Recent progresses in three-dimensional (3D) bioprinting technology accelerate the coming of the era of personalized medicine. With various printing approaches and materials developed, 3D bioprinting may have a broad range of medical applications, including the fabrication of delicate tissues/organs for the clinical use in the future or for the establishment of tissues in disease models. The principal advantages of 3D bioprinting are personalized design and precise fabrication, which are of critical importance for tissue engineering. To date, several types of biomimetic tissues, such as cartilage, skin, and vascular tissues have been fabricated by 3D bioprinting (Liaw and Guvendiren, 2017).
With regard to the neural tissues, especially in the central nervous system (CNS), the native microenvironment limits the regenerative capacity after injury in mammals. So far, the classical therapies for patients of spinal cord injury and other neurodegenerative diseases are passive abatement of symptoms rather than recovery of damaged areas. Implantation of drug- or cell-laden tissue engineering scaffolds to the impaired location of CNS may be a potential therapeutic treatment (Willerth and Sakiyama-Elbert, 2007). However, the artificial neural or brain tissues applied to neural regeneration are still rare and difficult to fabricate.
Brain is an organ highly demanding oxygen and nutrition, and therefore requires complete vascular functions. Formation of neural circuits in the brain is accompanied and guided by the vascular system during development, and vascular endothelial cells establish a protective gate (called blood-brain barrier) to control the influx of materials in the CNS (Tata et al., 2015). Therefore, not only neural cells but also the vascular-related cells are essential components for 3D bioprinted neural tissues to have long-term functions. On the other hand, neural cells in the tissue constructs need innervation to the neurons of the local neural circuit. The materials applied to fabrication of neural tissue or mini-brain should facilitate the establishment of neural network.
To fabricate a mini-brain by 3D bioprinting, the cell types and the choice of bioink are of primary concerns. Neural stem cells (NSCs) or progenitor cells (NPCs) are the favorable choices for recovering the functions of impaired neural tissues, and several clinical trials have been demonstrated in human (Gage and Temple, 2013). However, the amount of autologous NSCs/NPCs may not be sufficient enough to generate a customized neural tissue by 3D bioprinting because of the gradual reduction of NSCs/NPCs with age. Alternative cell sources are desired for fabrication of 3D neural tissues. One of the potential candidates is the adipose tissue. Adipose-derived stem cells (ADSCs) are relatively abundant and easier to obtain compared to the other types of stem cells. With the appropriate induction by neural growth factors or chitosan-based 3D scaffolds, ADSCs may be differentiated toward neuron-like cells (Gao et al., 2014). Meanwhile, fibroblasts may be another potential cell source after the appropriate reprogramming procedures (Hou and Lu, 2016).
The microextrusion bioprinting system may be suitable for the fabrication of mini-brain tissue. During the process of microextrusion bioprinting, the cells were mixed and then deposited with hydrogels by air-pressure or other driven force. After deposition, the hydrogels were crosslinked by physical or chemical methods to stabilize the constructs. Based on the models and printing parameters employed, the constructs with different geometries can be obtained by the microextrusion approach. Furthermore, the constructs containing multiple cell types can be achieved by the microextrusion-based 3D bioprinter equipped with multi-nozzles. As mentioned, establishment of vascular network is a bottleneck needed to be overcome in 3D tissues. The shape, function, size, or thickness of 3D-printed tissues is still fully limited because of the lack of vascularization within the printed tissues. To generate a mini-brain by 3D bioprinting, the establishment of vasculature within artificial neural tissues is the next step should be conquered. By using the microextrusion bioprinting system, neural and vascular cells can be separately pre-mixed with appropriate hydrogel (bioink) before printing, and aligned in the resulting construct with desired arrangement. Meanwhile, the growth factors could be incorporated into the bioink with uniform or gradient concentration to induce the cell growth and guide the formation of vascular/neural networks within the constructs. On the other hand, the greater density of cells embedded and thickness of the printed construct are the advantages of the microextrusion approach, compared to the other bioprinting systems, such as inkjet and laser-assisted bioprinting approaches (Pedde et al., 2017).
Since the crosstalk of neural- and vascular-related cells regulates the proliferation and fate determination of NSCs in CNS, the sufficient interaction between neural- and vascular-related cells within the printed neural tissues may accelerate the formation of the mini-brain construct. Formation of cellular spheroids is an efficient approach to promote cell-cell interaction, which also results in alteration of physiological properties of cells (Hsu et al., 2014). Homo- and hetero-spheroids can be generated by several methods, and positive effects on NSCs such as enhancement of self-renewal activity have been mentioned for the cellular spheroids (Ahmed, 2009). Bioprinting of the cellular co-spheroids from component cells required for generation of brain-like tissues, rather than dissociated cells, may be a potential strategy to create artificial mini-brain with neural and vascular networks due to the enhanced cellular crosstalk between neural- and vascular-related cells.
Bioprinting of cellular spheroids formed by neural- and vascular-related cells to produce a mini-brain or neural tissue may possess the other potential advantages. Cell-cell contact interaction within the material-embedded neurovascular spheroids may mimic the crosstalk of neural- and vascular-related cells occurred in the process of in vivo development or regeneration of CNS. As a disease model or drug screening platform, the outcome obtained from this self-organized neurovascular unit may be similar to that shown in the native condition. In addition, each spheroid encapsulated in the printed constructs can be considered as an independent neurovascular unit. After a period of culture, if the neural or vascular network could be established between the separated spheroids, these neurovascular spheroids may actively form connection with the in vivo neural network and repair neural functions after transplantation as a clinical neural tissue.
As mentioned above, delicate fabrication is one of the strength of 3D bioprinting for tissue engineering. A relative narrow nozzle is required for the production of high-resolution constructs. However, strong shear stress also simultaneously occurs to the embedded cells during the extrusion process, leading to dramatic cell loss. One solution is use of the bioink with low viscosity to reduce shear force to the cells as passing the nozzle (Pedde et al., 2017). In addition, the mechanical damage may be relieved as bioprinting the cells in the form of spheroids. Cellular spheroids are more elastic, and therefore interior cells in the spheroids are better protected from the shear stress produced by the extrusion procedure. The application of cellular spheroids in the fabrication of 3D-printed tissues may enhance the cellular crosstalk within the constructs as well as increase the cell survival rate, which is of essential importance for the tissue growth of 3D bioprinted constructs.
Physical and chemical properties of bioinks determine their scope in medical applications. Basic requirements for a bioink are printability, biocompatibility, and biodegradability. Bioinks should possess the capabilities of promoting the formation of personalized constructs after printing, as well as the structural stability to be used in bioprinting and additive manufacturing. Cells in bioink should maintain their proliferation, migration, and adhesion, so they can form a functional tissue construct. The biodegradability is particularly favorable for in vivo therapies. With regard to the bioink used to generate neural constructs, both natural and synthetic materials have been mentioned previously. For the natural bioink, polysaccharide-based (composed of alginate, carboxymethyl-chitosan, and agarose) hydrogel was recently applied to create 3D neural tissues. Cells such as NSCs displayed obvious cell proliferation and differentiation in this hydrogel. Meanwhile, the neural network may form within the construct, indicating the excellent biocompatibility of this hydrogel for generation of neural constructs (Gu et al., 2016). Synthetic biocompatible materials, such as polyurethane (PU), are also used as bioink to perform 3D bioprinting of NSCs. PU hydrogel was reported to promote the differentiation of NSCs (Hsieh et al., 2015). Theoretically, natural materials have better biocompatibility as compared to those of synthetic materials. Nevertheless, the relatively low cost and stable material source and composition are the critical advantages of synthetic materials like PU when employed in 3D bioprinting. Based on the existing literature, we suggest that an appropriate bioink to be applied in neural tissue printing should possess certain properties including biocompatibility to neural- and vascular-related cells, simple and non-toxic gelation procedure (such as thermal-sensitive hydrogel), suitable gel stiffness (~600 Pa), convenient incorporation of growth factors, and proper biodegradation.
The regenerative activity of impaired CNS is very limited in mammals. An effective treatment for CNS injury still needs to be developed. Due to its ability to fabricate biomimetic tissues with complicated and diverse cell/extracellular matrix (ECM) types and compositions, advanced 3D bioprinting technology has become a potential approach to generate a mini-brain or neural construct that reconnects and subsequently recovers the damaged neural circuit. Here, we propose a promising strategy to generate a mini-brain construct by 3D bioprinting (Figure 1). We have initially tested this strategy, and suggested that the neural tissues with vascular network could be generated in the near future by this approach. Briefly, neural- and vascular-related cells were organized into the cellular co-spheroids by the biomaterial-based substrates, and then the formed co-spheroids were collected and mixed with bioink (compatible for neural/vascular network formation). Mixture of the co-spheroids and bioink was subjected to 3D bioprinting. To serve as an in vitro tool, such as drug screening platform, the mixture could be directly bioprinted into the multi-well culture plates and developed into a high-throughput screening platform for neuroregenerative drugs. Meanwhile, the mixture could also be bioprinted with the customized geometries to be used as in vivo neural grafts after the appropriate induction. Sufficient interaction between neural- and vascular-related cells occurring in the cellular co-spheroids and appropriate growth environment provided by hydrogels may result in the formation of the brain-like structure. Future efforts will be focused on development of multiple bioinks and cells, employment of non-neural cells, introduction of vasculature into the artificial tissues, the active crosstalk of neural- and vascular-related cells, and the use of cellular spheroids.
Figure 1.
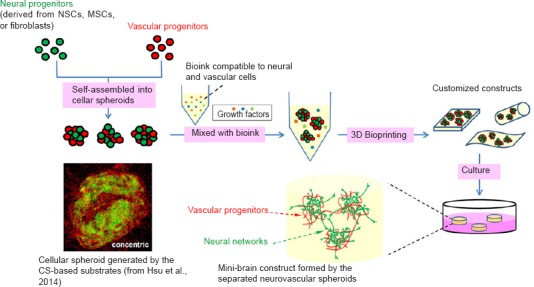
A potential strategy to generate mini-brain by 3D bioprinting of cellular spheroids.
To produce mini-brain, neural- and vascular-related cells is first assembled into cellular co-spheroids by the chitosan (CS)-based substrates or other approaches. Cellular co-spheroids are then mixed with the bioink, which is compatible to neural and vascular cells and has the appropriate physiochemical properties for development of neurovasculature after gelation. After 3D bioprinting, the customized constructs are further cultured to induce the self-organization of neural and vascular networks. The resulting mini-brain constructs may serve as in vitro research tools or in vivo neural grafts. NSCs: Neural stem cells; MSCs: mesenchymal stem cells; 3D: three-dimensional.
This research was supported by the Cutting-Edge Steering Research Project of National Taiwan University (NTU-CESRP-106R4000, grant under Ministry of Education) and National Health Research Institute (106-0324-01-10-07, grant under Ministry of Health and Welfare).
Footnotes
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Glenn S. Gerhard, Temple University, USA.
References
- Ahmed S. The culture of neural stem cells. J Cell Biochem. 2009;106:1–6. doi: 10.1002/jcb.21972. [DOI] [PubMed] [Google Scholar]
- Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron. 2013;80:588–601. doi: 10.1016/j.neuron.2013.10.037. [DOI] [PubMed] [Google Scholar]
- Gao S, Zhao P, Lin C, Sun Y, Wang Y, Zhou Z, Yang D, Wang X, Xu H, Zhou F, Cao L, Zhou W, Ning K, Chen X, Xu J. Differentiation of human adipose-derived stem cells into neuron-like cells which are compatible with photocurable three-dimensional scaffolds. Tissue Eng Part A. 2014;20:1271–1284. doi: 10.1089/ten.tea.2012.0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Tomaskovic-Crook E, Lozano R, Chen Y, Kapsa RM, Zhou Q, Wallace GG, Crook JM. Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Advanced healthcare materials. 2016;5:1429–1438. doi: 10.1002/adhm.201600095. [DOI] [PubMed] [Google Scholar]
- Hou S, Lu P. Direct reprogramming of somatic cells into neural stem cells or neurons for neurological disorders. Neural Regen Res. 2016;11:28–31. doi: 10.4103/1673-5374.169602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh FY, Lin HH, Hsu SH. 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials. 2015;71:48–57. doi: 10.1016/j.biomaterials.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Hsu SH, Ho TT, Huang NC, Yao CL, Peng LH, Dai NT. Substrate-dependent modulation of 3D spheroid morphology self-assembled in mesenchymal stem cell-endothelial progenitor cell coculture. Biomaterials. 2014;35:7295–7307. doi: 10.1016/j.biomaterials.2014.05.033. [DOI] [PubMed] [Google Scholar]
- Liaw CY, Guvendiren M. Current and emerging applications of 3D printing in medicine. Biofabrication. 2017;9:024102. doi: 10.1088/1758-5090/aa7279. [DOI] [PubMed] [Google Scholar]
- Pedde RD, Mirani B, Navaei A, Styan T, Wong S, Mehrali M, Thakur A, Mohtaram NK, Bayati A, Dolatshahi-Pirouz A, Nikkhah M, Willerth SM, Akbari M. Emerging biofabrication strategies for engineering complex tissue constructs. Adv Mater. 2017:29. doi: 10.1002/adma.201606061. [DOI] [PubMed] [Google Scholar]
- Tata M, Ruhrberg C, Fantin A. Vascularisation of the central nervous system. Mech Dev 138 Pt. 2015;1:26–36. doi: 10.1016/j.mod.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerth SM, Sakiyama-Elbert SE. Approaches to neural tissue engineering using scaffolds for drug delivery. Adv Drug Deliv Rev. 2007;59:325–338. doi: 10.1016/j.addr.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


