Abstract
Colon cancer is the most common type of gastrointestinal cancer. A number of specific and sensitive biomarkers facilitate the diagnosis and monitoring of patients with colon cancer. Fusion genes are typically identified in cancer and a majority of the newly identified fusion genes are oncogenic in nature. Therefore, fusion genes are potential biomarkers and/or therapy targets in cancer. In the present study, the regulation of specific candidate fusion genes were investigated using Brother of the Regulator of Imprinted Sites (BORIS) in the HCT116 colon cancer cell line, which is a paralog of the fusion gene regulator CCCTC-binding factor (CTCF). The copy number of BORIS increased correspondingly to the progression of colorectal carcinoma from the M0 to the M1a stage. It was identified that EIF3E(e1)-RSPO2(e2), EIF3E(e1)-RSPO2(e3), PTPRK(e1)-RSPO3(e2), PTPRK(e7)-RSPO3(e2), TADA2A-MEF2B and MED13L-CD4 are fusion transcripts present in the transcriptome of the HCT116 colon cancer cell line. CDC42SE2-KIAAO146 is a genomic fusion transcript, which originates from DNA arrangement in HCT116 cells. BORIS suppresses the expression of EIF3E, RSPO2, PTPRK, RSPO3, TADA2A and CD4 to inhibit the expression of fusion transcripts in HCT116 cells. It was hypothesized that the fusion transcripts investigated in the present study may not be oncogenic in HCT116 cells. As BORIS is not colorectal carcinoma-specific, the fusion genes investigated may be a biomarker assemblage for monitoring the progression of colorectal carcinoma.
Keywords: fusion gene, colorectal carcinoma, Brother of the Regulator of Imprinted Sites, 5-aza-2′-deoxycytidine, HCT116
Introduction
Colon cancer is the most common type of gastrointestinal cancer. The risk of the colon cancer is associated with lifestyle, inherent cause and colorectal adenoma. Diagnosis of colon cancer includes laboratory endoscopy, biopsy, exfoliative cytopathology and carcinoembryonic antigen (CEA) testing. The CEA test measures the amount of this protein that may appear in the blood of certain people who have certain types of cancer, particularly cancer of the large intestine (colon and rectal cancer). However, high CEA levels do not indicate cancer cell metastasis, as low CEA levels were also detected in patients with metastatic colon cancer. Exfoliative cytopathology is not frequently used in clinics, because an ideal exfoliative sample was difficult to obtain. Specific and sensitive biomarkers or a distinct biomarker assemblage may facilitate the diagnosis and monitoring of patients with colon cancer.
Fusion genes are often identified in cancer and numerous newly identified fusion genes have oncogenic properties (1). Therefore, fusion genes are potential biomarkers or therapeutic targets in cancer (2). Fusion genes are a novel type of gene that are a full or partial fusion of two genes and usually result from chromosomal rearrangements (1). Recently, RNA sequencing (RNA-seq) was applied in the study of transcriptomes and novel fusion genes were identified, including SLC45A3-ELK4 and PAX3-FOXO1, which were ascertained to promote cancer progression (3–5). Using transcriptome sequencing, Seshagiri et al (6) identified IF3E-RSPO2 and PTPRK-RSPO3 in clinical colon cancer samples and predicted that these fusion genes are involved in colon cancer progression.
CCCTC-binding factor (CTCF) was previously revealed to suppress expression of the fusion transcript SLC45A3-ELK4 in prostate cancer by binding to the insulators on the genome between SLC45A3 and ELK4 (7). CTCF binds to enhances or insulators on chromosomes to inhibit the spread of heterochromatin and regulate gene expression (8,9). As there are ~15,000 binding sites for CTCF in the human genome (10), CTCF may regulate the expression of numerous other fusion genes. Qin et al (11) identified that CTCF regulates the expression of fusion transcripts that are not unique to cancer cells. CTCF is widely expressed in normal tissues, so may not drive the onset and progression of cancer (10). Brother of the Regulator of Imprinted Sites (BORIS) is a paralog of CTCF and is expressed in normal testis, ovary and skin cells; however, it is abnormally expressed in breast, prostate and colon cancer cells (12–17). As BORIS has the same zinc-finger domains as those of CTCF (13), BORIS may bind to CTCF-binding sites and regulate fusion transcripts. Considering the potential effect of BORIS on fusion genes and its carcinogenicity, the regulation of fusion genes identified using RNA-seq by Seshagiri et al (6) was evaluated using BORIS in the HCT116 colon cancer cell line. The copy number of BORIS increased as the colorectal carcinoma progressed from the M0 to the M1a stage (www.oncomine.org) (18). The HCT116 colon cancer cell line possesses EIF3E(e1)-RSPO2(e2), EIF3E(e1)-RSPO2(e3), PTPRK(e1)-RSPO3(e2), PTPRK(e7)-RSPO3(e2), TADA2A-MEF2B and MED13L-CD4 fusion transcripts within the transcriptome. CDC42SE2-KIAAO146 is a genomic fusion transcript originating from DNA arrangement in HCT116 cells. BORIS suppresses the expression of EIF3E, RSPO2, PTPRK, RSPO3, TADA2A and CD4 to inhibit the expression of fusion transcripts in HCT116 cells. It was hypothesized that the fusion transcripts investigated here may not have oncogenic functions in HCT116 cells. As BORIS is not colorectal carcinoma-specific, the fusion genes investigated may constitute a biomarker assemblage for monitoring the progression of colorectal carcinoma.
Materials and methods
Cell culture
The human HCT116 colon carcinoma cell line and the K562 chronic myelogenous leukemia cell linewere cultivated in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Cells were seeded at a density of 1×105 into 6-well plates prior to drug treatment. When cells reached 70% confluence, 5 µM 5-aza-2′-deoxycytidine (5-Aza-dC; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was applied to treat the cells for 48 h, following which an equal volume was added to re-treat the cells for 48 h. Acetic acid (50%) in water was used as the negative control. RNA from the cells was extracted using TRIzol® reagent (Thermo Fisher Scientific, Inc. Waltham, MA, USA), according to the manufacturer's protocol.
siRNA silencing and transfection
Negative siRNA and BORIS siRNA were synthesized by Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). BORIS siRNA targeting; forward, 5′-AACGACGAUGCCGAACCAAUU-3′ was used for silencing BORIS. The target of negative siRNA is not homologous with any sequence of the human genome. The BORIS overexpression plasmid (p-BORIS) was obtained from OriGene Technologies, Inc. (Rockville, MD, USA). Cells were seeded onto a 6-well plate for transfection. Lipofectamine® RNAiMAX reagent and Lipofectamine® 3000 reagent (Thermo Fisher Scientific, Inc.) were applied to, respectively, transfect the siRNA and the plasmid, and to silence and induce the expression of BORIS in HCT116 cells. RNA was extracted 3 days after transfection.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA was extracted using TRIzol® (Thermo Fisher Scientific, Inc.) for subsequent RT using TransScript®One-Step gDNA Removal and cDNA Synthesis SuperMix (Beijing Transgen Biotech Co., Ltd., Beijing, China). The transcript amount was quantified using RT-qPCR and calculated using the 2−ΔΔCq method (19). The UltraSYBR Mixture was purchased from cwbiotech (Beijing CWBIO Biotech Co., Ltd., Beijing, China) for RT-qPCR reaction. Primers are listed in Table I. RT-qPCR was conducted using the Applied Biosystems® 7500 Real-Time PCR System (Thermo Fisher Scientific, Inc.).
Table I.
Primers used in the present study.
| Fusion gene | Forward primer (5′-3′) | Reverse primer (5′-3′) | Transcript length, bp |
|---|---|---|---|
| EIF3E(e1)-RSPO2(e2) | ACTACTCGCATCGCGCACT | GGGAGGACTCAGAGGGAGAC | 155 |
| EIF3E(e1)-RSPO2(e3) | ACTACTCGCATCGCGCACT | TGCAGGCACTCTCCATACTG | 205 |
| PTPRK(e1)-RSPO3(e2) | AAACTCGGCATGGATACGAC | GCTTCATGCCAATTCTTTCC | 226 |
| PTPRK(e7)-RSPO3(e2) | TGCAGTCAATGCTCCAACTT | GCCAATTCTTTCCAGAGCAA | 250 |
| ETV6-NTRK3 | AAGCCCATCAACCTCTCTCA | GGGCTGAGGTTGTAGCACTC | 206 |
| CDC42SE2-KIAA0146 | AGGGCCAGATTTGAGTGTGT | AAACTGAAAATCCCCGCTGT | 188 |
| TADA2A-MEF2B | GCTCTTTGGCGCGGATTA | GGAGCTACCTGTGGCCCT | 152 |
| MED13L-CD4 | GTGTATGGCGTCGTGATGTC | TCCCAAAGGCTTCTTCTTGA | 151 |
The transcript length indicates the amplicon length achieved using the primers listed. All primers are from Seshagiri et al (6).
Genomic DNA extraction and genomic fusion detection
HCT116 and K562 cell genomic DNA was extracted using the phenol-chloroform extraction method (7). Primers used to test fusion genes are listed in Table I. Taq DNA polymerase purchased from Takara Biotechnology Co., Ltd., Dalian, China was utilized for PCR. HCT116 and K562 genomic DNA were applied as templates to detect the putative fusions. The PCR amplicons were separated by 1.5% agarose gel electrophoresis and visualized by BIORAD ChemiDoc imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Clinical data
The copy number amplification frequency of BORIS in two TCGA colorectal carcinoma datasets was summarized by the cBio Cancer Genomics Portal (cBioPorta, the date of access is September 24th, 2015) (20,21). Associations between the copy number of BORIS and the progression of colorectal carcinoma were analyzed using Oncomine 4.4 (© 2015 Thermo Fisher Scientific Inc.).
Statistical analysis
All experimental data are presented as the mean ± standard deviation. Data in the TCGA Colorectal 2 dataset exported from the Oncomine database were replotted and the significance was calculated using analysis of variance by the software of SPSS Statistics 17.0.0 (SPSS Inc., Chicago, IL, USA). The significance of up- or downregulation was calculated using Student' t-tests type 2 analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
BORIS copy number alteration in colorectal carcinoma
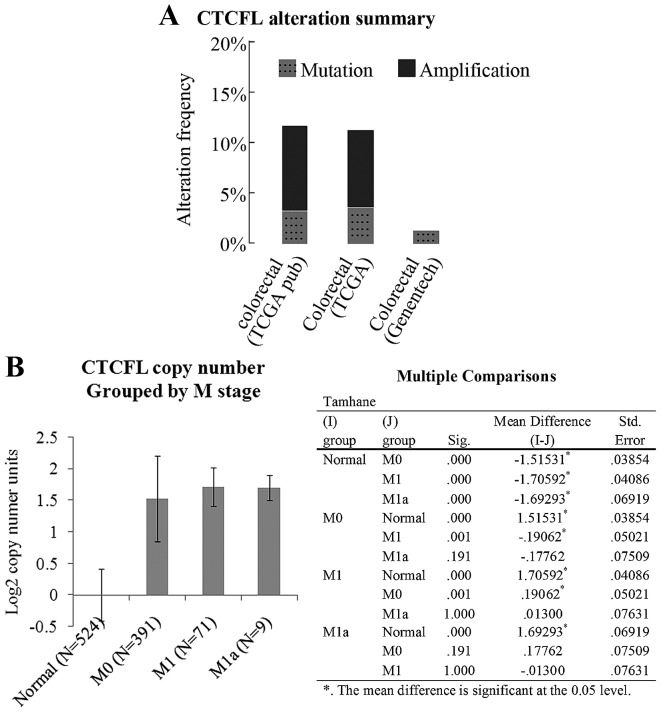
BORIS is expressed in the testis, ovary and skin cells of healthy individuals (13,22). Prior studies have identified the abnormal expression of BORIS in a number of somatic cancer types, including breast cancer and prostate cancer (15–17,23). Fig. 1A indicates a copy number amplification frequency of ~10% for BORIS in two TCGA colorectal carcinoma datasets, as summarized by cBioPortal (20,21). Fig. 1B indicates that the copy number of BORIS is increased according to M stage progression. The data were analyzed using Oncomine. It was considered that the increased expression of BORIS may be associated with colorectal carcinoma progression.
Figure 1.
Copy number abnormality of BORIS in colorectal carcinoma. (A) Data were summarized and counted using cBioPortal. ‘Mutation’ indicates the mutation ratio of BORIS in the genome of clinic samples to the corresponding database on the x-axis. ‘Amplification’ indicates the ratio of the patients who gained numerous copies of BORIS in the genome to the corresponding database on the x-axis. (B) The TCGA Colorectal 2 dataset, which includes the data from 524 healthy volunteers, 391 M0 stage, 71 M1 stage and 9 M1a colorectal cancer patients, was summarized by Oncomine. The CTCFL copy number was grouped by M stage. CTCFL, CTCF-like. The table presents the multiple comparisons between M stage groups using analysis of variance. Sig., significance; Std., standard.
Genomic fusion gene examination
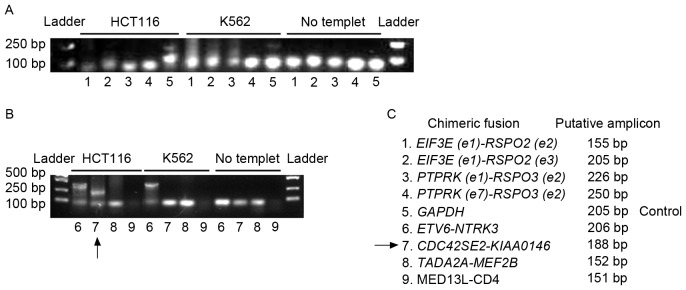
Seshagiri et al (6) analyzed 70 pairs of colon cancer and adjacent non-cancerous tissues using RNA-seq. This study identified multiple fusion transcripts, including recurrent gene fusions, involving the R-spondin family members RSPO2 and RSPO3 that together occur in ~10% of patients with colon cancer. By fusing with exon one of EIF3E or PTPRK, the expression of RSPO2 or RSPO3 was increased, and the Wnt signaling pathway was correspondingly activated. To investigate the fusion genes in colon cancer, the existence of fusion genes in the genome of HCT116 cells was initially evaluated. Genomic DNA was extracted from HCT116 and K562 cells. The primers listed in Table I were used to determine the presence of the fusion genes. Putative lengths of the amplicons are listed in Table I and Fig. 2. As the K562 cell line is BCR-ABL fusion gene-positive and its genome is unstable (24), it was investigated whether K562 cells possess the same genomic fusion genes that were identified in colon cancer, in order to validate the specificity of the investigated fusion genes in colon cancer. ‘No template’ in Fig. 2 indicates the negative control of PCR, which did not contain template to preclude the contamination of the PCR system. GAPDH was used as a positive control for PCR amplification. The arrow in Fig. 2B indicates the amplicon of CDC42SE2-KIAAO146 from the HCT116 genome. Fig. 2B indicates that a larger band of the ETV6-NTRK3 amplicon was detected in the HCT116 and the K562 genome. The lowest bands on the gels were primer dimers (Fig. 2A and B). The fusion between ETV6 and NTRK3 is beyond the range of prediction. Furthermore, the ETV6-NTRK3 amplicon is not specific for colon cancer (Fig. 2B). Therefore, ETV6-NTRK3 may not be worthy of further study. Although Seshagiri et al (6) demonstrated that fusion genes involving RSPO2 and RSPO3 existed in the genome of a limited number of patients with colon cancer, genomic fusion of EIF3E(e1)-RSPO2(e2), EIF3E(e1)-RSPO2(e3), PTPRK(e1)-RSPO3(e2), PTPRK(e7)-RSPO3(e2), TADA2A-MEF2B and MED13L-CD4 was not identified in the genome of HCT116 or K562 (Fig. 2A and B). However, the expression of these fusion genes was detected in the transcriptome of HCT116 (Figs. 3–6).
Figure 2.
Genomic fusion gene examination. Regular PCR was applied to determine the existence of fusion genes in the genome of HCT116 cells and K562 cells. (A) Detection for the existence of fusions 1 to 5. (B) Detection for the existence of fusions 6 to 9. PCR results were shown by agarose gel electrophoresis. ‘No template’ indicated the negative control of PCR, which was not supplied with template to preclude the contamination of the PCR system. GAPDH was used as a positive control for PCR amplification. The arrow indicates the amplicon of CDC42SE2-KIAAO146 from the HCT116 genome. The ETV6-NTRK3 fusion gene was detected in the HCT116 and K562 genome. (C) The code designation of fusion genes and the putative length of PCR amplicons were listed. PCR, polymerase chain reaction; GAPH, glyceraldehyde 3-phosphate dehydrogenase.
Figure 3.
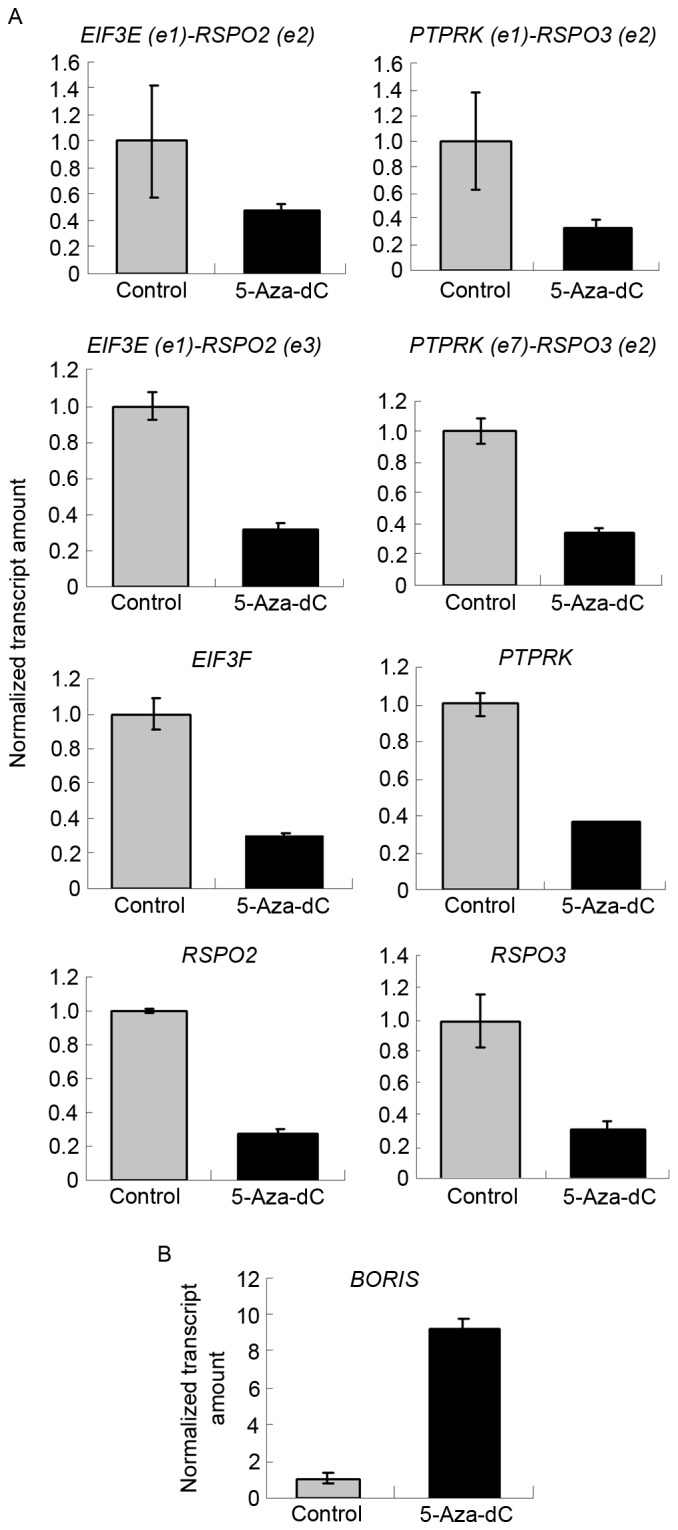
Overall, 5-Aza-dC suppressed the expression of fusion transcripts and the parent genes. (A) 5-Aza-dC suppressed the expression of fusion transcripts and their parent genes. The expression of targets investigated in control samples was set as 1. The expression levels of targets investigated in 5-Aza-dC-treated samples are presented as the normalized transcript amount compared with the control. (B) 5-Aza-dC induced the expression of BORIS. 5-Aza-dC, 5-aza-2′-deoxycytidine; BORIS, Brother of the Regulator of Imprinted Sites.
Figure 6.
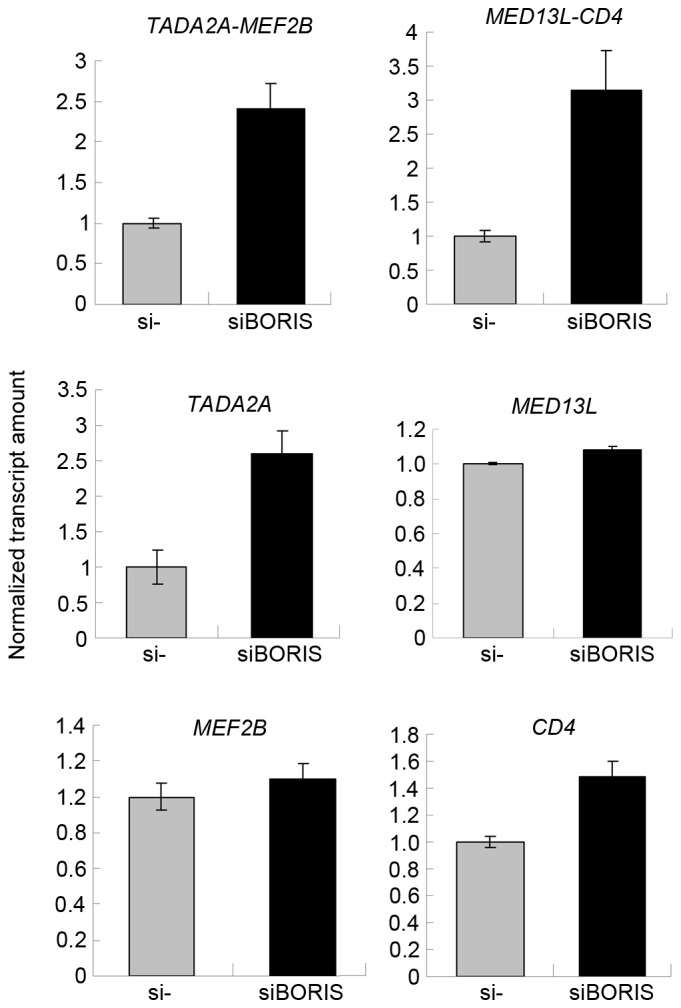
BORIS silencing induced the expression of fusion transcripts and their parent genes. The expression of tested targets in control samples was set as 1. The expression of targets investigated in BORIS-silenced samples are presented as a normalized transcript amount when compared with the negative control. BORIS, Brother of the Regulator of Imprinted Sites; si, short interfering.
5-Aza-dC induces the expression of BORIS to downregulate the expression of fusion transcripts
BORIS is the homolog of CTCF and is expressed abnormally in colorectal carcinoma (Fig. 1A). As CTCF regulates the expression of SLC45A3-ELK4 and other fusion transcriptsin prostate cancer (11), BORIS may also regulate fusion transcripts in colon cancer. In the present study, 5-Aza-dC was used to induce the expression of BORIS (14). BORIS was upregulated by demethylation, which is induced by 5-Aza-dC treatment (Fig. 3B). The results demonstrated that EIF3E(e1)-RSPO2(e2), EIF3E(e1)-RSPO2(e3), PTPRK(e1)-RSPO3(e2) and PTPRK(e7)-RSPO3(e2) were suppressed by 5-Aza-dC treatment (Fig. 3A). The expression of BORIS was upregulated, and that of EIF3E, RSPO2, PTPRK and RSPO3 was downregulated. This suggests that 5-Aza-dC promotes the expression of BORIS to suppress the expression of the investigated fusion transcriptsand their parent genes (Fig. 3).
Overexpression of BORIS inhibits the expression of fusion transcripts
Although 5-Aza-dC treatment induced the expression of BORIS, 5-Aza-dC induced apoptosis (25). 5-Aza-dC may regulate other genes, in addition to BORIS, to suppress the expression of fusion transcripts. To exclude this possibility, BORIS was overexpressed by transfecting the pBORIS plasmid into HCT116 cells. The empty vector was set as the negative control. BORIS overexpression efficiency is indicated in Fig. 4B. The results revealed that the fusion transcripts and fusion parent genes were all downregulated (Fig. 4A). These results were in agreement with those of 5-Aza-dC treatment, and suggested that BORIS suppresses the expression of fusion transcripts by downregulating the parent genes.
Figure 4.
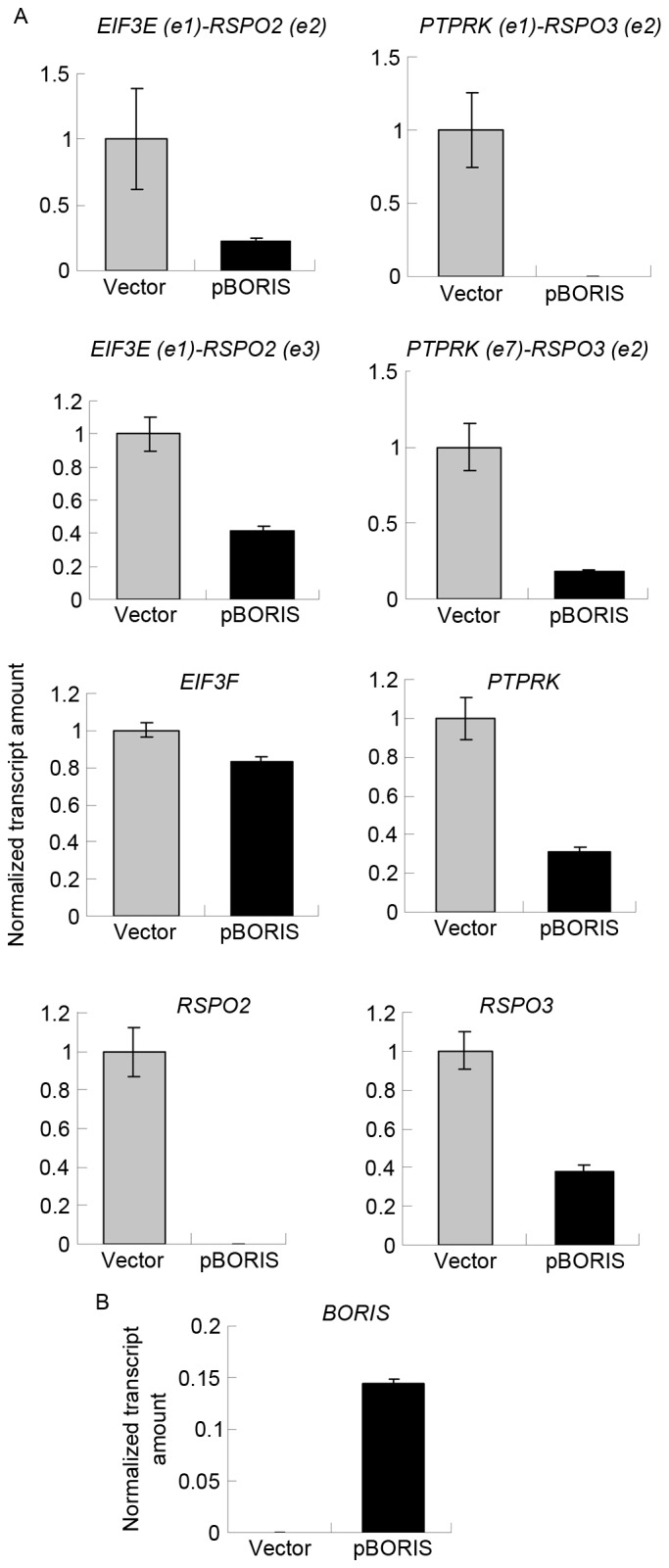
BORIS overexpression suppressed the expression of fusion transcripts and the parent genes. (A) BORIS overexpression suppressed the expression of fusion transcripts and their parent genes. The expression of targets investigated in control samples was set as 1. The expression of targets investigated in BORIS-overexpression samples are presented as the normalized transcript amount when compared with the control. (B) Efficiency of BORIS overexpression. BORIS, Brother of the Regulator of Imprinted Sites.
Silencing of BORIS upregulates fusion transcripts by promoting the parent genes
To confirm the function of BORIS in the regulation of fusion transcripts, BORIS was silenced in HCT116 cells. Silencing efficiency was ~70% following transfecting siRNA of BORIS into HCT116 cells (Fig. 5B). The expression of EIF3E and PTPRK was upregulated (Fig. 5A). Owing to the potential toxicity of transfection, the expression of RSPO2, RSPO3 and the fusion transcripts were undetected. Therefore, the expression of another two fusion transcripts TADA2A-MEF2Band MED13L-CD4 identified by Seshagiri et al (6) in colon cancer was investigated. As the fusions of TADA2A-MEF2B and MED13L-CD4 was not detected in the genome of HCT116 (Fig. 2B), TADA2A-MEF2B and MED13L-CD4 may be regulated by BORIS in the transcriptome of HCT116 cells. The RNA of siBORIS-silenced HCT116 cells was extracted and reversed-transcribed into cDNA. RT-qPCR was applied to determine the expression of certain transcripts. The results revealed that the fusion parent genes TADA2A and CD4 were upregulated and the fusion transcripts of TADA2A-MEF2B and MED13L-CD4 were upregulated (Fig. 6). These results suggested that the downregulation of BORIS promotes the expression of the investigated fusion transcripts via upregulating the expression of the parent genes.
Figure 5.
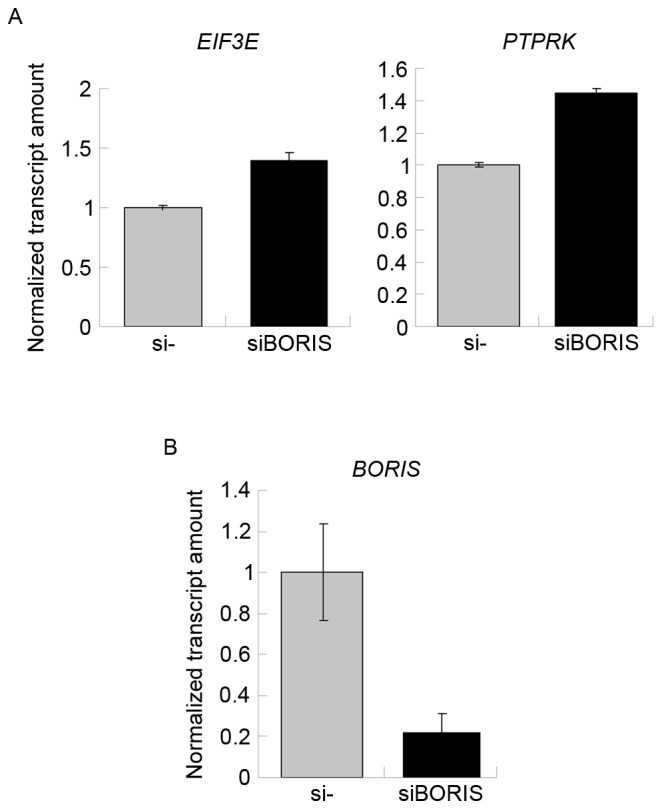
(A) BORIS silencing induced the expression of fusion parent genes. The expression of targets investigated in the control samples was set as 1. The expression of targets investigated in BORIS-silenced samples are presented as normalized transcript amounts compared with the negative control. (B) BORIS silencing efficiency. BORIS, Brother of the Regulator of Imprinted Sites; si, short interfering.
Discussion
Chimeric fused nucleic acids were classified as fusion DNA in the genome and as fusion RNA transcripts in the transcriptome. As a rule, fusion products in the genome are formed through DNA rearrangement (26–28). Fusion RNA transcripts in the transcriptome comprised of cis-splicing of adjacent genes and trans-splicing (fusion between genes on different strands of the same chromosome or different chromosomes) (4,7,11,29). Our study revealed that the existence of fusion RNA transcripts was not always accompanied by DNA rearrangements.
As genomic fusion genes are not typically regulated, the type of fusion investigated in the present study was first confirmed to be DNA rearrangement or RNA fusion. CDC42SE2-KIAAO146 was detected in the HCT116 genome (Fig. 2B). A larger band, in comparison with the putative ETV6-NTRK3 amplicon, was detected in HCT116 and K562 cell lines (Fig. 2B). The fusion between ETV6 and NTRK3 is out of the prediction range. In addition, ETV6-NTRK3 is not specific for colon cancer, therefore the regulation of ETV6-NTRK3 was not examined in HCT116 by BORIS. The other fusion candidates investigated were not detected in the genome of HCT116. The appearance of the fusions at the RNA level, whilst a lack of existence at the genomic DNA level, suggests that they are fusion transcripts on transcriptome.
Two parent transcripts joined to be one fusion transcript. Therefore, the expression of fusion transcripts may be affected by the expression of the parent genes. The regulation of the parent genes was examined in the present study and BORIS was identified to suppress the parent gene expression to inhibit the expression of the fusion transcripts (Figs. 3–6).
The statistical data analyzed using cBioPortal indicatedthat the copy number of BORIS was amplified significantly in colorectal cancer. However, a rare mutation of BORIS was detected (Fig. 1A) that suggests that high expression levels of BORIS may be associated with colon cancer.
BORIS is the homolog of CTCF. CTCF regulates the expression of fusion RNA transcripts, including SLC45A3-ELK4 and ADCK4-NUMBL chimeric RNA transcripts (7,11). The cis-splicing of adjacent genes was identified to be regulated by the binding events of CTCF to genome. BORIS and CTCF share the same zinc-finger DNA binding domains (13), therefore BORIS may regulate fusion transcripts by binding to similar genomic regions to CTCF. Furthermore, BORIS has a role in chromatin organization and gene expression; it demethylates chromatin to regulate the gene expression of cancer testis antigens, and recruits H3K4 methyltransferase to promote the expression of MYC and BRCA1 (30,31). Thus, BORIS may also regulate fusion transcripts by affecting the expression of the parent genes. In the present study, BORIS was identified to inhibit the expression of EIF3E, RSPO2, PTPRK, RSPO3, TADA2A and CD4 (Figs. 3–6). The underlying molecular mechanism requires further study.
The colon cancer genomic fusion gene CDC42SE2-KIAAO146 was detected in the present study (Fig. 2B). Whether it may be used as target for diagnosis, prognosis and therapy requires further examination in other colon cancer cell lines and clinic samples. The expression of EIF3E (e1)-RSPO2(e2), EIF3E(e1)-RSPO2(e3), PTPRK(e1)-RSPO3(e2), PTPRK(e7)-RSPO3(e2), TADA2A-MEF2B and MED13L-CD4 was detected in the HCT116 transcriptome. The expression of the fusion transcripts was affected by the parent genes, which were inhibited by BORIS (Figs. 3–6). High expression of BORIS frequently associated with cancer and BORIS promotes cancer cell proliferation. On the other hand, chimeric fusion nuclear acids were traditionally considered abnormal products of cancer. In addition, it was predicted that BORIS promotes the expression of abnormal chimeric fusions. However, BORIS did not induce or suppress the expression of the tested chimeric fusions in our study. Therefore, the association between cancer and chimeric fusion RNA transcripts needs further determination. Clinical investigation of the copy number alteration of BORIS in colorectal carcinoma patients suggested that the BORIS copy number is increased according to M stage progression (Fig. 1B). As BORIS is not colorectal carcinoma-specific, the fusion genes investigated may constitute a biomarker assemblage for monitoring colorectal carcinoma progression.
Acknowledgements
The present study was supported by National Natural Science Foundation of China (grant no. 81301782) and the Project supported for Returned Overseas Chinese Scholars. The authors wish to thank Mr. Xiaoliang Zheng and Mrs. Dongmei Yan of the Zhejiang Academy of Medical Science (Hangzhou, China) for their discussion and support.
References
- 1.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 2.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and Safety of a Specific Inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 3.Kumar-Sinha C, Kalyana-Sundaram S, Chinnaiyan AM. SLC45A3-ELK4 chimera in prostate cancer: Spotlight on cis-splicing. Cancer Discov. 2012;2:582–585. doi: 10.1158/2159-8290.CD-12-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rickman DS, Pflueger D, Moss B, Van Doren VE, Chen CX, de la Taille A, Kuefer R, Tewari AK, Setlur SR, Demichelis F, Rubin MA. SLC45A3-ELK4 is a novel and frequent erythroblast transformation-specific fusion transcript in prostate cancer. Cancer Res. 2009;69:2734–2738. doi: 10.1158/0008-5472.CAN-08-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maher CA, Kumar-Sinha C, Cao X, Kalyana-Sundaram S, Han B, Jing X, Sam L, Barrette T, Palanisamy N, Chinnaiyan AM. Transcriptome sequencing to detect gene fusions in cancer. Nature. 2009;458:97–101. doi: 10.1038/nature07638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seshagiri S, Stawiski EW, Durinck S, Modrusan Z, Storm EE, Conboy CB, Chaudhuri S, Guan Y, Janakiraman V, Jaiswal BS, et al. Recurrent R-spondin fusions in colon cancer. Nature. 2012;488:660–664. doi: 10.1038/nature11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Gong M, Yuan H, Park HG, Frierson HF, Li H. Chimeric transcript generated by cis-splicing of adjacent genes regulates prostate cancer cell proliferation. Cancer Discov. 2012;2:598–607. doi: 10.1158/2159-8290.CD-12-0042. [DOI] [PubMed] [Google Scholar]
- 8.Zlatanova J, Caiafa P. CTCF and its protein partners: Divide and rule? J Cell Sci. 2009;122:1275–1284. doi: 10.1242/jcs.039990. [DOI] [PubMed] [Google Scholar]
- 9.Lobanenkov VV, Nicolas RH, Adler VV, Paterson H, Klenova EM, Polotskaja AV, Goodwin GH. A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5′-flanking sequence of the chicken c-myc gene. Oncogene. 1990;5:1743–1753. [PubMed] [Google Scholar]
- 10.Kim TH, Abdullaev ZK, Smith AD, Ching KA, Loukinov DI, Green RD, Zhang MQ, Lobanenkov VV, Ren B. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell. 2007;128:1231–1245. doi: 10.1016/j.cell.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin F, Song Z, Babiceanu M, Song Y, Facemire L, Singh R, Adli M, Li H. Discovery of CTCF-sensitive Cis-spliced fusion RNAs between adjacent genes in human prostate cells. PLoS Genet. 2015;11:e1005001. doi: 10.1371/journal.pgen.1005161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vatolin S, Abdullaev Z, Pack SD, Flanagan PT, Custer M, Loukinov DI, Pugacheva E, Hong JA, Morse H, III, Schrump DS, et al. Conditional expression of the CTCF-paralogous transcriptional factor BORIS in normal cells results in demethylation and derepression of MAGE-A1 and reactivation of other cancer-testis genes. Cancer Res. 2005;65:7751–7762. doi: 10.1158/0008-5472.CAN-05-0858. [DOI] [PubMed] [Google Scholar]
- 13.Loukinov DI, Pugacheva E, Vatolin S, Pack SD, Moon H, Chernukhin I, Mannan P, Larsson E, Kanduri C, Vostrov AA, et al. BORIS, a novel male germ-line-specific protein associated with epigenetic reprogramming events, shares the same 11-zinc-finger domain with CTCF, the insulator protein involved in reading imprinting marks in the soma; Proc Natl Acad Sci USA; 2002; pp. 6806–6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann MJ, Müller M, Engers R, Schulz WA. Epigenetic control of CTCFL/BORIS and OCT4 expression in urogenital malignancies. Biochem Pharmacol. 2006;72:1577–1588. doi: 10.1016/j.bcp.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 15.D'Arcy V, Abdullaev ZK, Pore N, Docquier F, Torrano V, Chernukhin I, Smart M, Farrar D, Metodiev M, Fernandez N, et al. The potential of BORIS detected in the leukocytes of breast cancer patients as an early marker of tumorigenesis. Clin Cancer Res. 2006;12:5978–5986. doi: 10.1158/1078-0432.CCR-05-2731. [DOI] [PubMed] [Google Scholar]
- 16.D'Arcy V, Pore N, Docquier F, Abdullaev ZK, Chernukhin I, Kita GX, Rai S, Smart M, Farrar D, Pack S, et al. BORIS, a paralogue of the transcription factor, CTCF, is aberrantly expressed in breast tumours. Br J Cancer. 2008;98:571–579. doi: 10.1038/sj.bjc.6604181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dougherty CJ, Ichim TE, Liu L, Reznik G, Min WP, Ghochikyan A, Agadjanyan MG, Reznik BN. Selective apoptosis of breast cancer cells by siRNA targeting of BORIS. Biochem Biophys Res Commun. 2008;370:109–112. doi: 10.1016/j.bbrc.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 18.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM. ONCOMINE: A cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/S1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Method. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosa-Garrido M, Ceballos L, Alonso-Lecue P, Abraira C, Delgado MD, Gandarillas A. A cell cycle role for the epigenetic factor CTCF-L/BORIS. PLoS One. 2012;7:e39371. doi: 10.1371/journal.pone.0039371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin-Kleiner I. BORIS in human cancers-a review. Eur J Cancer. 2012;48:929–935. doi: 10.1016/j.ejca.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Weber A, Borghouts C, Brendel C, Moriggl R, Delis N, Brill B, Vafaizadeh V, Groner B. Stat5 exerts distinct, vital functions in the cytoplasm and nucleus of Bcr-Abl+ K562 and Jak2(V617F)+ HEL leukemia cells. Cancers. 2015;7:503–537. doi: 10.3390/cancers7010503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider-Stock R, Diab-Assef M, Rohrbeck A, Foltzer-Jourdainne C, Boltze C, Hartig R, Schönfeld P, Roessner A, Gali-Muhtasib H. 5-Aza-cytidine is a potent inhibitor of DNA methyltransferase 3a and induces apoptosis in HCT-116 colon cancer cells via Gadd45- and p53-dependent mechanisms. J Pharmacol Exp Ther. 2005;312:525–536. doi: 10.1124/jpet.104.074195. [DOI] [PubMed] [Google Scholar]
- 26.Rabbitts TH. Chromosomal translocations in human cancer. Nature. 1994;372:143–149. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- 27.Rowley JD. The role of chromosome translocations in leukemogenesis. Semin Hematol. 1999;36(4 Suppl 7):S59–S72. [PubMed] [Google Scholar]
- 28.Heim S, Mitelman F. Molecular screening for new fusion genes in cancer. Nat Genet. 2008;40:685–686. doi: 10.1038/ng0608-685. [DOI] [PubMed] [Google Scholar]
- 29.Yuan H, Qin F, Movassagh M, Park H, Golden W, Xie Z, Zhang P, Sklar J, Li H. A chimeric RNA characteristic of rhabdomyosarcoma in normal myogenesis process. Cancer Discov. 2013;3:1394–1403. doi: 10.1158/2159-8290.CD-13-0186. [DOI] [PubMed] [Google Scholar]
- 30.Bhan S, Negi SS, Shao C, Glazer CA, Chuang A, Gaykalova DA, Sun W, Sidransky D, Ha PK, Califano JA. BORIS binding to the promoters of cancer testis antigens, MAGEA2, MAGEA3, and MAGEA4, is associated with their transcriptional activation in lung cancer. Clin Cancer Res. 2011;17:4267–4276. doi: 10.1158/1078-0432.CCR-11-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen P, Bar-Sela G, Sun L, Bisht KS, Cui H, Kohn E, Feinberg AP, Gius D. BAT3 and SET1A form a complex with CTCFL/BORIS to modulate H3K4 histone dimethylation and gene expression. Mol Cell Biol. 2008;28:6720–6729. doi: 10.1128/MCB.00568-08. [DOI] [PMC free article] [PubMed] [Google Scholar]