Abstract
Vascular pathology and protein accumulation both contribute to cognitive decline, whereas exercise can slow vascular degeneration and improve cognitive function. Recent investigations suggest that glymphatic clearance measured in aged mice while anesthetized is enhanced following exercise. We predicted that exercise would also stimulate glymphatic activity in awake, young mice with higher baseline glymphatic function. Therefore, we assessed glymphatic function in young female C57BL/6J mice following five weeks voluntary wheel running, as compared to sedentary mice. The active mice ran a mean distance of 6 km daily. We injected fluorescent tracers in cisterna magna of awake behaving mice and in ketamine/xylazine anesthetized mice, and later assessed tracer distribution in coronal brain sections. Voluntary exercise consistently increased CSF influx during wakefulness, primarily in the hypothalamus and ventral parts of the cortex, but also in the middle cerebral artery territory. While glymphatic activity was higher under ketamine/xylazine anesthesia, we saw a decrease in glymphatic function during running by awake mice after five weeks of wheel running. In summary, daily running increases CSF flux in widespread areas of mouse brain, which may mediate the pro-cognitive effects of exercise.
Keywords: Exercise, hypothalamus, glymphatic system, convection, astrocyte, cardiovascular changes
Introduction
Exercise affects several basic physiological functions such as sleep, appetite, and mood. Regular exercise can protect against chronic diseases and has been suggested as a therapeutic intervention for dementia because of the robust enhancement of cognitive performance following exercise [1–3]. The enhanced post-exercise cognitive boost correlates with modulation of cerebrovascular function. For example, vessel surface area and perimeter [4] as well as vessel density [5] in the rodent dentate gyrus increase after a bout of exercise. These benefits may be mediated by increased levels of insulin-like growth factor 1 (IGF-1), which has a role in vascular remodeling [6]. Indeed, serum levels of IGF-1 and other growth factors, such as brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF), are elevated in humans and rodents after a bout of exercise, both in athletic and untrained individuals performing aerobic or anaerobic exercise [7–10]. These growth factors are involved in processes such as neurogenesis, angiogenesis, and synaptic plasticity, which may contribute causally to improved cognition. However, the nature of these relationships is complex, since habitual training lowers the baseline BDNF level in humans, as suggested by negative correlations between maximal aerobic performance (VO2 max) and BDNF levels [7,8,11]. Similar findings has been reported for VEGF levels in relation to fitness training [12]. Furthermore, rodent studies do not universally show an increased neurogenesis upon exercise [13]. While a positive relationship between growth factors levels and enhanced cognition seems well-established, others have argued that there is rather a correlation between exercise intensity and BDNF levels than a causal effect on cognition per se [14].
The glymphatic system is a pathway for circulation of cerebrospinal fluid through the interstitial space, which mediates clearance of intracerebral neurotoxic waste to the peripheral circulation [15]. Glymphatic activity measured in anesthetized aged mice was shown to increase following exercise, in association with some rescue of spatial memory performance [16]. The glymphatic system is partly driven by the cardiorespiratory system, the function of which is indeed altered by exercise [3], such that the glymphatic system could be a factor participating in the pro-cognitive effects of exercise. However, anesthesia itself affects glymphatic activity, and corresponding effects of exercise on glymphatic activity in awake mice are unknown. In this study, we measure the influx of cerebrospinal fluid (CSF) into the brain parenchyma to characterize the dynamic relationship between exercise and the glymphatic activity in healthy, young, and awake mice. In a separate experiment, we tested the acute effects of running during the tracer uptake phase in mice.
Materials and Methods
Animals
All experiments were approved by the Danish Animal Experiments Inspectorate. Nine weeks old female mice (C57BL/6JRj, Janvier) were pair-housed to avoid the negative effects of social deprivation [17]. The mice had ad libitum food and water access in an environment with a 12:12 hour light/dark cycle (lights on at 6 a.m.). Two running wheels (Columbus Instruments) with inner diameter of 9.2 cm (29 cm circumference) were mounted in the cages and the mice had free running wheel access for the subsequent five weeks. Control mice had similar individually ventilated cages (IVC) with enrichment, but no running wheels. Each turn of the running wheel was registered by a magnet and activity was sampled at 10 seconds intervals (software from Columbus Instruments). We calculated the mean running distance per mouse for each cage.
Cisterna Magna Injection
The cisterna magna (CM) cannulations were performed as described previously [18]. The mice were anesthetized with isoflurane, and received preoperative buprenorphine (0.1 mg/kg i.p.), along with local anesthesia at the surgical site (0.25 ml lidocaine (1 mg/ml) and bupivacaine (0.25 mg/ml)). After positioning the needle tip in the CM, the needle was fixed by application of superglue and dental cement, and the animals were returned to their home cage. The following day, the ketamine/xylazine (KX) groups were anesthetized (100 mg/kg ketamine and 20 mg/kg xylazine, i.p.), whereas the awake groups were untreated. The study was of 2×2 design and included four groups (control awake (n=10), control KX (n=10), exercise awake (n=6), exercise KX (n=8)). All mice then received a CM injection of 10 µl 1% fluorescein-conjugated dextran (FITC, 3 kDa, Thermofisher D3305) and 0.5% Alexa Fluor 647-conjugated ovalbumin (AF647, 45 kDa, Thermofisher O34784) in artificial CSF, injected over a period of ten minutes. At 25 or 180 minutes after the end of tracer infusion, mice were euthanized by cervical dislocation. For glymphatic clearance mice were 10–14 weeks old (n=4). Brains were rapidly removed and fixed by immersion overnight in 4% PFA at 4 °C degrees before cutting 100 µm coronal sections on a vibratome. Seven sections spaced across the A-P axis were chosen for microscopic analysis, stained with DAPI, and then mounted with PROLONG Anti-Fade Gold (Life Technologies).
Acute Running
To investigate whether acute running affects tracer distribution, exercised mice were CM cannulated as described above. During CM injection, the mice were either active in their running wheel or moved freely around their cage (groups referred to as ‘Running’ vs. ‘Awake’, respectively).
Tracer Analysis
Multi-channel whole-slice montages were acquired with the ND Acquisition tool of Nikon at constant exposure levels throughout the study. Images were analyzed in ImageJ software (NIH), with uniform thresholding at a pixel intensity of 50 (out of 255), and subtraction of background fluorescence. The thresholded area was expressed as percentage of the entire slice area, and the mean tracer coverage for the seven slices was averaged within each animal to generate a single biological measure. For the regional analysis, the section −1.7 mm from bregma was divided into regions of interest (hippocampus, thalamus, hypothalamus, dorsal, lateral, and ventral cortex). To illustrate the average tracer distribution by group, we selected and overlaid one coronal section (bregma −0.3) from each mouse.
Immunohistochemistry
The sections were blocked in goat serum before overnight incubation with primary antibodies at 4° C. After washing, fluorescent secondary antibody incubation was undertaken for two hours at room temperature. Primary antibodies were anti-aquaporin-4 (AQP4) (EMD Millipore, AB3594; 1:500) and anti-glial fibrillary acidic protein (GFAP) (Thermofisher, PA1-10004; 1:400). Secondary antibodies were goat anti-rabbit AF568 (Thermofisher, A11011; 1:500) and goat anti-chicken AF568 (Thermofisher, A11041; 1:500), respectively. For both hemispheres, we acquired 10 µm z-stacks from six mice using a Nikon Instruments C2+ confocal laser-scanning microscope equipped with a 40× 1.3 NA oil immersion objective. The excitation sources were 405, 488, and 565 nm solid-state diode laser lines. Maximum intensity projections were analyzed in ImageJ software.
Physiological Measurements
We used a small animal physiological monitoring system (75–1500, Harvard Apparatus) to measure heart and respiratory rate. Anesthesia was induced with 3% isoflurane and maintained at 1.5% for five-minute acclimatization followed by three-minute recordings.
Statistics
Data are presented as mean ±SEM. All statistics were performed with the software Prism (GraphPad). The statistical treatment of each dataset is described in the corresponding figure legends. A p-value of <0.05 was considered significant for rejection of the null hypothesis.
Results
Wheel running
After introducing running wheels in the cage, the running distance increased steadily for the first six days and thereafter maintained at a plateau (Figure 1A) corresponding to a mean running distance of 6.7±0.2 km/day (n=8), with running activity for a mean of 4.6±0.1 hours per night cycle.
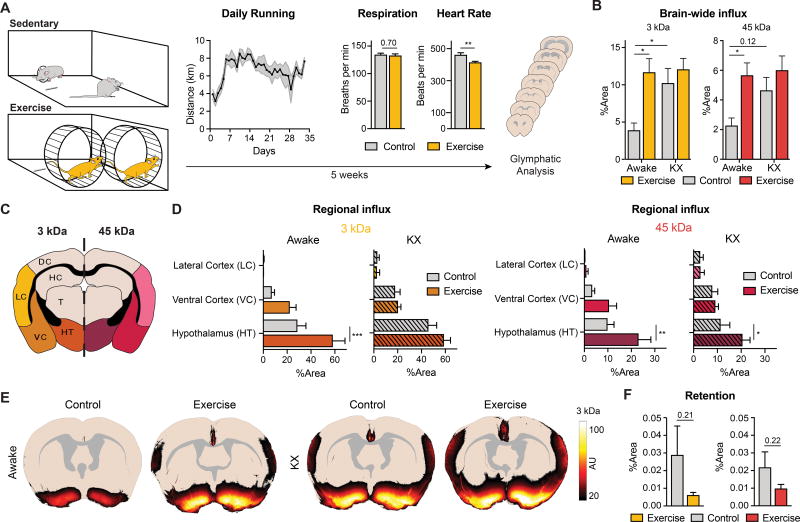
Figure 1. Exercise increases CSF tracer influx in awake, behaving mice.
A. Female C57BL/6J mice are pair housed for five weeks before analysis of their glymphatic system and subsequent immunohistochemistry. The activity of the mice is measured as average daily running distance per cage. Mean distance per day is 6.7 km (n=8). The respiration and heart rate measured under isoflurane anesthesia (n=7–8) (Student’s t-test, P=0.7 and P=0.0061, respectively). B. Total CSF tracer concentration measured as percentage coverage of 7 coronal sections for a small (FITC-dextran, 3 kDa) and large (ovalbumin-AF647, 45 kDa) tracer (n=6–10). (Two-way ANOVA with Tukey’s correction, * = P<0.05). C. A schematic representation of the regional analysis. D. Regional analysis of the CSF tracers (3 and 45 kDa). The fluorescence intensities are measured as the percentage area coverage (Two-way ANOVA with Sidak correction, ** = P<0.01, *** = P<0.001). Regions without tracer fluorescence were omitted from the figure. E. Overlay of fluorescence influx in coronal sections −0.3 mm from bregma for the four groups (n=6–10). F. Retention of CSF tracers (3 and 45 kDa) assessed after 3 hours of circulation in awake mice (n=4) (Student’s t-test, P=0.21 and P=0.22).
Physiological measurements
Since exercise enhances basic physiological mechanisms to improve oxygen delivery, we measured heart and respiration rate for the groups after five weeks of voluntary running. Under isoflurane anesthesia, there was no group difference for the respiratory rate (134±2.7 vs. 132±3.1 breaths per minute, P=0.7, n=7–8), perhaps due to confounding effects of isoflurane anesthesia. However, heart rate decreased significantly in the exercised group (461±13.3 vs. 414±6.9 beats per minute, P=0.006, n=7–8) (Figure 1A), consistent with prior reports [19].
Exercise increases glymphatic activity
The glymphatic tracer influx in awake exercised mice was more than 2-fold that in awake littermates without access to a running wheel (Figure 1B). In contrast, CSF tracer influx did not differ between the exercise and control group subjected to KX anesthesia (P=0.85 for FITC-3 kDa and P=0.66 for AF647 45 kDa). Thus, the potency by which exercise increased the glymphatic activity in the awake state could be compared to the effect of KX anesthesia on brain-wide tracer influx.
Regional fluorescence intensity differs
We next analyzed the group differences in regional distribution and intensity of tracers across several different brain regions (Figure 1C). Exercised and sedentary awake groups primarily showed tracer uptake in ventral parts of the brain (hypothalamus and ventral cortex), albeit at a significant higher intensity in the exercised group (Figure 1D). The two KX anesthetized groups displayed a strong enhancement of CSF tracer intensity along the lateral cortex, consistent with previous reports [20]. Strikingly, the awake exercise group also exhibited tracer uptake in this area, implying that exercise increases peri-arterial CSF influx along the middle cerebral artery (MCA) in awake mice (Figure 1E).
Glymphatic clearance increases after exercise
In a separate experiment, the tracer distribution 180 minutes after CM injection was assessed in awake animals. The analysis showed a trend towards reduced retention of the tracers, indicating that both tracers were cleared more quickly in the exercise group (Figure 1F). Since CSF tracer influx was increased in the voluntary running group (Figure 1B), these observations suggest that exercise enhances both influx and efflux of CSF tracers.
Wheel running impairs tracer influx
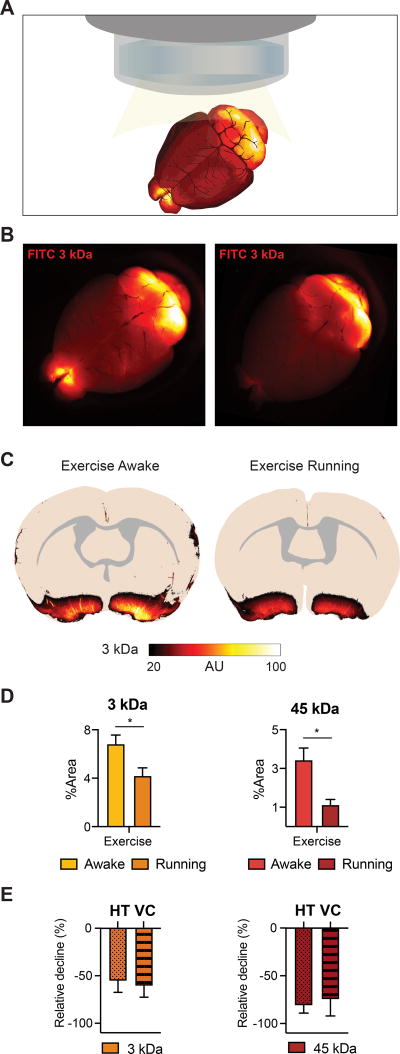
Next, we analyzed the influx of CSF tracers in exercised mice actively running in their home cage. The CM injection was started when they voluntarily began to run. The overall tracer influx was suppressed by acute running compared to awake behaving, exercised mice (Figure 2A–C). Quantitative image analysis showed that tracer influx was suppressed both with regard to brain-wide and regional tracer distribution (Figure 2D–E). The increase in glymphatic activity in the awake exercised group must therefore be attributable to a physiological adaptation to regular physical activity, rather than an acute effect of exercise.
Figure 2. Active running reduces CSF tracer influx.
A. Schematic representing the dorsal macroscopic view of the whole brain after CM injection. B. Macroscopic image of the FITC (3 kDa) tracer distribution in the exercise awake and exercise running groups. C. Overlay of fluorescence influx in coronal sections (bregma −0.3 mm) for exercise awake and exercise running mice (n=3–4). D. The tracer intensity (FITC, 3 kDa and AF647, 45 kDa) as percentage coverage averaged over 7 coronal sections for the exercise running group (n=4) compared to awake exercised mice (n=3). (Student’s ttest, * = P<0.05). E. Relative difference in regional distribution in hypothalamus (HT) and ventral cortex (VC) for the running exercise group compared to the awake exercise group (normalized values, n=3–4).
Immunohistochemistry
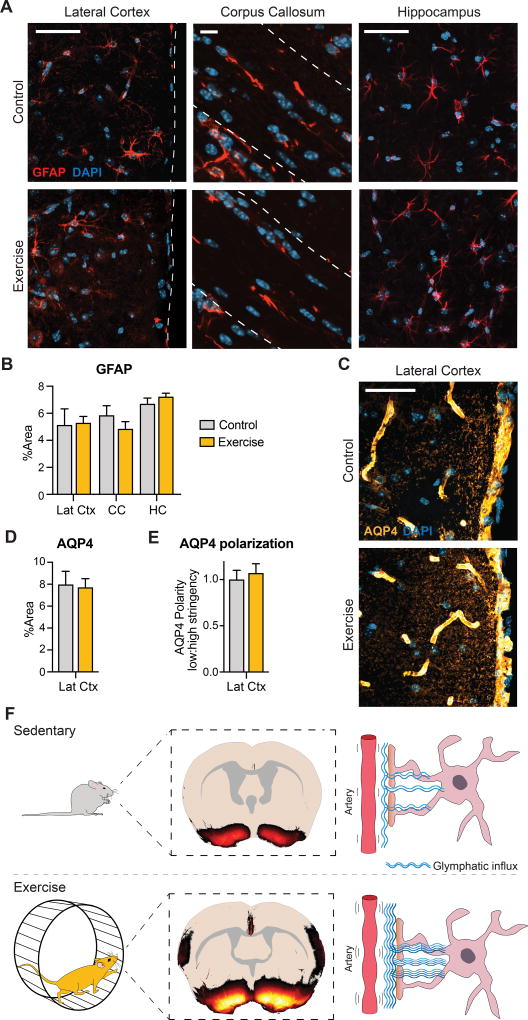
Since astrocytes are key players in the glymphatic system, we used immunohistochemistry to assess effects of voluntary running on expression of the astrocytic marker glial fibrillary protein (GFAP) and the water channel, aquaporin-4 (AQP4). The levels of GFAP (Figure 3A–B) and AQP4 (Figure 3C–D) were similar between the groups. Furthermore, a trend towards an exercise-induced polarization of AQP4 was noted (Figure 3E).
Figure 3. Astrocytic expression of GFAP and AQP4 is unaltered.
A. Confocal images of GFAP expression shown as maximum intensity projection of 10 µm z-stack, 40×. B. Glial fibrillary acidic protein (GFAP) expression in layer 1 of the lateral cortex (Lat Ctx), corpus callosum (CC), and CA3 of the hippocampus (HC) quantified as percentage area coverage (n=6) (Multiple t-testing with FDR, P=0.3–0.9). C. Representative confocal images of aquaporin-4 AQP4 expression (40×). D. AQP4 expression quantified as percentage area coverage in layer 1 of the lateral cortex (n=6) (Student’s t-test, P=0.8). E. AQP4 polarization calculated as low to high stringency as previously described [31]. Data are normalized. No significant difference (n=6) (Student’s t-test, P = 0.6). F. Our proposed model of glymphatic activity following five weeks of voluntary wheel running. When CSF tracers are injected into the cisterna magna of exercised awake animals, the glymphatic influx is increased compared to sedentary control mice.
Discussion
This study shows that voluntary exercise increases the influx of CSF tracers into the brain of young, freely behaving, awake mice relative to sedentary mice. In fact, the exercised-induced enhancement of glymphatic activity was of similar magnitude to the effect of K/X anesthesia on tracer uptake compared to control mice (Figure 1B) [21]. However, a detailed regional analysis revealed that the tracer influx induced by exercise training was most pronounced in the hypothalamus and ventral parts of the cortex. The influx of tracers along the lateral cortex in the acutely exercising awake group is suggestive of the cardiovascular changes in the territory of the MCA in response to exercise, which is consistent with the well-known beneficial effects of exercise on cardiac functions (Figure 1A). Habitual training modulates the cardiovascular system by lowering the heart rate, increasing the pulse pressure (systolic-diastolic values) as well as decreasing arteriolar resistance in order to optimize blood flow [22,23]. The anesthetized groups exhibit the highest influx of tracer along the MCA, but our analysis shows that a similar pattern also occurs in the awake state upon five weeks of voluntary running. In support of this, He et al. recently demonstrated increased glymphatic influx and clearance rates in aged exercising mice [16].
Contrary to findings in anesthetized exercised mice, we find that glymphatic influx is low while the mice are running. We suggest that this acute suppression of glymphatic influx is a downstream effect of increased norepinephrine (NE) signaling in the brain during exercise [24,25]. It could be hypothesized that brain metabolite clearance is simply not prioritized during running. The high glymphatic activity in sleep state correlates with low tonic activity of the noradrenergic locus coeruleus, and that pharmacological treatment with NE antagonists enhances glymphatic activity, consistent with a tonic NE-ergic inhibition of glymphatic function [21].
It was recently reported that exercise increases clearance of amyloid-beta from the brains of aged mice by an upregulation of glymphatic activity, which was measured in the anesthetized state [16]. Nonetheless, we failed in the present study to discern significant changes in glymphatic flow in the anesthetized state after exercise. We used K/X anesthesia, which strongly suppresses NE release, whereas He et al. in the earlier study used chloral hydrate, which has minimal effect on the sympathetic nervous system [26]. Since the activity of noradrenergic neurons in the locus coeruleus is a key factor regulating glymphatic activity, we suppose that choice of anesthetic agent could explain the discrepancy between studies [21]. However, mice used by He et al. were considerably older than in the present study (14 months vs. 3 months). It is possible that exercise can rescue glymphatic activity in the anesthetized state in old mice due to vascular dysfunction that has been reported to develop in aged rodents with sedentary lifestyle [27].
In young rat brain, exercise has been shown to increase glial fibrillary acidic protein (GFAP) expression and induce astrocytic proliferation [28,29]. However, in young sedentary and exercised mice, we found comparable levels of GFAP in different brain regions (Figure 3A–B), consistent with another recent study in adult mice brain [30]. Polarized AQP4 facilitates the CSF flux from the perivascular space, leading us to predict more extensive AQP4 polarization in the hypothalamus of the exercised group, in proportion to their increased glymphatic function in ventral structures. Astrocytic AQP4 polarization normally decreases with age, but can be rescued by exercise [16]. Nonetheless, we did not see exercise-induced rescue of polarization in our mice, perhaps due to high prevailing polarization noted previously in mice of similar young age [20].
Regular exercise is increasingly showing benefits for many aspects of brain health, including the maintenance of cerebrovascular integrity. We now show that free access to a running wheel of five weeks increases perivascular influx of CSF in young mice in a manner unrelated to effects of anesthesia. We propose that heightened glymphatic activity induced by exercise may favor brain health by enhancing clearance of neurotoxic waste products from brain.
Highlights.
We assessed glymphatic activity in young mice after five weeks of voluntary running.
Exercise increases glymphatic influx in awake but not in anesthetized animals.
Glymphatic influx is enhanced in hypothalamus, ventral, and lateral cortex.
CSF tracer influx declines during running itself.
Acknowledgments
The project is part of the research program “Emerging Roles of Astrocytes in Health and Disease” supported by The Novo Nordisk Foundation and was further supported by Lundbeckfonden, Læge Sofus Carl Emil Friis og Hustru Olga Friis’ Legat, as well as NIH. We would like to thank Dan Xue for help with graphical design and Humberto Mestre for the brain-wide integration of coronal tracer distribution. We thank Paul Cumming for comments and edits of the manuscript.
Abbreviations
- AQP4
Aquaporin-4
- BDNF
Brain-derived neurotrophic factor
- CM
Cisterna magna
- CSF
Cerebrospinal fluid
- GFAP
Glial fibrillary acidic protein
- IGF-1
Insulin-like growth factor 1
- KX
Ketamine/xylazine
- MCA
Middle cerebral artery
- VEGF
Vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions statement
M.N. conceived of the study and edited the manuscript. N.C.P. analyzed running data and reviewed the manuscript. S.H.R. carried out the experiments, analyzed and interpreted the data, and conceived of the manuscript.
Competing interests statement
The authors have no competing interests to declare.
References
- 1.Chennaoui M, Arnal PJ, Sauvet F, Léger D. Sleep and exercise: A reciprocal issue? Sleep Med. Rev. 2015;20:59–72. doi: 10.1016/j.smrv.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Booth FW, Roberts CK, Laye MJ. Lack of Exercise Is a Major Cause of Chronic Diseases. Compr. Physiol. 2012;2:1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Recommendations on Physical Activity for Health. [Accessed 05/05/2017];WHO. 2010 May;:1–60. http://apps.who.int/iris/bitstream/10665/44399/1/9789241599979_eng.pdf. [PubMed]
- 4.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptaions to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19:937–950. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc. Natl. Acad. Sci. 2004;101:9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Currie J, Ramsbottom R, Ludlow H, Nevill A, Gilder M. Cardio-respiratory fitness, habitual physical activity and serum brain derived neurotrophic factor (BDNF) in men and women. Neurosci. Lett. 2009;451:152–155. doi: 10.1016/j.neulet.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 8.Babaei P, Damirchi A, Mehdipoor M, Tehrani BS. Long term habitual exercise is associated with lower resting level of serum BDNF. Neurosci. Lett. 2014;566:304–308. doi: 10.1016/j.neulet.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Uysal N, Kiray M, Sisman aR, Camsari UM, Gencoglu C, Baykara B, Cetinkaya C, Aksu I. Effects of voluntary and involuntary exercise on cognitive functions, and VEGF and BDNF levels in adolescent rats. Biotech. Histochem. 2015;90:55–68. doi: 10.3109/10520295.2014.946968. [DOI] [PubMed] [Google Scholar]
- 10.Carro E, Nuñez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 2000;20:2926–2933. doi: 10.1016/j.tins.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung SH, Kim J, Davis JM, Blair SN, Cho HC. Association among basal serum BDNF, cardiorespiratory fitness and cardiovascular disease risk factors in untrained healthy Korean men. Eur. J. Appl. Physiol. 2011;111:303–311. doi: 10.1007/s00421-010-1658-5. [DOI] [PubMed] [Google Scholar]
- 12.Gu J-W, Gadonski G, Wang J, Makey I, Adair TH. Exercise increases endostatin in circulation of healthy volunteers. BMC Physiol. 2004;4:2. doi: 10.1186/1472-6793-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambrogini P, Orsini L, Mancini C, Ferri P, Ciaroni S, Cuppini R. Learning may reduce neurogenesis in adult rat dentate gyrus. Neurosci. Lett. 2004;359:13–16. doi: 10.1016/j.neulet.2003.12.123. [DOI] [PubMed] [Google Scholar]
- 14.Ferris LT, Williams JS, Shen C-L. The Effect of Acute Exercise on Serum Brain-Derived Neurotrophic Factor Levels and Cognitive Function. 2007;39:728–734. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- 15.Jessen NA, Munk ASF, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015;40:2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He X, Liu D, Zhang Q, Liang F, Dai G, Zeng J, Pei Z, Xu G, Lan Y. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front. Mol. Neurosci. 2017;10:1–14. doi: 10.3389/fnmol.2017.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry A, Bellisario V, Capoccia S, Tirassa P, Calza A, Alleva E, Cirulli F. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology. 2012;37:762–772. doi: 10.1016/j.psyneuen.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes. Including Amyloid, Sci. Transl. Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stypmann J. Doppler Ultrasound in Mice. Echocardiography. 2007;24:97–112. doi: 10.1111/j.1540-8175.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- 20.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014;76:845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep Drives Metabolite Clearance from the Adult Brain. Science (80-.) 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baggish AL, Wood MJ. Athlete’s Heart and Cardiovascular Care of the Athlete: Scientific and Clinical Update. Circulation. 2011;123:2723–2735. doi: 10.1161/CIRCULATIONAHA.110.981571. [DOI] [PubMed] [Google Scholar]
- 23.Bevegård BS, Shepherd JT. Regulation of the Circulation During Exercise in Man. Physiol. Rev. 1967;47:178–213. doi: 10.1152/physrev.1967.47.2.178. http://www.ncbi.nlm.nih.gov/pubmed/5342871. [DOI] [PubMed] [Google Scholar]
- 24.Stranahan AM, Lee K, Mattson MP. Central Mechanisms of HPA Axis Regulation by Voluntary Exercise. NeuroMolecular Med. 2008;10:118–127. doi: 10.1007/s12017-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziegler DR, Cass WA, Herman JP. Excitatory influence of the locus coeruleus in hypothalamic-pituitary-adrenocortical axis responses to stress. J. Neuroendocrinol. 1999;11:361–369. doi: 10.1046/j.1365-2826.1999.00337.x. [DOI] [PubMed] [Google Scholar]
- 26.Tremoleda JL, Kerton A, Gsell W. Anaesthesia and physiological monitoring during in vivo imaging of laboratory rodents: considerations on experimental outcomes and animal welfare. EJNMMI Res. 2012;2:44. doi: 10.1186/2191-219X-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in Cerebral Microvasculature with Age are Associated with the Decline in Growth Hormone and Insulin-Like Growth Factor 1*. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- 28.Saur L, Baptista PPA, de Senna PN, Paim MF, Do Nascimento P, Ilha J, Bagatini PB, Achaval M, Xavier LL. Physical exercise increases GFAP expression and induces morphological changes in hippocampal astrocytes. Brain Struct. Funct. 2014;219:293–302. doi: 10.1007/s00429-012-0500-8. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Ding Y-H, Rafols JA, Lai Q, McAllister JP, Ding Y. Increased astrocyte proliferation in rats after running exercise. Neurosci. Lett. 2005;386:160–164. doi: 10.1016/j.neulet.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Fahimi A, Baktir MA, Moghadam S, Mojabi FS, Sumanth K, McNerney MW, Ponnusamy R, Salehi A. Physical exercise induces structural alterations in the hippocampal astrocytes: exploring the role of BDNF-TrkB signaling. Brain Struct. Funct. 2017;222:1797–1808. doi: 10.1007/s00429-016-1308-8. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Iliff JJ, Liao Y, Chen MJ, Shinseki MS, Venkataraman A, Cheung J, Wang W, Nedergaard M. Cognitive Deficits and Delayed Neuronal Loss in a Mouse Model of Multiple Microinfarcts. J. Neurosci. 2012;32:17948–17960. doi: 10.1523/JNEUROSCI.1860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]