Abstract
Despite strong anti-smoking efforts, at least 12% of American women cannot quit smoking when pregnant resulting in more than 450,000 smoke-exposed infants born yearly. Smoking during pregnancy is the largest preventable cause of childhood respiratory illness including wheezing and asthma. Recent studies have shown a protective effect of vitamin C supplementation on the lung function of offspring exposed to in utero smoke in a non-human primate model and an initial human trial. Vitamin C to Decrease the Effects of Smoking in Pregnancy on Infant Lung Function (VCSIP) is a randomized, double-blind, placebo-controlled trial to evaluate pulmonary function at 3 months of age in infants delivered to pregnant smokers randomized to 500 mg/day of vitamin C versus placebo during pregnancy. Secondary aims evaluate the incidence of wheezing through 12 months and pulmonary function testing at 12 months of age. Women are randomized between 13–23 weeks gestation from clinical sites in Portland, Oregon at Oregon Health & Science University and PeaceHealth Southwest Medical Center and in Indianapolis, Indiana at Indiana University and Wishard Hospital. Vitamin C supplementation occurs from randomization to delivery. Monthly contact with participants and monitoring of medical records is performed to document medication adherence, changes in smoking and medical history, and adverse events. Pulmonary function testing of offspring occurs at 3 and 12 months of age and incidence of wheezing and respiratory illness through 12 months is captured via at least quarterly questionnaires. Ancillary studies are investigating the impact of vitamin C on placental blood flow and DNA methylation.
Keywords: In utero smoke, Lung development, Pulmonary function test, Asthma, Wheeze, Vitamin C, Forced expiratory flows
1. Introduction
Smoking during pregnancy is the largest preventable cause of childhood respiratory illness1–3 and is a large public health issue since more than 50% of smokers who become pregnant continue to smoke despite the Surgeon General’s warning of the associated health problems.4;5 This equates to at least 12% of American women continuing to smoke when pregnant, resulting in more than 450,000 smoke-exposed infants born yearly.6 Maternal smoking during pregnancy adversely affects lung development as seen by lifelong decreases in pulmonary function and increased risk of wheezing, respiratory tract infections, and asthma.1;2 Nearly 20% of expenditures for childhood respiratory illness are caused by maternal smoking in the United States (US), amounting to over $1 billion annually in current health care dollars.7 Although in utero damage caused by tobacco smoke is preventable with smoking cessation, the reality is that smoking is a unique morbidity in that it is addictive and heavily advertised; 8–10 moreover, individuals with certain genotypes have a significantly increased likelihood of nicotine addiction and failure to quit.11–13 Statistics from the Center for Disease Control show that decreases in smoking rates during pregnancy have plateaued and rates have even increased in some states, especially among younger women.14 Thus, finding ways to lessen the effect of smoking during pregnancy is of great public health importance.
Recent studies have shown a protective effect of vitamin C supplementation on the lung function of offspring exposed to in utero nicotine/ smoke in both a non-human primate model 15 and an initial trial in humans16 measuring newborn pulmonary function tests. However, one of the key effects of maternal smoking is a decrease in the forced expiratory flows17–20 (FEFs) of the offspring which was not measured in the aforementioned initial clinical trial. This report describes the development and methodology for the VCSIP (“Vitamin C to Decrease the Effects of Smoking in Pregnancy on Infant Lung Function”) study (ClinicalTrials.gov number NCT01723693), a randomized, double-blind, placebo controlled intervention of vitamin C supplementation to pregnant smokers with measurements of their offspring’s forced expiratory flows at 3 months of age as the primary outcome. VCSIP (www.vcsip.com) is supported by a R01 HL105447 from the National Heart, Lung, and Blood Institute with co-funding from the Office of Dietary Supplements.
2. Background studies
Newborns of smokers have decreased pulmonary function when measured after delivery and before significant exposure to postnatal smoke, confirming the importance of in utero exposure.21;22 These changes include decreases in FEFs, tidal breathing parameters, and respiratory compliance.16;20–23 Decreased pulmonary function early in life is associated with increased respiratory illnesses early in life, and maternal smoking during pregnancy is a major contributor to these adverse respiratory outcomes.24–27 Since pulmonary function tracks from infancy to early adulthood along percentiles established very early in life, it is critical to develop early life strategies to maximize lung growth and development, which begins early in gestation.22
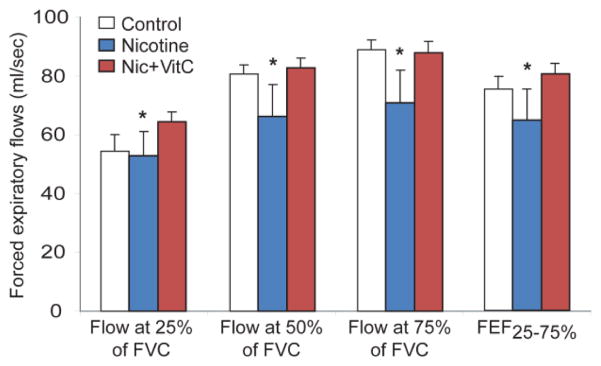
Our underlying hypothesis is that the effect of in utero smoke exposure on lung development and offspring lung function is mediated by oxidant mechanisms and therefore can be prevented by maternal vitamin C supplementation. A primary mediator of smoking-induced oxidant injury is nicotine.28–31 This is supported by data shown in Figure 1, in which prenatal nicotine exposure in pregnant non-human primates leads to decreased pulmonary function of the offspring similar to that seen in infants born after in utero smoke exposure 15,32 and discussed in a recent review.33 In the non-human primate model, maternal vitamin C supplementation significantly lessened the effects on pulmonary function, while decreasing the effects of nicotine on lung surfactant and elastin expression.15
Figure 1.
Forced expiratory flows in non-human primates treated as shown (Mean ± SEM).
*p< 0.05 for overall comparison of Nicotine-treated group to control and Nicotine + vitamin C-treated groups by MANOVA. FEF25%–75% = the average flow between 25% and 75% of forced expired volume. N= 20 total animals. Used by permission from Proskocil et al.15
Based on the above results, we performed an initial study16 of the potential for supplemental vitamin C to block the effects of maternal smoking on offspring respiratory health. In this study, 159 pregnant smokers were randomly allocated in a double blind design to an additional 500 mg of vitamin C per day versus placebo, alongside a reference group of pregnant nonsmokers. Within 72 hours of age, newborn pulmonary function testing was done to assess tidal breathing flows of the time to peak tidal flow to expiratory time (TPTEF: TE) and passive respiratory system compliance (Crs). As shown in Table 1, maternal smoking during pregnancy was associated with decreases in TPTEF: TE, Crs, and Crs/kg (Crs normalized for newborn’s weight in kilograms), and the supplemental vitamin C blocked these decreases.16
Table 1.
Newborn pulmonary function tests after in utero vitamin C to randomized smokers
| Newborns of nonsmokers (n=76)a | Newborns of placebo treated smokers (n=83) | Newborns of vitamin C treated smokers (n=76) | Mean Difference (95% CI) (Vitamin C- Placebo) † | P value† | |
|---|---|---|---|---|---|
| TPTEF:TE* | 0.399 ± 0.077 | 0.345 ± 0.078 | 0.383 ± 0.084 | 0.036 (0.011 to 0.062) | 0.006 |
| Crs/kg* (mL/cmH2O/kg) | 1.36 ± 0.30 | 1.20 ± 0.24 | 1.32 ± 0.30 | 0.11 (0.02 to 0.20) | 0.01 |
| Total Crs* (mL/cmH2O) | 4.46 ± 1.10 | 3.92 ± 0.81 | 4.16 ±1.13 | 0.32 (0.02 to 0.62) | 0.04 |
Abbreviations: Crs, passive respiratory compliance; TPTEF:TE, ratio of time to peak tidal expiratory flow to expiratory time;
Mean ± SD.
Nonsmokers are reference group only and not included in formal statistical analysis;
Values adjusted for gestational age at randomization, birth weight, and gestational age < 37 weeks. Used by permission from McEvoy et al.16
Used by permission from McEvoy et al.16
The incidence of wheezing through 1 year of age as assessed by a standardized respiratory questionnaire in the infants of smokers allocated to supplemental vitamin C versus placebo was significantly decreased (Table 2).16
Table 2.
Respiratory outcomes in randomized infants through one year of age
| Newborns of non smokers (n=70)a | Newborns of placebo treated smokers (n=77) | Newborns of vitamin C treated smokers (n=70) | Relative Risk (95% CI)* | P value * | |
|---|---|---|---|---|---|
| At least 1 episode of wheeze, No.(%) | 19 (27) | 31 (40) | 15 (21) | 0.56 (0.33 to 0.95) | 0.03 |
| Medication for wheeze, No.(%) | 7 (10) | 17 (22) | 9 (13) | 0.56 (0.27 to 1.18) | 0.13 |
Nonsmokers are reference group only and not included in formal statistical analysis;
Values adjusted for gestational age at randomization, birth weight, and gestational age < 37 weeks.
Used by permission from McEvoy et al.16
Although our initial study showed significantly improved newborn pulmonary function tests (PFTs) in the offspring of smokers allocated to vitamin C, we did not measure forced expiratory flows (FEFs) of the offspring which are considered the most sensitive measure of peripheral airway function. 20;34;35 It is difficult to measure FEFs in the newborn period due to instability of newborn lung volumes in the first weeks of life, sedation needs, and separation from family. In order to assess pulmonary FEFs, we initiated a larger, multicenter randomized clinical trial (VCSIP) that includes measures of FEFs and other clinical signs and symptoms of infant respiratory health through one year of age. We are measuring FEFs at 3 months of age to balance the need to minimize possible side effects from the required chloral hydrate sedation with postnatal smoke exposure.36–38 With the addition of Indianapolis, Indiana, a second geographically distinct study location, this new trial includes a more diverse population. It also robustly examines clinical outcomes, specifically the measurement of FEFs at 3 and 12 months of age and the incidence of wheeze through one year of age among infants born to pregnant smokers randomly allocated to vitamin C versus placebo.
3. Study design and methods
3.1. Study aims
The primary aim of the VCSIP trial is to determine if vitamin C supplementation as compared to placebo improves pulmonary function, measured by forced expiratory flows and specifically FEF75 (forced expiratory flows at 75% of forced vital capacity (FVC)) at 3 months of age, in infants delivered to mothers who continue to smoke cigarettes at least through the first trimester of pregnancy. We hypothesize that vitamin C supplementation during pregnancy will block the adverse effects of maternal smoking on infant pulmonary function measured at 3 months of age in infants born to pregnant smokers.
A secondary aim is to compare the incidence of wheezing through 12 months of age in infants of mothers allocated to vitamin C versus placebo during pregnancy. We hypothesize that vitamin C supplementation during pregnancy will decrease wheeze in the first 12 months of life in offspring of smokers. An additional secondary aim is to demonstrate improved pulmonary function at 12 months of age in infants delivered to pregnant smokers who are allocated to supplemental vitamin C versus placebo during pregnancy as assessed by forced expiratory flows. We hypothesize that vitamin C supplementation during pregnancy blocks the adverse effects of maternal smoking on infant pulmonary function measured at 12 months of age (forced expiratory flows) in infants born to smoking mothers.
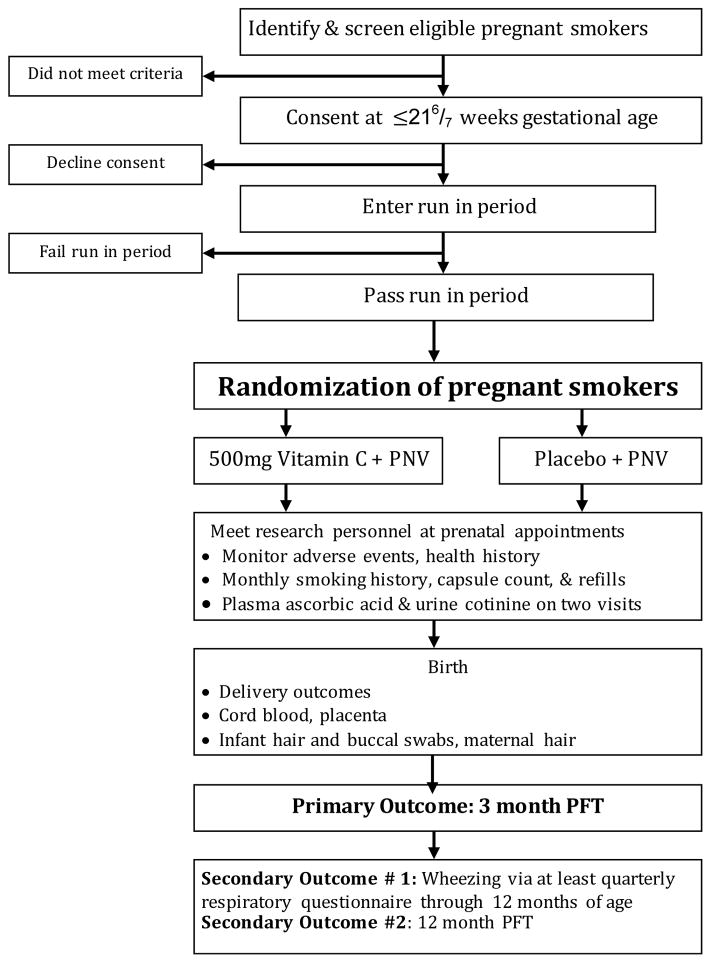
3.2. Study design summary (Figure 2)
Figure 2.
The VCSIP Study Design
Abbreviations: PNV: prenatal vitamin; PFT: pulmonary function test
The study is a placebo-controlled, double-blind randomized trial conducted at the following clinical locations: Portland, OR metropolitan area (clinics delivering at Oregon Health & Science University (OHSU) and PeaceHealth Southwest Medical Center) and Indianapolis, IN (recruiting at Indiana University and Wishard Hospital). After screening, consent, and successful completion of a run in phase, pregnant smokers less than 230/7 weeks of gestation are randomized to one of two groups: Vitamin C (500 mg)/day or matching placebo. Smoking cessation is actively encouraged at each prenatal visit. During pregnancy, women are monitored with a set of serial biomarkers to assess nicotine exposure and medication adherence including fasting plasma ascorbic acid levels, urine cotinine levels, smoking questionnaires, pill counts, and exhaled carbon monoxide levels. Adverse events are monitored through delivery for mothers, and through 1 year of age for infants. Pulmonary function tests are performed at 3 and 12 months of age and the offspring respiratory history is documented at least quarterly through 12 months of age.
3.3. Study design population
Potential study participants are pregnant women who report cigarette smoking at ≤216/7 weeks and are receiving care at clinics affiliated with the following hospital locations: OHSU (Portland, Oregon), PeaceHealth Southwest Medical Center (Vancouver, Washington), Wishard or Indiana University (IU) Hospitals or surrounding clinics (Indianapolis, Indiana) and meet other eligibility criteria at randomization as follows.
3.3.1. Inclusion criteria (applied to pregnant women at randomization)
Singleton gestation
≥15 years old
Gestational age between 130/7 and 226/7 weeks based on clinical information and ultrasound
Receiving prenatal care at clinics delivering at OHSU, PeaceHealth Southwest Hospital, Wishard, or Indiana University (IU) Hospital or surrounding clinics
Current cigarette smoking of at least 1 cigarette in last week
English speaking
3.3.2. Exclusion criteria at randomization
Gestational age ≥230/7 weeks
Multiple gestation
Documented major fetal congenital anomalies
Current use of illicit drugs
Current alcohol abuse as defined by questionnaire (≥3 drinks on ≥5 days per week during this pregnancy or any hospitalization for alcohol abuse or complications of alcohol abuse or outpatient visit for acute alcohol intoxication during the past year)
Use of vitamin C (≥500 mg/day) > 3 days per week since LMP
Refusal to abstain from vitamin or supplements containing significant vitamin C other than those provided through the study or approved by study staff
History of kidney stone
Insulin dependent diabetes
Complex maternal medical conditions
Participation in other conflicting research projects
Unable to demonstrate stable method of communication or incarcerated
Pregnancy by in-vitro fertilization
Plan to terminate pregnancy
Failure of run in phase / medication adherence trial (see section 3.7 below)
Failure to return in designated period during run-in
BMI > 50 at screening
3.4. Intervention: vitamin C supplement
VCSIP is testing a supplemental ascorbic acid (vitamin C) dose of 500 mg per day versus placebo. The vitamin C and placebo medications are manufactured in organoleptically similar tablets at an outside site (Magno-Humphries Laboratories INC, Tigard, OR) and dispensed through the research pharmacy at OHSU. Each vitamin C tablet contains 500 mg of ascorbic acid powder; the placebo tablet contains microcrystalline cellulose and 100 mg of citric acid to mimic the taste of vitamin C. The tablets are otherwise identical in appearance, size and shape. Both medications are dispensed in 21 and 100 tablet quantities (21 count during the run in/adherence period and 100 count during the treatment period). All pregnant women also receive 60 mg of vitamin C through a standard prenatal vitamin (Prenavite, Rugby Laboratories, Duluth, GA, 100 count) supplied to them by the study.
Vitamin C is a common antioxidant and the Institute of Medicine states that daily doses of up to 2000 mg are likely to pose no risks of adverse effect.39 A concern over a potentially harmful effect of supplemental vitamin C during pregnancy on prematurity has been described. A 2005 Cochrane review of vitamin C supplementation in pregnancy concluded that women supplemented with vitamin C were at an increased risk of preterm birth (RR 1.38, 95% CI 1.04 to 1.82, 3 trials, 583 women).40 This meta-analysis was significantly influenced by one trial,41 the only trial of the three to demonstrate this pattern of increased preterm deliveries in the treated group. Of note, 43% of the randomized patients were smokers in this study,41 and there was no significant difference in the incidence of prematurity within the subgroups of smokers by treatment group. A trial of 10,154 women who were allocated to vitamins C and E in pregnancy versus placebo did not show any significant difference in preterm births.42 Also, an updated 2015 Cochrane review of vitamin C supplementation in pregnancy evaluating data from 16 studies and 22,250 patients found no evidence of increased or decreased preterm delivery.43
Our initial study16 also lends support for the safety of 500 mg of vitamin C daily in pregnancy as there was no significant difference in maternal, fetal, or neonatal adverse outcomes between the randomized arms. Similarly, there were no significant differences in prematurity or gestational age between babies born to smoking mothers randomized to vitamin C versus placebo.
The 500mg daily dose of vitamin C is further justified within our initial trial16 showing that at 28–30 weeks of gestation, the vitamin C treated smokers had a higher average fasting plasma ascorbic acid level than the placebo-treated smokers (58.9 vs 39.8 μmol/L [95% CI for the difference between the means, 11.23–26.95]; p <0.001) which was comparable to the level of 57.8 μmol/L measured in the reference group of nonsmokers. This is congruent with data in non-human primates exposed to just nicotine, in which prenatal nicotine exposure also decreased ascorbic acid levels in both cord blood and amniotic fluid (Spindel, unpublished). Therefore we selected this dose of vitamin C to restore ascorbic acid levels in the pregnant smoker to be similar to that in the pregnant nonsmoker. This supplementation does not significantly increase vitamin C levels compared to the pregnant nonsmoker who is not consuming a supplement.
3.5. Identification/ screening for eligibility
Recruitment is maximized by assigning a research coordinator to each clinical recruitment site to screen and consent eligible pregnant smokers. Women are recruited by provider referral and by self-referral from posted study flyers. Additionally, research staff routinely query the clinics’ electronic medical records to identify obstetrical patients who fit initial eligibility criteria, including cigarette smoking, which is a standard question on all obstetrical intake forms.
3.6. Counseling and enrollment
The screening visit primarily occurs at the subject’s prenatal visit between 11 and 216/7 weeks gestation. At the screening visit, research staff present a written description of the study and review eligibility criteria. Once eligibility is confirmed, informed consent is obtained prior to any study procedures.
Because of the importance of emphasizing smoking cessation, research staff discuss this with the patient and provide a pregnancy-specific pamphlet. The 5 A’s for smoking cessation are assessed and documented: ask about tobacco use; advise every smoking patient to quit; assess willingness to make a quit attempt; assist in quit attempt; arrange for follow-up and future support.44 We follow best practices and strongly encourage the patient to participate in a smoking cessation program; because of this protocol, we believe that this population may receive greater encouragement to quit smoking than the general population. We included a specialist from the OHSU Smoking Cessation Center as a co-investigator on the study45;46 who trained research personnel about cessation techniques. Only women truly unable to quit smoking are involved in the study; a substantial proportion of pregnant smokers will stop spontaneously before they begin prenatal care and those who are active smokers at their first prenatal visit are less likely to change their smoking habits.47–49 If a patient indicates that she has quit smoking after randomization, this is noted and they are instructed to continue to take the study medication.
3.7. Run-in phase
This study includes a run-in phase. Upon consent, to exclude highly noncompliant subjects, patients enter into a medication adherence trial of 14 ± 7 days. The research pharmacy dispenses 21 days of the placebo medication, and all subjects are given an appointment to return within three weeks. Non-adherence is defined by failing to return for the subsequent visit within 14 ± 7 days or consuming < 75% of the required tablets.
3.8. Gestational age determination
Gestational age is first estimated by reviewing the results of the first available ultrasound examination. The minimum days required to complete the run-in are 7, thus women >22 weeks of gestation are too advanced to complete the run-in and be randomized prior to 23 weeks of gestation and are excluded. When an ultrasound examination has not been performed, gestational age is estimated using available information and an ultrasound is scheduled. Gestational age is based on the earliest obstetrical ultrasound estimate as determined at each clinical center.
3.9. Randomization
The second study visit includes assessment of the run-in phase (medication adherence trial). If the patient is adherent(≥75% tablets consumed and returns within 14 ± 7 days of first visit) the detailed smoking questionnaire is administered, fasting blood, urine, and hair samples are collected, an exhaled carbon monoxide test is done, and the patient is randomized into the study. See Table 3 for summary of procedures done at the various visits.
Table 3.
Summary of procedures in VCSIP
| Study Visit (weeks gestation) | Enrollment (12 to <22) | Randomization (13 to < 23) | Monthly visits | 2nd trimester (24 to 28 ) | Monthly visits | 3rd trimester (30 to 34) | Delivery | 3 months after delivery | 12 months after delivery |
|---|---|---|---|---|---|---|---|---|---|
| Health/Medical History | X | ||||||||
| Demographic Information | X | ||||||||
| Height, weight, blood pressure | X | X | X | X | X | X | X | ||
| Smoking questionnaire | X | X | X | X | X | X | X | ||
| Fasting plasma ascorbic acid 1 | X | X | X | ||||||
| Urine for cotinine | X | X | X | ||||||
| Blood/urine for biorepository 2 | X | X | X | ||||||
| Exhaled carbon monoxide 3 | X | X | X | X | X | ||||
| Maternal hair for nicotine | X | X | X | X | |||||
| Adverse events | X | X | X | X | X | X | |||
| Umbilical cord blood 1, 2 | X | ||||||||
| Placenta samples | X | ||||||||
| Infant hair for nicotine | X | X | X | ||||||
| Infant buccal swabs4 | X | X | X | ||||||
| Pulmonary function test | X | X | |||||||
| Respiratory questionnaire (quarterly) | X | X | |||||||
| Ages & stages questionnaire on infant | X |
Blood for ascorbic acid (1 green top heparin tube)
Blood for DNA extraction and genotyping (2 purple top tubes)
Measured with Smokelyzer, Bedfont Scientific, London, UK
For DNA extraction and genotyping
Randomization is performed at the Data Coordinating Center (DCC) using permuted block randomization stratified by gestational age at time of randomization (< 18 weeks versus > 18 weeks gestation) and clinical site (Oregon, Washington, or Indiana); we used random block size. Gestational age was selected for stratification to limit potential bias by spontaneous abortion or unequal distribution of gestational age among study arms. It is also a potentially critical covariate because of the effect of duration and total dose of vitamin C on the developing infant. A randomization schedule is given to the research pharmacy for preparation of the medication to dispense to research subjects. The study medication is labeled with study identification and a consecutive study code for the patient, aligned with the randomization scheme. After confirming all relevant data in the database, an intervention assignment corresponding to the sequenced medication is returned to the study coordination.
Participants are provided a bottle of study drug which contains 100 tablets of either vitamin C or placebo dispensed by the research pharmacies at the respective study locations. In addition, each participant is also dispensed a standard prenatal vitamin (Prenavite, Rugby Laboratories, Duluth, GA ) and instructed to take one study tablet daily and one prenatal vitamin, preferably with meals. Women are reminded frequently to bring medication bottles back to each study visit and at delivery.
3.10. Prenatal visits/contact
After randomization, the research staff coordinates interval study visits with routine prenatal appointments that occur approximately every four weeks, then more frequently as delivery approaches. Smoking is assessed with a standardized questionnaire at each visit. Brief smoking cessation counseling as per the American College of Obstetricians and Gynecologists guidelines50 occurs and is documented at each prenatal visit. Urine, maternal hair, fasting maternal blood samples and exhaled carbon monoxide levels are obtained at 26 ± 2 weeks, and 32 weeks ± 2 weeks of gestation. Adherence with dispensed medication (vitamin C or placebo) is assessed via monthly pill count by VCSIP study staff and at return of each medication bottle dispensed. The research staff conduct monthly reviews of the electronic medical record to check for pregnancy complications.
3.11. Delivery and follow-up visits
Research personnel attend each participant’s delivery and collect cord blood and placenta samples. During hospitalization for delivery, study personnel collect infant anthropometric measurements, neonatal data, and maternal and newborn hair samples and buccal swabs. After delivery, research staff contacts participants every 3 months to inquire about the infant’s respiratory status via a standardized respiratory questionnaire. Subjects and their offspring are seen when the infant is 3 and 12 months of age for pulmonary function testing. At these visits the infant health, maternal smoking, and environmental smoke exposure assessments are done in person, hair samples are collected from both the mother and infant, an exhaled carbon monoxide measurement is obtained from the mother and buccal swabs are obtained from the infant. At the last visit the Ages and Stages Questionnaire51;52 and the study disposition form is completed. The study visits and contacts are outlined in Table 3 below.
3.12. Participant retention
Frequent, consistent, and personal contact is critical to retain this vulnerable population in a research protocol.53 Interval phone calls, text messages, and emails to patients are employed monthly. To maximize retention, multiple contact information is collected, private messaging through social media is utilized, and small travel reimbursements are provided.
4. Outcome procedures / assessments
The primary outcome of this study is the measurement of infant pulmonary function tests (PFTs) at 3 months of age, specifically, the measurement of forced expiratory flow at 75% of the expired volume (FEF75) using the raised volume rapid thoracic compression (RVRTC) technique. Secondary outcomes include: the measurement of forced expiratory flows at 12 months of age; the incidence of wheezing through 12 months of age via at least quarterly documentation of respiratory symptoms and treatments; other measurements of FEFs obtained at the 3 month PFT, including FEF25-75, FEF50, and FEF85, and forced expiratory volumes including forced vital capacity (FVC), forced expired volume in the initial 0.4 sec (FEV0.4) and 0.5 sec (FEV0.5) and the ratio of FEV0.5/FVC.
4.1. Pulmonary function testing
Forced expiratory flow measurements were chosen as the primary outcome in this study since they are the parameters of airway function shown to be most sensitive to the effects of maternal smoking in infants and most predictive of increased risk of future pulmonary disease. 20;34;35 All pulmonary function tests in the VCSIP study are performed in the controlled infant pulmonary function testing laboratory located at OHSU/Doernbecher Children’s Hospital, the James Whitcomb Riley Hospital for Children, or the PFT laboratory at Peace Health/ Southwest Medical Center. These laboratories are the few across the country equipped to perform this testing in infants and have a history of successful studies in the area of infant pulmonary function testing.16;54;55 All labs are staffed with skilled technicians who perform pulmonary function tests and sedation under physician supervision. Testing is performed following the American Thoracic Society/ European Respiratory Society criteria for performance and acceptance 56 and measurements are reported as absolute values.
Infant pulmonary function testing is performed at 3 months (the age range of testing allowed is 12 weeks up to 6 months of age) and at 12 months (range of 10 up to 15 months of age). Since growth/length is an important determinant of the FEFs, the pulmonary function tests are performed as close as possible to 12 weeks and 12 months post term age. However, as testing cannot be done within 3 weeks of a respiratory illness, a time range for testing has been established.
The same pulmonary function testing equipment is used at all sites (Jaeger/Viasys Master Screen BabyBoy; Yorba Linda, California). Operational procedures were rigorously calibrated across study sites according to the manufacturer’s directions and outlined explicitly in the study’s manual of operations. In addition, cross training and certification of PFT laboratories assures the same testing techniques and acceptance criteria are applied across sites. All PFT test results are reviewed by a blinded trained reviewer. The respective hospital pediatric sedation protocol is followed, and the infant is given 50 to 100 milligrams per kilogram of chloral hydrate by mouth, with a maximum dose of 1 gram. The parents are counseled about possible side effects and asked to sign a separate consent for sedation as per respective hospital policy, and are given patient discharge instructions. Adverse events are reported as outlined below. The results of the 3 and 12 month PFTs are faxed to the patient’s physician.
4.2. Incidence of wheeze through 12 months of age
To compare the incidence of wheezing between the infants of smoking mothers randomized to vitamin C and placebo, a detailed standardized infant respiratory questionnaire57 is administered at least quarterly to the infant’s primary caretaker, either by phone or in person at the 3 and 12 month PFT. This questionnaire is the same as that administered quarterly in the Vitamin D Antenatal Asthma Reduction Trial (VDAART) study57;58 in which one of the primary outcomes was the parental report of physician diagnosis of asthma or occurrence of recurrent wheeze in the child’s first 3 years of life. This questionnaire is used in the VCSIP to document the incidence of wheezing in the infants through one year of age by the caretaker and by doctor report, as well as other respiratory symptoms (lower respiratory infections), medications of interest including inhaled or oral bronchodilators and/ or steroids, and leukotriene modifiers and a clinician’s diagnosis of bronchitis, bronchiolitis and asthma.
5. Sample collections
5.1. Blood collection
Fasting blood samples for ascorbic acid levels are collected from the mothers at randomization, at 26 ± 2, and at 32 ± 2 weeks of gestation, as a measure of medication adherence. At one of these time points, additional blood is drawn for genotyping.
Plasma ascorbic acid levels are tightly controlled around steady state levels with transient peaks after oral intake therefore necessitating fasting levels.59;60 The sample at randomization will establish the baseline ascorbic acid level prior to beginning the study supplementation and the subsequent levels will allow us to confirm adherence to the medication and evaluate levels achieved for the study dose.
At delivery, cord blood samples are collected to allow us to compare the ascorbic acid levels in the newborn to the maternal levels. A sample is also collected for genotyping. Near the time of delivery, women are customarily not allowed to eat, and study personnel request that they do not drink any liquid high in vitamin C in the hours prior to delivery. Study personnel carefully document the date and time of last study medication intake and dietary intake.
After obtaining consent, the mother and her infant are genotyped for the α5 nAChR D398N structural polymorphism (rs16969968), the decrease of function allele for GSTP1 (rs1695), and for the null alleles for GSTM1 and GSTT1. Remaining DNA is stored for further genetic analyses of smoking-induced changes. The α5 nAChR structural polymorphism (rs16969968) has been associated with increased risk of lung cancer, nicotine addiction, and chronic lung disease61 and in our initial study, offspring of mothers homozygous for this at risk allele had the greatest decrease in their PFTs, and the most benefit from the vitamin C supplementation.16
The types of blood samples collected are shown in Table 3.
5.2 Urine collection
Urine samples are collected for assessment of cotinine at baseline, 26 ± 2, and 32 ± 2 weeks of gestation. Within one hour of collection, the urine is aliquoted into eight cryovials and placed in −80°C freezer and shipped on dry ice to Dr. Eliot Spindel’s laboratory where it is stored in a −80°C freezer until tested for cotinine levels with additional samples stored for future use.
5.3. Exhaled carbon monoxide
Non-invasive measurements of exhaled carbon monoxide (CO) are obtained from the mother at baseline, 26 ± 2, and 32 ± 2 weeks of gestation and at the 3 and 12 month PFTs (Smokelyzer, Bedfont Scientific, London, UK) 62 to assess recent cigarette smoke exposure. The mother is instructed to inhale deeply and hold her breath for 15 seconds and then blow into the disposable mouthpiece of the Smokerlyzer until her lungs are completely empty. The highest level of the parts per million (ppm) of carbon monoxide and the percent of carboxyhemoglobin (COHb) are then recorded. Detection of the exhaled CO is performed by an electrochemical sensor which has a range of detection of 0–500 ppm, a sensitivity of 1 ppm and repeatability of readings of ± 3%. Nonsmokers who have no exposure to second hand smoke having readings of < 2 ppm. Calibration of the equipment is performed prior to study initiation and every six months as per the manufacturer specification.
5.4 Hair nicotine from mothers and offspring
Hair nicotine gives a longer representation of smoke exposure with hair growing on average 1 cm per month and the hair closest to the scalp representing the most recent exposure to nicotine. Small samples of the mother’s hair are taken at entry into the study. Additionally, mother and infant hair samples are collected at delivery, 3 months and 12 months of age. An area for hair collection, ideally on the occiput is identified. The hair is sectioned off by hand and least 20 strands are gently cut with scissor as close to the scalp as possible. A tie is placed on the cut end to identify the root, and the sample is placed in a piece of tissue paper and then into an anti-static bag and stored at room air until processed.
5.5 Placenta
The placenta is processed as soon as possible after delivery (within two hours) by study personnel. It is weighed on the same balance at each location and the maximum diameter (x-axis) and minimum diameter (y-axis) recorded to the nearest millimeter. It is then positioned with fetal side facing up and fetal membranes folded around the edge and the four quadrants of the placenta are sampled twice per quadrant using a stainless steel placenta biopsy tool. Each tissue sample is divided in half with one sample flash frozen and the other sample placed into either RNA later or neutral buffered formalin to be fixed in paraffin. The tissue in the RNA later tubes are incubated at 4°C for 16–72 hours and then transferred to tin foil and stored at −80°C. The time of all sampling processing is documented and samples are placed at −80°C until transferred on dry ice to storage at −80°C in the sample repository at Dr. Spindel’s laboratory for future study.
5.6 Buccal swabs on offspring
Buccal swabs are collected on the offspring at birth, 3 months, and 12 months of age to investigate DNA methylation patterns that may mediate subsequent respiratory disease after prenatal exposure to nicotine.63 Buccal swabs are taken using Epicentre Catch-All wrapped soft foam swabs. Swabs are performed by rolling the swab on the inside of the infant’s cheek, approximately 10–20 times on each side, making certain to roll the brush over the entire cheek. After collection the swab is placed in the collection tube and stored at −20°C. To increase yield of cells a separate swab is used for each cheek surface and each cheek is swabbed separately twice.
6. Sample assays/analysis
6.1. Ascorbic acid/green top heparin tubes
Seven mL of blood are collected and the contents of the tubes are gently inverted eight to ten times immediately after collection, are protected from the light by either wrapping in tin foil or inverting a box over the tube, and placed in wet ice for up to a maximum of 60 minutes until the tubes are centrifuged. The blood is spun at ≤1300 x g at 4°C for 10 minutes in a refrigerated centrifuge to separate blood cells from plasma. Four aliquots of 750 μL of plasma are then pipetted into separate 2 mL flip-cap microcentrifuge tubes and placed into a -80°C freezer at Dr. Spindel’s lab until shipped on dry ice to the Linus Pauling Institute for analysis. All blood samples are processed as soon as possible and within 2 hours of sample collection; a minimum plasma sample of at least 200 μL is required for ascorbic acid assay.
Plasma ascorbic acid levels are performed at the Linus Pauling Institute, Oregon State University. Human plasma is stabilized by the 1:1 (v/v) addition of 15% perchloric acid (PCA; EMD Millipore, Billerica, MA) containing 1mM diethylenetriaminepentaacetic acid (DTPA; Sigma-Adrich, St. Louis, MO) to chelate excess iron. Acid extracts are prepared for ascorbate analysis using high performance liquid chromatography as previously described.64 Briefly, extracts are diluted in sodium acetate/methanol/water mobile phase (0.3%/7.5%/92% w/v) containing Q12 ion pairing reagent (Regis Technologies, Morton Grove, IL) and pH-adjusted with 2.58 M KH2PO4 buffer (pH 9.8). Samples are separated on Watters 2695 using an LC-8 Supelco column (Sigma-Aldrich, St. Louis, MO) under an applied potential of 600 mV using an electrochemical detector (BAS, West Layfayette, IN). Under these conditions, both urate and ascorbate are resolved and quantified by comparisons to authentic standards with a detection limit of approximately 10 nM (inter-assay CV <15%). For human plasma samples, urate values are used to normalize ascorbate values to minimize any variations detected (intra-assay CV <5%).
6.2 Urine cotinine assay
Urine analysis for cotinine 65 are performed with a widely used ELISA (Enzyme Linked Immunesorbant Assay) kit following the vendor’s protocol (Calbiotech, Sping Valley, CA) in Dr. Eliot Spindel’s laboratory. The detection limit is 2 ng/mL.
6.3 Genotyping processing lavender-top tubes (EDTA) for whole blood
Two 3 mL lavender-top (EDTA) tubes are used to collect blood for maternal and infant genotyping. Two mL of blood are placed in each tube and gently inverted eight to ten times immediately after collection. The samples are then placed directly into a −80º C freezer and shipped to the Spindel laboratory and stored in −80º C freezers.
Genotyping for the α5 nAChR D398N structural polymorphism (rs16969968) and the decrease of function allele for GSTP1 (rs1695) are performed using predesigned qPCR SNP genotyping reagents from Thermo Fisher (Applied Biosystems, Foster City, CA). Genotyping of the mothers and infants for the null alleles for GSTM1 and GSTT1 are performed using predesigned qPCR copy number assays from Applied Biosystems. Remaining DNA is stored for further genetic analyses of smoking-induced changes. Genomic DNA is isolated from 0.35 mL of blood using automated protocols from Promega (Madison, WI) or Qiagen (Hilden, Germany).
6.4. Hair nicotine
Hair nicotine is determined by a modification of the methods of Hegstad et al 66 and Pichini et al67 by tandem mass spectrometry (LC-MS/MS) using deuterated internal standards.
7. Quality of study medication and blinding
Maintaining the integrity of randomization and blinding is the most important asset of the study and is the primary goal of the DCC as outlined above. To ensure integrity of the medication preparation, potency testing of the vitamin C tablets was done prior to the initiation of the study. To assist in assessing the effectiveness of blinding, participants are asked at the 12 month PFT which treatment group they believe they were assigned during the study.
7.1. Assessment of medication adherence- Pill counts at prenatal visits
Patients are reminded frequently to bring their study medication in to every prenatal visit and at delivery. Adherence is calculated based on number of study medication tablets dispensed and number of study medication tablets returned (number of tablets taken divided by number of tablets that should have been taken X 100 = percent compliant). However given the greater reliability of a biomarker to detect adherence in comparison to pill count, we selected ascorbic acid levels as our primary measure of adherence.
8. Analysis plan and statistical power
8.1. Analysis plan
This is a randomized, double blinded, multi-center, placebo controlled clinical trial of supplemental vitamin C to pregnant smokers to improve pulmonary function and decrease wheeze in their offspring. The primary outcome is the measurement of FEF75 at 3 months of age. Secondary outcomes include the incidence of wheeze through 12 months of age and FEF75 at 12 months of age.
The statistical analyses are based on intention to treat. The primary analysis of the FEF75 will compare the FEF75 of infants born to mothers randomized to Vitamin C versus placebo, using regression analysis adjusting for the infant’s sex, race, and age and length at the time of FEF75 measurement, and the stratification variables (site and gestational age at randomization i.e.≤18 weeks versus > 18 weeks). FEFs are highly correlated with body length and are a standard component in the interpretation of FEF75 and similar measures.54;56. Since FEF75 is not normally distributed, we will use the natural logarithmic transformation of FEF75 for the primary analysis to reduce expected skewness.68 Assessment of outliers and influential points will be conducted and reported.
8.2. Statistical power
The trial was designed to detect a 15% increase in FEF75 at 3 months of age in the vitamin C supplemented versus placebo group. The estimates of sample size and power were obtained using our experience in the initial vitamin C study16 and on measures of variability of FEF75 in 155 healthy infants of smoking and nonsmoking women as published by co-investigator, Dr. Tepper.54 The estimated standard deviation from a regression model comparing infants of non-smoking to smoking women was 0.28, using the natural logarithm of FEF75 as the outcome. The estimated increase in FEF75 for infants of nonsmoking women was 17.6% greater than that for infants born to smoking women. For power calculations, we use this value along with increases in FEF75 of 15.0% and 12.5%.
The PFT data from our initial study showed an increase of 11% (p<0.01) in the measurement of the time to peak tidal flow to expiratory time (TPTEF:TE) and a 10% increase in respiratory compliance normalized per kilogram (Crs/kg) between babies born to smoking pregnant women randomized to vitamin C versus placebo. Both have clinical significance, particularly the change in TPTEF:TE. A decreased TPTEF:TE measured in the initial weeks of life has been shown to precede and predict wheezing.15;21;34;69;70 The current VCSIP study employs FEFs, a more sensitive and reproducible measure of peripheral airway function.20;34;35 Thus, we powered VCSIP to show a 15% difference in FEF75 between randomized patients. We chose this as clinically significant as the NHLBI asthma guidelines71 define a positive response to a bronchodilator as increases in FEV1 of this magnitude. Table 4 shows the sample sizes needed to show a 15% difference in FEF75 with a 80% and 90% power at a significance level 0.05 using the estimated standard deviation of 0.28 as published from Jones and Tepper et al.54 as well as a 90% upper confidence bound for the standard deviation, 0.30 using the chi-squared distribution of the estimated SD. Calculations are based on two-sided tests.
Table 4.
Sample sizes per group for FEF75 with 90 (80%) power using a two-sided test at level of 0.05.
| Mean difference in ln(FEF75) between infants in the vitamin C and the placebo groups. | Ratio of FEF75 for infants in mothers allocated to vitamin C relative to placebo. | Sample size per group needed for 90% (80%) power using the estimated SD of 0.28. | Sample size per group for 90% (80%) power using estimated 90% upper confidence bound for SD (0.30). | Sample size per group for 90% (80%) power using estimated 90% upper confidence bound for SD: 0.30 and assuming 4% non-adherence/crossover |
|---|---|---|---|---|
| 0.162 | 17.6% | 64 (48) | 75 (56) | 82 (61) |
| 0.1398 | 15.0% | 86 (64) | 100 (75) | 109 (82) |
| 0.1178 | 12.5% | 120 (90) | 140 (105) | 152 (114) |
Conservatively, using the 90% upper confidence bound for the standard deviation, with a sample size of 100 mothers per group, we would have 90% power at level 0.05 to detect an increase of 15% in FEF75 for infants randomized to Vitamin C compared to those randomized to placebo and measured at three months of age. Adjusting for adherence data from our initial study showing only 4% of patients were non-compliant (took <50% of their medications), we increased our sample size to 109 patients per group studied at the 3 month PFT. Figure 3 below shows our original projected study recruitment to achieve this desired sample size based on recruitment and cohort loss data from our initial randomized trial.
Figure 3.
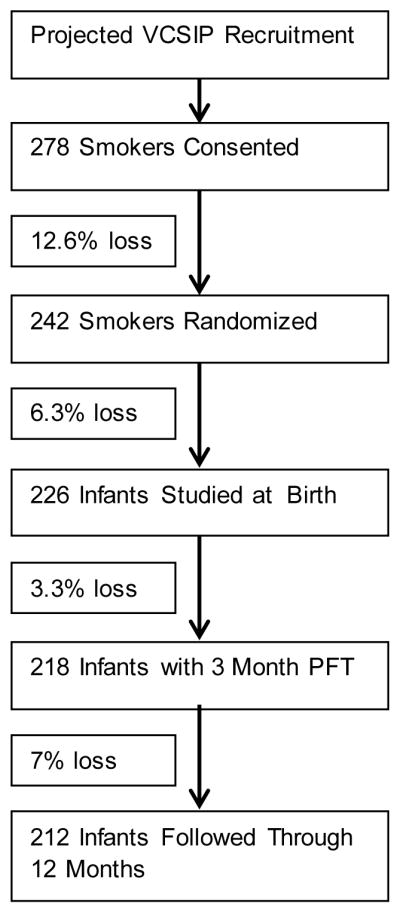
Original projection of study recruitment
With regard to aim 2, preliminary data from our initial study on 80 infants from mothers allocated to vitamin C versus placebo suggested a difference in the incidence of wheezing in their infants through 1 year of age: placebo 48%, vitamin C 26% (p=0.06). The incidence of wheezing at 1 year of age in 101 infants of pregnant smokers randomized to the placebo was 45% which was similar to that reported by Dezateux.35 The incidence of wheezing in infants of non-smoking mothers in our pilot study was 14%. Based on these data from our initial study and from that of Dezateux,35 Table 5 shows the sample sizes needed at delivery to detect various hazard ratios for wheezing through 1 year of age with 80% and 90% power with a two-sided significance level of 0.05. Calculations are based on the log-rank test. We estimate that our targeted sample size of 113 infants per group at delivery allows excellent power of 80–90% for detection of hazard ratios of 0.45–0.50 with a 0.5–1% drop-out per month.
Table 5.
Sample sizes per group to detect wheezing incidence with 80% (90%) power
| Proportion wheeze in placebo group within first year | Proportion wheeze in vitamin C group within first year | Hazard Ratio vitamin C group relative to placebo | Sample size per group needed at delivery for 90% (80%) power with drop-out of 0.5% per month | Sample size per group needed at delivery for 90% (80%) power with drop-out of 1% per month |
|---|---|---|---|---|
| 0.48 | 0.14 | 0.23 | 35 (27) | 36 (28) |
| 0.48 | 0.20 | 0.34 | 57(43) | 59 (45) |
| 0.48 | 0.26 | 0.46 | 99 (74) | 101 (77) |
| 0.45 | 0.14 | 0.25 | 41 (32) | 42 (33) |
| 0.45 | 0.20 | 0.37 | 71 (54) | 73 (55) |
| 0.45 | 0.26 | 0.50 | 131 (99) | 135 (102) |
| 0.40 | 0.14 | 0.30 | 54 (44) | 58 (45) |
| 0.40 | 0.20 | 0.44 | 108 (81) | 111 (84) |
9. Trial Monitoring
An external Data Safety and Monitoring Board (DSMB) was appointed by the National Heart, Lung, and Blood Institute (NHLBI) and consists of experts in the fields relevant to this study. The DSMB approved the trial protocol. During the conduct of the study, the DSMB monitors trial performance and safety, and protocol adherence. The DSMB receives quarterly reports detailing adverse events, patient recruitment, cohort retention, medication adherence, baseline patient characteristics, and center performance information with respect to data quality, timeliness of data submission and protocol adherence (in addition to safety and efficacy data). It also approves all manuscripts prior to submission for peer review. VCSIP has approval by the Institutional Review Boards of each of the participating Clinical Coordinating Centers and the Data Coordinating Center.
Monitoring the safety of the vitamin C dose is a high priority. We actively monitor the following: inability to tolerate ingestion of the study medication; any allergic or adverse responses; the unlikely development of kidney stones; confirmation of adherence to exclusion criteria of no other vitamin C supplementation aside from study medication and supplied prenatal vitamin; incidence of any complications of pregnancy; incidence of intrauterine growth retardation and large for gestational age; incidence of prematurity and associated respiratory morbidities; presence of any congenital malformations. This is done at each prenatal visit (at least monthly) and at delivery. The study medication is terminated under the following conditions: side effect presumed to be from study medication; termination of pregnancy; urolithiasis; serious complication of pregnancy where continuation of study medication is deemed inappropriate or impossible.
10. Adverse event reporting
All subjects are monitored for adverse events (AEs). AEs in this study are identified by interviewing the subject, review of the subject’s electronic medical record, and by physical exam/observations (including vital signs monitoring during sedation). Research staff review AEs in real-time with study principal investigators to grade the AEs as to their expectedness and attribution (unrelated, possibly, probably or definitely related to the protocol) and AE/SAE forms are completed and faxed to the DCC. All serious adverse events (SAEs) and unexpected problems (UPs) are reported by clinical sites to the DCC within one business day. All AEs are reported within 7 days. The DCC evaluates each event and determines reporting requirements. All SAEs and UPs that are deemed related and unexpected require expedited reporting and are reported to the DSMB within 7 business days. In addition, the following has expedited reporting: maternal death through delivery; fetal loss, neonatal or infant death through 1 year of age; any sedation related event requiring significant resuscitation and/or hospitalization. All other SAEs are reported to the DSMB within 30 business days and all other AE’s are summarized quarterly for the DSMB. Summary AE and SAE reports are submitted to the respective clinical site IRBs annually.
11. Data collection, forms and entry
Data for the VCSIP study is collected on standardized case report forms with independent double data entry into a REDCap database. Site personnel complete the paper-based case report forms, perform pre-processing checks for completeness and consistency of key data fields, and independently enter data into the study database via secure web-based forms. Duplicate data entry occurs at the DCC with adjudication as required.
12. Data management
Data is collected within each study site by a combination of study-specific forms that capture clinical information not routinely recorded in patient charts, by abstraction of standard clinical information and outcomes from the records of patients, and by patient self-reports. The timely entry of data and faxing of case report forms is monitored by maintaining a study calendar using the database’s study and participant calendar feature and reviewing expected visits, forms, and specimens on a weekly basis.
The discrepancy management process includes a variety of important data quality tasks. These include running production edit checks, reviewing data discrepancies produced by edit checks, generating queries, resolving discrepancies, and updating any relevant data in the database. In addition to the computerized checks, the data management staff conduct manual reviews of the data throughout the study. All discrepancies identified are reviewed by the DCC staff who then either resolve the discrepancies or forward queries to each site for clarification or resolution on data clarification forms. The sites return the data clarification forms after resolution. The data team then modifies the data with a full audit trail generated for each modification.
13. Ancillary studies
13.1. Placental blood flow
Preliminary data in non-human primates has recently demonstrated decreased placental blood flow in nicotine-exposed fetal monkeys and has demonstrated that supplemental vitamin C decreases this effect.72 Based on this data, an ancillary study funded NHLBI with co-funding from the Office of Dietary Supplements was obtained. In this ancillary study, extra ultrasound views are done during a routinely ordered prenatal ultrasound to specifically measure umbilical blood flow using color and pulsed/wave Doppler, uterine artery and placental volume blood flow. 73;74 These extra views are done at 34 ± 2 weeks of gestation in a subset or randomized smokers in the main study.
13.2 Placenta and offspring buccal swabs analysis
Epigenetic changes that occur in utero may explain the lifelong detrimental health effects observed in individuals whose mother smoked during pregnancy. Maternal smoking during pregnancy has been associated with both modification of specific genes75;76 and changes in indices of global DNA methylation.76;77 We received ancillary funding to longitudinally follow methylation changes examined in the placenta and serial buccal smears from offspring of smokers, and to determine if there are methylation changes that are blocked by maternal vitamin C supplementation and which appear to associate with decreased risk of asthma. This will allow functional connection of the methylation changes with mechanisms underlying asthma development. This ancillary study is funded by catalyst funding through the Oregon Clinical and Translational Research Institute from OHSU.
Discussion
VCSIP is designed to determine if prenatal vitamin C supplementation (500mg/day) administered to women unable to quit smoking during pregnancy can increase the pulmonary function tests/ FEFs of their offspring measured at 3 months of age as compared to the pulmonary function tests from offspring of placebo allocated women. Its secondary outcomes evaluate the ability of supplemental vitamin C to decrease the incidence of wheeze in their offspring through 12 months of age and to improve the forced expiratory flows of the offspring at 12 months of age. Multiple studies clearly show that maternal smoking during pregnancy leads to altered lung development manifested by impaired pulmonary function and reflected by increased respiratory illness in early life.17;20;78 Adverse respiratory outcomes related to maternal smoking during pregnancy track from childhood and into adulthood.2 VCSIP uniquely intervenes in utero, a critical time period for lung development, and measures the response to vitamin C exposure through infant FEFs, a sensitive measure of peripheral airway function.20;34 Strengths of our trial include a low-risk and simple yet novel intervention to potentially block the harmful effects of nicotine during fetal lung development, and quantification of lung function with skilled infant pulmonary function tests.
Most pregnant smokers will continue to smoke and given nicotine’s addictive nature, the low socio-economic status of the study population, and the constant advertising by tobacco companies, smoking during pregnancy will continue to adversely affect millions of babies worldwide. Statistics from the Center for Disease Control show that decreases in smoking rates have plateaued79 and even increased in recent years for teenagers.80 Multiple studies have shown, that upon learning of pregnancy, approximately 50% of smokers will immediately quit; the other 50% will continue to smoke no matter the intervention.9 This unfortunate reality makes finding ways to lessen the impact of smoking during pregnancy of vital importance. Smoking cessation remains our foremost goal, and another strength of VCSIP is that smoking cessation counseling continues throughout the study. Only women truly unable to quit smoking are involved in the study. If a woman quits smoking after randomization, this is noted and they are instructed to continue to participate in the study, as the analytic design is intention-to-treat.
The strengths of VSCIP include the application of infants PFTs to quantify the infant’s physiologic response to the intervention and the correlation to clinical respiratory outcomes. Longitudinal studies 69;81;82 have demonstrated that PFTs can quantify the “root” i.e. beginning of infant lung disease, and patients who begin life with PFTs below the median continue to track below the median throughout life with increased wheezing, asthma and a predisposition to the development of chronic obstructive pulmonary disease as the normal aging process occurs. Our initial study showed that vitamin C significantly improved the PFTs of newborns born to randomized pregnant smokers and that these offspring also showed a lower incidence of wheezing at 1 year of age. In addition, VCSIP is powered at a minimum of 90% power for the pulmonary function outcomes and between 80 to 90% power for the clinical outcome of the incidence of wheezing.
Limitations include a knowledge gap regarding the ideal time to initiate the vitamin C supplementation to block the effects of in utero smoke on the offspring’s lung function. However, data from our initial study supports that 23 weeks gestation may be early enough for vitamin C supplementation to improve PFTs. There is also animal data83 to support that the critical period of exposure to nicotine in terms of impacting lung development is mid gestation, and all of our patients will be randomized prior to that time period. Ideally, VSCIP would have multiple study arms to assess different combinations of in utero and postnatal exposure to vitamin C supplementation with and without smoke exposure, but this is not practical. Postnatal maternal smoking and offspring exposures to environmental smoke are carefully documented through 1 year of age and the randomization design will allow VCSIP investigators to compare postnatal exposures between treatment groups. Lastly, VSCIP is designed to follow offspring through 1 year of age. As a clinically relevant asthma diagnosis usually occurs at more than 5 years of age, we intend to follow the cohort through 5 years of age.
The VSCIP study has successfully incorporated important ancillary objectives. In particular, genotype likely plays a key role in the development of asthma, the sensitivity to maternal smoking, and the difficulty in quitting smoking. Common deletions or structural polymorphisms in the glutathione transferase (GST) genes increase both the risk of asthma and the sensitivity of the fetus to maternal smoking.63;84–87 The D398N (rs16969968) structural polymorphism of the α5 nicotinic receptor increases nicotine addiction, makes quitting more difficult, increases the risk of lung cancer and COPD 88 and has been associated with increased growth retardation in offspring.89;90 These ancillary studies will assist in elucidating mechanistic links between vitamin C supplementation and offspring pulmonary function and potential genetic factors associated with high rates of maternal smoking in this patient population.
VSCIP will provide critical information regardless of whether a difference in pulmonary function tests is observed at three months post-delivery between randomized groups. However our initial trial of vitamin C supplementation to pregnant smokers is very supportive for a positive result in the present VCSIP. In addition, the VCSIP prospective cohort of offspring of pregnant smokers is also uniquely phenotyped prenatally and postnatally to address important scientific questions related to maternal smoking and childhood respiratory health, which is highly relevant to the overall goals of NHLBI, and thus can have a major impact upon public health.
Acknowledgments
Foremost, the VCSIP research team deeply thanks the women who participate in our study. VCSIP is supported by R01 HL105447 from the NHLBI with cofounding from the Office of Dietary Supplements (ODS). The ancillary study “Can Supplemental Vitamin C Lessen the Effects of Smoking in Pregnancy on the Placenta” is supported by a supplement to this award by the NHLBI with cofunding from the ODS. The ancillary study “Blocking Lung Disease and the Epigenetic Changes in Childhood Caused by Maternal Smoking During Pregnancy” is funded by a pilot award from the Oregon Clinical and Translational Research Institute (OCTRI) ; we received additional support for data coordination through for the study was provided through OCTRI which is funded by the National Center for Advancing Translational Sciences (UL1TR000128). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The VCSIP team also thanks and acknowledges the members of the Vitamins for Early Lung Health (VITEL) DSMB for their advice, support, and data monitoring during the trial.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.US Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General—Executive Summary. Centers for Disease Control and Prevention; 2006. pp. 1–27. [Google Scholar]
- 2.Hayatbakhsh MR, Sadasivam S, Mamun AA, Najman JM, O'callaghan MJ. Maternal smoking during and after pregnancy and lung function in early adulthood: A prospective study. Thorax. 2009;64:810–814. doi: 10.1136/thx.2009.116301. [DOI] [PubMed] [Google Scholar]
- 3.Best D. From the American Academy of Pediatrics: Technical report--Secondhand and prenatal tobacco smoke exposure. Pediatrics. 2009;124:e1017–e1044. doi: 10.1542/peds.2009-2120. [DOI] [PubMed] [Google Scholar]
- 4.Filion KB, Abenhaim HA, Mottillo S, et al. The effect of smoking cessation counselling in pregnant women: a meta-analysis of randomised controlled trials. BJOG. 2011;118:1422–1428. doi: 10.1111/j.1471-0528.2011.03065.x. [DOI] [PubMed] [Google Scholar]
- 5.Schneider S, Huy C, Schutz J, Diehl K. Smoking cessation during pregnancy: a systematic literature review. Drug Alcohol Rev. 2010;29:81–90. doi: 10.1111/j.1465-3362.2009.00098.x. [DOI] [PubMed] [Google Scholar]
- 6.Tong VT, Jones JR, Dietz PM, D'Angelo D, Bombard JM. Trends in smoking before, during, and after pregnancy - Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000–2005. MMWR Surveill Summ. 2009;58:1–29. [PubMed] [Google Scholar]
- 7.Stoddard JJ, Gray B. Maternal smoking and medical expenditures for childhood respiratory illness. Am J Public Health. 1997;87:205–209. doi: 10.2105/ajph.87.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiFranza JR, Wellman RJ, Sargent JD, Weitzman M, Hipple BJ, Winickoff JP. Tobacco promotion and the initiation of tobacco use: assessing the evidence for causality. Pediatrics. 2006;117:e1237–e1248. doi: 10.1542/peds.2005-1817. [DOI] [PubMed] [Google Scholar]
- 9.Anderson SJ, Glantz SA, Ling PM. Emotions for sale: cigarette advertising and women's psychosocial needs. Tob Control. 2005;14:127–135. doi: 10.1136/tc.2004.009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schroeder SA. Tobacco control in the wake of the 1998 master settlement agreement. N Engl J Med. 2004;350:293–301. doi: 10.1056/NEJMsr031421. [DOI] [PubMed] [Google Scholar]
- 11.Wang JC, Cruchaga C, Saccone NL, et al. Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet. 2009;18:3125–3135. doi: 10.1093/hmg/ddp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saccone SF, Hinrichs AL, Saccone NL, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bierut LJ, Madden PA, Breslau N, et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong VT, Dietz PM, Morrow B, et al. Trends in smoking before, during, and after pregnancy--Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000–2010. MMWR Surveill Summ. 2013;62:1–19. [PubMed] [Google Scholar]
- 15.Proskocil BJ, Sekhon HS, Clark JA, et al. Vitamin C Prevents the Effects of Prenatal Nicotine on Pulmonary Function in Newborn Monkeys. Am J Respir Crit Care Med. 2005;171:1032–1039. doi: 10.1164/rccm.200408-1029OC. [DOI] [PubMed] [Google Scholar]
- 16.McEvoy CT, Schilling D, Clay N, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 2014;311:2074–2082. doi: 10.1001/jama.2014.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham J, Dockery DW, Speizer FE. Maternal smoking during pregnancy as a predictor of lung function in children. Am J Epidemiol. 1994;139:1139–1152. doi: 10.1093/oxfordjournals.aje.a116961. [DOI] [PubMed] [Google Scholar]
- 18.Hanrahan JP, Tager IB, Segal MR, et al. The effect of maternal smoking during pregnancy on early infant lung function. Am Rev Respir Dis. 1992;145:1129–1135. doi: 10.1164/ajrccm/145.5.1129. [DOI] [PubMed] [Google Scholar]
- 19.Tager IB, Weiss ST, Munoz A, Rosner B, Speizer FE. Longitudinal study of the effects of maternal smoking on pulmonary function in children. N Engl J Med. 1983;309:699–703. doi: 10.1056/NEJM198309223091204. [DOI] [PubMed] [Google Scholar]
- 20.Tager IB, Hanrahan JP, Tosteson TD, et al. Lung function, pre- and post-natal smoke exposure, and wheezing in the first year of life. Am Rev Respir Dis. 1993;147:811–817. doi: 10.1164/ajrccm/147.4.811. [DOI] [PubMed] [Google Scholar]
- 21.Hoo AF, Henschen M, Dezateux C, Costeloe K, Stocks J. Respiratory function among preterm infants whose mothers smoked during pregnancy. Am J Respir Crit Care Med. 1998;158:700–705. doi: 10.1164/ajrccm.158.3.9711057. [DOI] [PubMed] [Google Scholar]
- 22.Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med. 2013;1:728–742. doi: 10.1016/S2213-2600(13)70118-8. [DOI] [PubMed] [Google Scholar]
- 23.Lodrup Carlsen KC, Jaakkola JJ, Nafstad P, Carlsen KH. In utero exposure to cigarette smoking influences lung function at birth. Eur Respir J. 1997;10:1774–1779. doi: 10.1183/09031936.97.10081774. [DOI] [PubMed] [Google Scholar]
- 24.Rantakallio P. Relationship of maternal smoking to morbidity and mortality of the child up to the age of five. Acta Paediatr Scand. 1978;67:621–631. doi: 10.1111/j.1651-2227.1978.tb17813.x. [DOI] [PubMed] [Google Scholar]
- 25.Taylor B, Wadsworth J. Maternal smoking during pregnancy and lower respiratory tract illness in early life. Archives of Disease in Childhood. 1987;62:786–791. doi: 10.1136/adc.62.8.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burke H, Leonardi-Bee J, Hashim A, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129:735–744. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 27.Neuman A, Hohmann C, Orsini N, et al. Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med. 2012;186:1037–1043. doi: 10.1164/rccm.201203-0501OC. [DOI] [PubMed] [Google Scholar]
- 28.Bruin JE, Petre MA, Lehman MA, et al. Maternal nicotine exposure increases oxidative stress in the offspring. Free Radic Biol Med. 2008;44:1919–1925. doi: 10.1016/j.freeradbiomed.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Gunes T, Koklu E, Gunes I, Narin F, Koklu S. Influence of maternal nicotine exposure on neonatal rat oxidant-antioxidant system and effect of ascorbic acid supplementation. Hum Exp Toxicol. 2008;27:781–786. doi: 10.1177/0960327107082229. [DOI] [PubMed] [Google Scholar]
- 30.Jaimes E, Tian RX, Raij L. Nicotine: The Link Between Cigarette Smoking and the Progression of Renal Injury? Am J Physiol Heart Circ Physiol. 2006 doi: 10.1152/ajpheart.00693.2006. [DOI] [PubMed] [Google Scholar]
- 31.Ozokutan BH, Ozkan KU, Sari I, Inanc F, Guldur ME, Kilinc M. Effects of Maternal Nicotine Exposure during Lactation on Breast-Fed Rat Pups. Biol Neonate. 2005;88:113–117. doi: 10.1159/000086130. [DOI] [PubMed] [Google Scholar]
- 32.Sekhon HS, Keller JA, Benowitz NL, Spindel ER. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am J Respir Crit Care Med. 2001;164:989–994. doi: 10.1164/ajrccm.164.6.2011097. [DOI] [PubMed] [Google Scholar]
- 33.Spindel ER, McEvoy CT. The Role of Nicotine in the Effects of Maternal Smoking during Pregnancy on Lung Development and Childhood Respiratory Disease. Implications for Dangers of E-Cigarettes. Am J Respir Crit Care Med. 2016;193:486–494. doi: 10.1164/rccm.201510-2013PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N Engl J Med. 1988;319:1112–1117. doi: 10.1056/NEJM198810273191702. [DOI] [PubMed] [Google Scholar]
- 35.Dezateux C, Stocks J, Dundas I, Fletcher ME. Impaired Airway Function and Wheezing in Infancy. The influence of maternal smoking and a genetic predisposition to asthma. Am J Respir Crit Care Med. 1999;159:403–410. doi: 10.1164/ajrccm.159.2.9712029. [DOI] [PubMed] [Google Scholar]
- 36.Cote CJ, Zaslavsky A, Downes JJ, et al. Postoperative apnea in former preterm infants after inguinal herniorrhaphy. A combined analysis. Anesthesiology. 1995;82:809–822. doi: 10.1097/00000542-199504000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Malviya S, Voepel-Lewis T, Ludomirsky A, Marshall J, Tait AR. Can we improve the assessment of discharge readiness?: A comparative study of observational and objective measures of depth of sedation in children. Anesthesiology. 2004;100:218–224. doi: 10.1097/00000542-200402000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Mayers DJ, Hindmarsh KW, Sankaran K, Gorecki DK, Kasian GF. Chloral hydrate disposition following single-dose administration to critically ill neonates and children. Dev Pharmacol Ther. 1991;16:71–77. [PubMed] [Google Scholar]
- 39.Institute of Medicine NAoS. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Vol. 2000. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 40.Rumbold A, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database Syst Rev. 2005:CD004072. doi: 10.1002/14651858.CD004072.pub2. [DOI] [PubMed] [Google Scholar]
- 41.Steyn PS, Odendaal HJ, Schoeman J, Stander C, Fanie N, Grove D. A randomised, double-blind placebo-controlled trial of ascorbic acid supplementation for the prevention of preterm labour. J Obstet Gynaecol. 2003;23:150–155. doi: 10.1080/014436103000074673. [DOI] [PubMed] [Google Scholar]
- 42.Roberts JM, Myatt L, Spong CY, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362:1282–1291. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rumbold A, Ota E, Nagata C, Shahrook S, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database Syst Rev. 2015:CD004072. doi: 10.1002/14651858.CD004072.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.United States Department of Health and Human Services. Treating Tobacco Use and Dependence. 2000. [Google Scholar]
- 45.Gonzales D, Bjornson W, Durcan MJ, et al. Effects of gender on relapse prevention in smokers treated with bupropion SR. Am J Prev Med. 2002;22:234–239. doi: 10.1016/s0749-3797(02)00419-1. [DOI] [PubMed] [Google Scholar]
- 46.Hays JT, Hurt RD, Rigotti NA, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. a randomized, controlled trial. Annals of Internal Medicine. 2001;135:423–433. doi: 10.7326/0003-4819-135-6-200109180-00011. [DOI] [PubMed] [Google Scholar]
- 47.Ershoff DH, Quinn VP, Boyd NR, Stern J, Gregory M, Wirtschafter D. The Kaiser Permanente prenatal smoking-cessation trial: when more isn't better, what is enough? Am J Prev Med. 1999;17:161–168. doi: 10.1016/s0749-3797(99)00071-9. [DOI] [PubMed] [Google Scholar]
- 48.O'Campo P, Davis MV, Gielen AC. Smoking cessation interventions for pregnant women: review and future directions. [Review] [37 refs] Seminars in Perinatology. 1995;19:279–285. doi: 10.1016/s0146-0005(05)80042-4. [DOI] [PubMed] [Google Scholar]
- 49.Van't Hof SM, Wall MA, Dowler DW, Stark MJ. Randomised controlled trial of a postpartum relapse prevention intervention. Tob Control. 2000;9(Suppl–6) doi: 10.1136/tc.9.suppl_3.iii64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.American College of Obstetricians and Gynecologists. Smoking cessation during pregnancy. Committee Opinion No 471. Obstet Gynecol. 2013;116:1241–1244. doi: 10.1097/AOG.0b013e3182004fcd. [DOI] [PubMed] [Google Scholar]
- 51.McDonald S, Kehler H, Bayrampour H, Fraser-Lee N, Tough S. Risk and protective factors in early child development: Results from the All Our Babies (AOB) pregnancy cohort. Res Dev Disabil. 2016;58:20–30. doi: 10.1016/j.ridd.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Wood ME, Frazier JA, Nordeng HM, Lapane KL. Longitudinal changes in neurodevelopmental outcomes between 18 and 36 months in children with prenatal triptan exposure: findings from the Norwegian Mother and Child Cohort Study. BMJ Open. 2016;6:e011971. doi: 10.1136/bmjopen-2016-011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barnett J, Aguilar S, Brittner M, Bonuck K. Recruiting and retaining low-income, multi-ethnic women into randomized controlled trials: successful strategies and staffing. Contemp Clin Trials. 2012;33:925–932. doi: 10.1016/j.cct.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones M, Castile R, Davis S, et al. Forced expiratory flows and volumes in infants. Normative data and lung growth. Am J Respir Crit Care Med. 2000;161:353–359. doi: 10.1164/ajrccm.161.2.9903026. [DOI] [PubMed] [Google Scholar]
- 55.Tepper RS, Williams-Nkomo T, Martinez T, Kisling J, Coates C, Daggy J. Parental smoking and airway reactivity in healthy infants. Am J Respir Crit Care Med. 2005;171:78–82. doi: 10.1164/rccm.200406-711OC. [DOI] [PubMed] [Google Scholar]
- 56.Beydon N, Davis SD, Lombardi E, et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175:1304–1345. doi: 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- 57.Litonjua AA, Carey VJ, Laranjo N, et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA. 2016;315:362–370. doi: 10.1001/jama.2015.18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Litonjua AA, Lange NE, Carey VJ, et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp Clin Trials. 2014;38:37–50. doi: 10.1016/j.cct.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levine M, Conry-Cantilena C, Wang Y, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci U S A. 2001;98:9842–9846. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bierut LJ. Convergence of genetic findings for nicotine dependence and smoking related diseases with chromosome 15q24–25. Trends Pharmacol Sci. 2010;31:46–51. doi: 10.1016/j.tips.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fagerstrom KO. Assessment of the smoker who wants to quit. Monaldi Arch Chest Dis. 2001;56:124–127. [PubMed] [Google Scholar]
- 63.Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180:462–467. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benowitz NL, Hukkanen J, Jacob P., III Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hegstad S, Khiabani HZ, Kristoffersen L, Kunoe N, Lobmaier PP, Christophersen AS. Drug screening of hair by liquid chromatography-tandem mass spectrometry. J Anal Toxicol. 2008;32:364–372. doi: 10.1093/jat/32.5.364. [DOI] [PubMed] [Google Scholar]
- 67.Pichini S, Altieri I, Pellegrini M, Pacifici R, Zuccaro P. Hair analysis for nicotine and cotinine: evaluation of extraction procedures, hair treatments, and development of reference material. Forensic Sci Int. 1997;84:243–252. doi: 10.1016/s0379-0738(96)02068-3. [DOI] [PubMed] [Google Scholar]
- 68.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 69.Haland G, Carlsen KC, Sandvik L, et al. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682–1689. doi: 10.1056/NEJMoa052885. [DOI] [PubMed] [Google Scholar]
- 70.Yuksel B, Greenough A, Giffin F, Nicolaides KH. Tidal breathing parameters in the first week of life and subsequent cough and wheeze. Thorax. 1996;51:815–818. doi: 10.1136/thx.51.8.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.National Asthma Education and Prevention Program. Expert Panel Report. Guidelines for the diagnosis and management of asthma. Update on selected topics 2002. J Allergy Clin Immunol. 2002;110(5 suppl):S141–S219. [PubMed] [Google Scholar]
- 72.Lo JO, Schabel MC, Roberts VH, et al. Vitamin C supplementation ameliorates the adverse effects of nicotine on placental hemodynamics and histology in nonhuman primates. Am J Obstet Gynecol. 2015;212:370–378. doi: 10.1016/j.ajog.2014.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Acharya G, Erkinaro T, Makikallio K, Lappalainen T, Rasanen J. Relationships among Doppler-derived umbilical artery absolute velocities, cardiac function, and placental volume blood flow and resistance in fetal sheep. Am J Physiol Heart Circ Physiol. 2004;286:H1266–H1272. doi: 10.1152/ajpheart.00523.2003. [DOI] [PubMed] [Google Scholar]
- 74.Rasanen J, Wood DC, Debbs RH, Cohen J, Weiner S, Huhta JC. Reactivity of the human fetal pulmonary circulation to maternal hyperoxygenation increases during the second half of pregnancy: a randomized study. Circulation. 1998;97:257–262. doi: 10.1161/01.cir.97.3.257. [DOI] [PubMed] [Google Scholar]
- 75.Joubert BR, Haberg SE, Nilsen RM, et al. 450K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ Health Perspect. 2012;120:1425–1431. doi: 10.1289/ehp.1205412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suter M, Ma J, Harris A, et al. Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics. 2011;6:1284–1294. doi: 10.4161/epi.6.11.17819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilhelm-Benartzi CS, Houseman EA, Maccani MA, et al. In utero exposures, infant growth, and DNA methylation of repetitive elements and developmentally related genes in human placenta. Environ Health Perspect. 2012;120:296–302. doi: 10.1289/ehp.1103927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown RW, Hanrahan JP, Castile RG, Tager IB. Effect of maternal smoking during pregnancy on passive respiratory mechanics in early infancy. Pediatr Pulmonol. 1995;19:23–28. doi: 10.1002/ppul.1950190105. [DOI] [PubMed] [Google Scholar]
- 79.Cigarette smoking among adults and trends in smoking cessation - United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1227–1232. [PubMed] [Google Scholar]
- 80.Cigarette use among high school students--United States, 1991–2007. MMWR Morb Mortal Wkly Rep. 2008;57:686–688. [PubMed] [Google Scholar]
- 81.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–764. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martinez FD. The origins of asthma and chronic obstructive pulmonary disease in early life. Proc Am Thorac Soc. 2009;6:272–277. doi: 10.1513/pats.200808-092RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wongtrakool C, Wang N, Hyde DM, Roman J, Spindel ER. Prenatal nicotine exposure alters lung function and airway geometry through alpha7 nicotinic receptors. Am J Respir Cell Mol Biol. 2012;46:695–702. doi: 10.1165/rcmb.2011-0028OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wenten M, Li YF, Lin PC, et al. In utero smoke exposure, glutathione S-transferase P1 haplotypes, and respiratory illness-related absence among schoolchildren. Pediatrics. 2009;123:1344–1351. doi: 10.1542/peds.2008-1892. [DOI] [PubMed] [Google Scholar]
- 85.Murdzoska J, Devadason SG, Khoo SK, et al. In utero Smoke Exposure and Maternal and Infant GST Genes on Airway Responsiveness and Lung Function In Infancy. Am J Respir Crit Care Med. 2010;181:64–71. doi: 10.1164/rccm.200812-1887OC. [DOI] [PubMed] [Google Scholar]
- 86.Morales E, Sunyer J, Julvez J, et al. GSTM1 polymorphisms modify the effect of maternal smoking during pregnancy on cognitive functioning in preschoolers. Int J Epidemiol. 2009 doi: 10.1093/ije/dyp141. [DOI] [PubMed] [Google Scholar]
- 87.Gilliland FD, Li YF, Dubeau L, et al. Effects of glutathione S-transferase M1, maternal smoking during pregnancy, and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2002;166:457–463. doi: 10.1164/rccm.2112064. [DOI] [PubMed] [Google Scholar]
- 88.Berrettini W, Yuan X, Tozzi F, et al. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leermakers ET, Taal HR, Bakker R, Steegers EA, Hofman A, Jaddoe VW. A common genetic variant at 15q25 modifies the associations of maternal smoking during pregnancy with fetal growth: the generation R study. PLoS ONE. 2012;7:e34584. doi: 10.1371/journal.pone.0034584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tyrrell J, Huikari V, Christie JT, et al. Genetic variation in the 15q25 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) interacts with maternal self-reported smoking status during pregnancy to influence birth weight. Hum Mol Genet. 2012;21:5344–5358. doi: 10.1093/hmg/dds372. [DOI] [PMC free article] [PubMed] [Google Scholar]