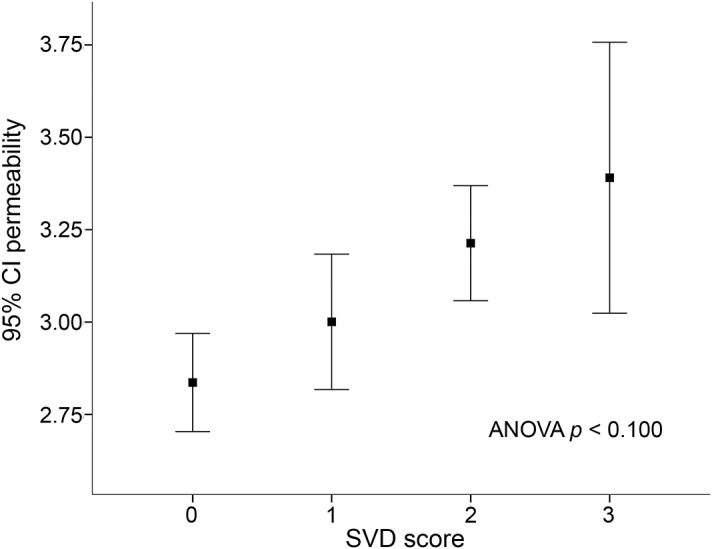
ANOVA = analysis of variance.
Table 2.
Linear regression analysis with blood–brain barrier (BBB) leakage as dependent variable
Regions remote to the acute ischemia and BBB derangement.
At baseline, 202 (95%) patients had evidence of BBB leakage in regions remote from the acute ischemia, with a mean (±SD) derangement of 2.87 (±0.64). Similarly to the analysis in the acute ischemic region, univariate analysis showed that BBB leakage increased with grade of white matter changes (p = 0.019) and brain atrophy (p < 0.001), whereas number of lacunes were not associated with BBB leakage (figure e-3). The combined SVD score was associated with increased BBB leakage (mean permeability 2.67 for SVD = 0; 2.97 for SVD = 1; 2.99 for SVD = 2; 3.33 for SVD = 3; p < 0.001; figure 3). Multivariate linear regression showed that brain atrophy was independently associated with BBB leakage (β = 0.25; p = 0.006), whereas white matter changes and lacunes were not. However, the combined SVD score confirmed the association with BBB leakage in the 2 multivariable models (β = 0.27; p = 0.003, β = 0.22; p = 0.013; table 2).
Figure 3. Blood–brain barrier leakage (mean and 95% confidence interval [CI]) within the nonischemic area across small vessel disease (SVD) score.
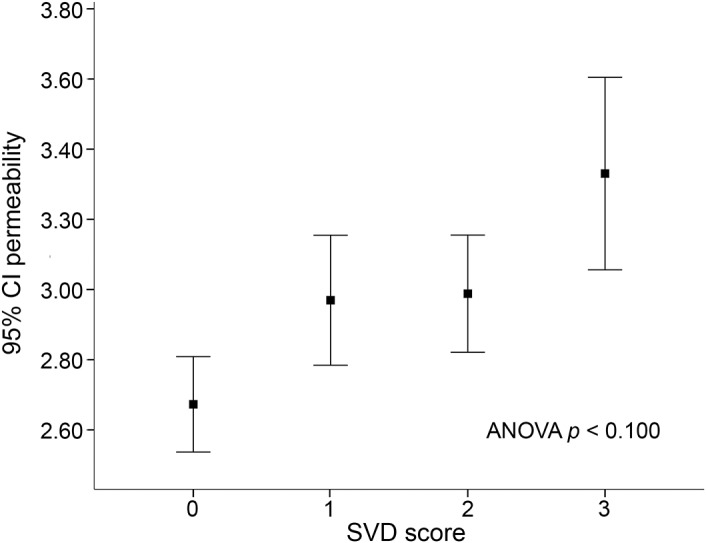
ANOVA = analysis of variance.
DISCUSSION
We investigated BBB leakage before IV rtPA in a population of ischemic stroke patients.
We found that BBB leakage in regions remote from the acute lesion occurred more frequently than BBB leakage in acute ischemic regions, even though the latter had higher values than the former. Brain atrophy and lacunes were associated with increased BBB leakage; however, a summed SVD score was more strongly associated with increasing BBB leakage throughout the brain, suggesting that the use of a combined score accounting for the global SVD burden may provide more complete information with regard to BBB leakage.
Consistently with prior reports,16–19 we found that some degree of BBB leakage was a common event in acute stroke patients. Remarkably, almost all patients (95%) had some amount of BBB leakage remotely from the acute ischemia compared with 80% in regions with acute ischemia. Although increasing amount of BBB derangement is likely pathologic, small amount of BBB leakage could be not related with disease, and this may explain the high frequency of patients with evidence of BBB disruption. Furthermore, BBB derangement increases with normal aging and in patients with SVD,17 and is thought to be one of the key pathologic changes of SVD.4,11,17 A recent consensus statement pointed out that increased BBB permeability may trigger SVD pathology,20 and our findings seems to corroborate this hypothesis, showing almost all patients with BBB leakage despite around half of our population with imaging signs of SVD.
SVD has a various imaging phenotype and we found that single features of SVD had a substantially different contribution to BBB leakage between chronic and acute damage. Conversely to a previous study,10 we did not found any association between white matter changes and BBB leakage. However, this may support the hypothesis that BBB disruption precedes the development of white matter disease rather than being primarily associated with established white matter changes.11,20 Brain atrophy was strongly associated with BBB leakage. This finding is in keeping with a systematic review and meta-analysis, which showed a more pronounced BBB leakage in patients with vascular dementia compared to Alzheimer disease or controls.21 Similarly to previous studies,6,18 we found that lacunes were associated with an increase of BBB leakage, although the association was independent only within the ischemic area. More importantly, we showed that a combined score accounting for the global SVD burden performed better than single SVD score components with regards to BBB disruption. The independent associations of the SVD score with increased BBB leakage in both hemispheres suggested that a combined score may reliably act as a useful surrogate marker of SVD and convey an good overall view of the pathology and its grade and effect on the brain. Although we conducted a cross-sectional study and cannot draw any conclusion about causal relationship, we found a linear relation between increasing SVD burden and BBB leakage, alluding to a dose-response effect between the 2 features, thus reinforcing the association. Future larger studies may assess validity of our methodology of BBB disruption and SVD and explore their long-term relation.
We found a more pronounced increase of BBB leakage in the ischemic area compared to the area remote to brain ischemia before rtPA treatment. Evidence suggests that rtPA boosts BBB permeability within the ischemic area in acute stroke patients treated with IV thrombolysis with successful recanalization, and may therefore contribute to the so-called reperfusion injury.19,22,23 As a consequence, BBB disruption within the ischemic area after rtPA treatment has been proposed as an intermediate marker of patients at risk to develop hemorrhage.24,25 However, our results showed that various degree of BBB leakage is present also before rtPA administration, and therefore independent from either rtPA itself or recanalization process. This is in keeping with a recent study that showed that a mild BBB leakage before thrombolytic treatment is reversible after reperfusion, while severe BBB leakage is associated with irreversible BBB rupture and subsequent increasing risk of post-thrombolysis intracranial hemorrhage.26 We demonstrated that preexisting characteristics of the brain, such as presence and grade of SVD, might modulate the amount of BBB permeability in the acutely ischemic area. Complex interactions among endothelial dysfunction,8 inflammation,16 and the integrity of the neurovascular unit27 might contribute to the worsening of BBB function in patients with SVD. This may be clinically relevant for 2 main reasons. First, SVD is frequent in patients with acute ischemic stroke (we found around one-third of the study population with moderate to severe global burden of SVD), and second, BBB leakage has been associated with hemorrhagic transformation after acute stroke treatment. Future studies might investigate whether SVD and BBB disruption interact with hemorrhagic transformation or worse outcomes after rtPA and endovascular procedures.
Strengths of our study are the large sample size, the standardized assessment of SVD with largely validated scales, and the blinded evaluation of both SVD and BBB leakage. However, we acknowledge several limits to our study. The analysis was performed on a de-identified dataset obtained from a public repository, thus the authors were not able to confirm the protocol used to enroll each patient. As a consequence, some selection bias interfering with the data is plausible. However, all the included patients were treated with IV rtPA after MRI, and experts should have excluded potential sources of bias (e.g., stroke mimics). The methodology we implemented for measuring BBB leakage utilized an MR sequence that can be routinely acquired as part of the evaluation of acute stroke patients within the time frame of recognized guidelines.28 Although our permeability method is limited to reflect a relative rather than an absolute measurement of the transfer constant, this has the advantage that the measurement is normalized to the scan it is obtained from, minimizing the effect of scanner variability. Despite this advantage, it is important to point out that the scan measures in this study varied substantially in acquisition parameters and strength of the magnet and the scanner manufacturers. This variability undoubtedly added noise to the dataset and may have obscured important associations other than those identified. Although the methodology used in this study is relatively new, it has been validated in different datasets in patients with stroke29,30 and brain tumors.31 Again, we acknowledge that the SVD score we used needs validation and appropriate statistical modeling for implementation in clinical practice. However, we provided a proof-of-concept for the SVD score as a tool for quantification of global cerebral SVD. The same score previously proved validity from 2 different datasets in relation to clinical outcomes after acute stroke14 and white matter perfusion.15 Finally, we did not have any information about pathogenic mechanism of the index strokes and we did not retain data on ischemic lesion characteristics (e.g., size, location). However, the aim of the study was to give a picture of the link between SVD and BBB immediately before rtPA treatment in the acute scenario, irrespectively from stroke pathogenesis. Whether stroke subtype may influence BBB could be investigated in future studies.
This study expands our knowledge about the relationship between SVD and BBB in acute ischemic stroke, showing that BBB leakage is frequent before rtPA treatment and that increasing SVD burden is consistently associated with higher BBB leakage also in the acutely ischemic area. Our results suggest that SVD and BBB leakage are interrelated processes in both acutely ischemic and nonischemic area. Further studies to investigate how SVD and BBB disruption interact with acute stroke therapy are warranted.
Supplementary Material
GLOSSARY
- ANOVA
analysis of variance
- BBB
blood–brain barrier
- FLAIR
fluid-attenuated inversion recovery
- MPD
mean permeability derangement
- MR
magnetic resonance
- NIHSS
NIH Stroke Scale
- OTT
onset-to-treatment time
- ROI
region of interest
- rtPA
recombinant tissue plasminogen activator
- SVD
small vessel disease
- TTP
time-to-peak
- VSS
Van Swieten Scale
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: STIR/VISTA Imaging Collaboration, Gregory W. Albers, Stephen M. Davis, Geoffrey A. Donnan, Marc Fisher, Anthony J. Furlan, James C. Grotta, Werner Hacke, Dong-Wha Kang, Chelsea Kidwell, Walter J. Koroshetz, Kennedy R. Lees, Michael H. Lev, David S. Liebeskind, A. Gregory Sorensen, Vincent N. Thijs, Götz Thomalla, Steven J. Warach, Joanna M. Wardlaw, and Max Wintermark
AUTHOR CONTRIBUTIONS
Francesco Arba: study concept, design, analysis of imaging data, draft manuscript. Richard Leigh: study design, analysis of imaging data, draft manuscript. Domenico Inzitari: study concept, analysis of imaging data, draft manuscript. Steven Warach: draft manuscript. Marie Luby: acquisition of data. Kennedy Lees: study design, draft manuscript.
STUDY FUNDING
Supported by the Department of Neurology, Dell Medical School, University of Texas at Austin.
DISCLOSURE
F. Arba received funding from Ente Cassa di Risparmio di Firenze (2010.06.03). R. Leigh is funded by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke (NINDS) of the NIH, Bethesda, MD. D. Inzitari, S. Warach, M. Luby, and K. Lees report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689–701. [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Smith EE, Biessels GJ, et al. ; Standards For Reporting Vascular Changes On Neuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gouw AA, Seewann A, van der Flier WM, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 2011;82:126–135. [DOI] [PubMed] [Google Scholar]
- 4.Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014;83:1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staals J, Booth T, Morris Z, et al. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging 2015;36:2806–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wardlaw JM, Sandercock PAG, Dennis MS, et al. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003;34:806–812. [DOI] [PubMed] [Google Scholar]
- 7.Ueno M, Tomimoto H, Akiguchi I, Wakita H, Sakamoto H. Blood-brain barrier disruption in white matter lesions in a rat model of chronic cerebral hypoperfusion. J Cereb Blood Flow Metab 2002;22:97–104. [DOI] [PubMed] [Google Scholar]
- 8.Poggesi A, Pasi M, Pescini F, Pantoni L, Inzitari D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: a review. J Cereb Blood Flow Metab 2016;36:72–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 2011;42:3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topakian R, Garrick TR, Howe FA, et al. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leukoaraiosis. J Neurol Neurosurg Psychiatry 2010;81:192–197. [DOI] [PubMed] [Google Scholar]
- 11.Huisa BN, Caprihan A, Thompson J, Prestopnik J, Qualls CR, Rosenberg GA. Long-term blood-brain barrier permeability changes in Binswanger disease. Stroke 2015;46:2413–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Swieten JC, Hijdra A, Koudstaal PJ, van Gijn J. Grading white matter lesions on CT and MRI: a simple scale. J Neurol Neurosurg Psychiatry 1990;53:1080–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.IST-3 Collaborative Group. Association between brain imaging signs, early and late outcomes, and response to intravenous alteplase after acute ischaemic stroke in the third International Stroke Trial (IST-3): secondary analysis of a randomised controlled trial. Lancet Neurol 2015;14:485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arba F, Inzitari D, Ali M, Warach SJ, Luby M, Lees KR; STIR/VISTA Imaging Collaboration. Small vessel disease and clinical outcomes after IV rt-PA treatment. Acta Neurol Scand 2017;136:72–77. [DOI] [PubMed] [Google Scholar]
- 15.Arba F, Mair G, Carpenter T, et al. ; IST-3 Trial Collaborators. Cerebral white matter hypoperfusion increases with small-vessel disease burden: data from the Third International Stroke Trial. J Stroke Cerebrovasc Dis 2017;26:1506–1513. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg GA. Neurological diseases in relation to the blood brain barrier. J Cereb Blood Flow Metab 2012;32:1139–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013;12:483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wardlaw JM, Makin S, Valdés Hernández MC, et al. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimer's Dement 2017;13:634–643. [Google Scholar]
- 19.Kaur J, Tuor UI, Zhao Z, Barber PA. Quantitative MRI reveals the elderly ischemic brain is susceptible to increased early blood–brain barrier permeability following tissue plasminogen activator related to claudin 5 and occludin disassembly. J Cereb Blood Flow Metab 2011;31:1874–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg GA, Wallin A, Wardlaw JM, et al. Consensus statement for diagnosis of subcortical small vessel disease. J Cereb Blood Flow Metab 2016;36:6–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiol Aging 2009;30:337–352. [DOI] [PubMed] [Google Scholar]
- 22.Kassner A, Roberts TP, Moran B, Silver FL, Mikulis DJ. Recombinant tissue plasminogen activator increases blood-brain barrier disruption in acute ischemic stroke: an MR imaging permeability study. AJNR Am J Neuroradiol 2009;30:1864–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kidwell CS, Latour L, Saver JL, et al. Thrombolytic toxicity: blood brain barrier disruption in human ischemic stroke. Cerebrovasc Dis 2008;25:338–343. [DOI] [PubMed] [Google Scholar]
- 24.Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke 2004;35:2659–2661. [DOI] [PubMed] [Google Scholar]
- 25.Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol 2004;56:468–477. [DOI] [PubMed] [Google Scholar]
- 26.Simpkins AN, Dias C, Leigh R; National Institutes of Health Natural History of Stroke Investigators. Identification of reversible disruption of the human blood-brain barrier following acute ischemia. Stroke 2016;47:2405–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Zoppo GJ. Aging and the neurovascular unit. NY Ann Acad Sci 2012;1268:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah S, Luby M, Poole K, et al. Screening with MRI for accurate and rapid stroke treatment: SMART. Neurology 2015;84:2438–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leigh R, Jen SS, Hillis AE, et al. Pretreatment blood-brain barrier damage and posttreatment intracranial hemorrhage in patients receiving intravenous tissue-type plasminogen activator. Stroke 2014;45:2030–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leigh R, Christensen S, Campbell BC, et al. ; DEFUSE 2 Investigators. Pretreatment blood-brain barrier disruption and post-endovascular intracranial hemorrhage. Neurology 2016;19:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kluge A, Lukas M, Toth V, Pyka T, Zimmer C, Preibisch C. Analysis of three leakage correction methods for DSC-based measurement of relative cerebral blood volume with respect to heterogeneity in human gliomas. Magn Reson Imaging 2016;34:410–421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.