Abstract
Attention deficit and hyperactivity disorder (ADHD) is a disorder characterized by behavioral symptoms including hyperactivity/impulsivity among children, adolescents, and adults. These ADHD related symptoms are influenced by the complex interaction of brain networks which were under explored. We explored age-related brain network differences between ADHD patients and typically developing (TD) subjects using resting state fMRI (rs-fMRI) for three age groups of children, adolescents, and adults. We collected rs-fMRI data from 184 individuals (27 ADHD children and 31 TD children; 32 ADHD adolescents and 32 TD adolescents; and 31 ADHD adults and 31 TD adults). The Brainnetome Atlas was used to define nodes in the network analysis. We compared three age groups of ADHD and TD subjects to identify the distinct regions that could explain age-related brain network differences based on degree centrality, a well-known measure of nodal centrality. The left middle temporal gyrus showed significant interaction effects between disease status (i.e., ADHD or TD) and age (i.e., child, adolescent, or adult) (P < 0.001). Additional regions were identified at a relaxed threshold (P < 0.05). Many of the identified regions (the left inferior frontal gyrus, the left middle temporal gyrus, and the left insular gyrus) were related to cognitive function. The results of our study suggest that aberrant development in cognitive brain regions might be associated with age-related brain network changes in ADHD patients. These findings contribute to better understand how brain function influences the symptoms of ADHD.
Keywords: nerve regeneration, attention deficit and hyperactivity disorder, cognitive function, connectivity, resting-state fMRI, Brainnetome Atlas, whole brain analysis, disease-aging interaction effect, neuroscience, neural regeneration
Introduction
Attention deficit and hyperactivity disorder (ADHD) is a brain disorder that is characterized by the symptoms of inattention and hyperactivity/impulsivity (Schneider et al., 2006; Subcommittee on Attention-Deficit/Hyperactivity Disorder, 2011; Castellanos and Proal, 2012). In addition to inattentive or hyperactive behaviors, ADHD is also known to be highly associated with cognitive dysfunction (Wilens et al., 1999; Segen, 2006; Rostain and Ramsay, 2006; Solanto et al., 2008; Knouse and Safren, 2010; Castellanos and Proal, 2012). Castellanos et al. (2006) suggested that ADHD-related studies should consider cognitive deficits in ADHD patients to better quantify their neurobehavioral symptoms. Previous studies have adopted cognitive behavioral treatment (CBT) approaches to treat ADHD patients (Wilens et al., 1999; Rostain and Ramsay, 2006; Solanto et al., 2008; Knouse and Safren, 2010). Solanto et al. (2008) found enhanced executive skills in ADHD patients who received CBT and others found a significant reduction in ADHD-related symptoms after receiving combined medication and CBT (Rostain and Ramsay, 2006). These studies suggested that ADHD is highly related to dysfunctions in cognitive processes.
ADHD is a lifetime mental disorder and it has been found that patients show distinct behavioral symptoms across different age groups (Bresnahan and Barry, 2002; Schneider et al., 2006; Hurtig et al., 2007; Subcommittee on Attention-Deficit/Hyperactivity Disorder, 2011; Castellanos and Proal, 2012; Park et al., 2016). These ADHD related symptoms are influenced by the complex interaction of brain networks which are typically explored using neuroimaging approaches (Zang et al., 2007; Tian et al., 2008; Cortese et al., 2012). Most ADHD studies have focused on exploring the differences in brain function in limited age groups (i.e., only in children or adolescents) and studies investigating brain networks among a wide spectrum of age groups (i.e., from children to adults) have been largely lacking (Wilens et al., 1999; Castellanos et al., 2006; Knouse and Safren, 2010; Konrad and Eickhoff, 2010; Uekermann et al., 2010). ADHD patients show age dependent alterations in brain networks which have not been fully explored. Here, we aimed to explore the age-related functional changes in brain networks in ADHD patients.
We explored the age-related brain network differences between ADHD patients and typically developing (TD) subjects using resting state functional magnetic resonance imaging (rs-fMRI). Rs-fMRI is an effective tool for analyzing neurobehavioral disorders such as ADHD (dos Santos Siqueira et al., 2014). One study reported that rs-fMRI demonstrated enhanced brain activation in the sensory-related cortices of adolescent ADHD patients (Tian et al., 2008). Another study found that a feature derived from rs-fMRI known as amplitude of low-frequency revealed significant differences between children with ADHD and TD children (Zang et al., 2007).
We assessed functional brain network differences using a network centrality measure which has been widely used to assess regional importance (Bullmore et al., 2009; Rubinov and Sporns, 2010; Ferreira and Busatto, 2013). We hypothesized that there would be age-related functional network differences between ADHD patients and TD subjects. In this study, we aimed to explore functional brain network changes related to ADHD among a wide spectrum of age groups.
Subjects and Methods
Subjects and imaging data
The Institutional Review Board (IRB) of Sungkyunkwan University approved our retrospective study (#2015-09-007). Our study was performed in full accordance with the principles of the Declaration of Helsinki, and informed consent was obtained from all subjects. We collected raw T1-weighted structural MRI and rs-fMRI data from the ADHD-200 database (ADHD-200 Consortium, 2012; Bellec et al., 2017). We also obtained structural and functional MRI data from the Human Connectome Project (HCP) database (Van Essen et al., 2013). The ADHD-200 database provided the child and adolescent data and the HCP database provided the adult data. The subjects were recruited via advertisement and further details were available (ADHD-200 Consortium, 2012; Van Essen et al., 2013). Scores related to ADHD symptoms were measured using Conner's Parent Rating Scale Revised, Long Version (CPRS-LV) for the ADHD-200 dataset and Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) for the HCP data (American Psychiatric Association, 1994; Conners et al., 1998). With both the ADHD-200 and HCP datasets, subjects with T-scores greater than or equal to 65 on at least one measure of the ADHD-related index were selected as ADHD patients. Subjects with a secondary diagnosis were excluded along with subjects who did not have ADHD-related scores. Based on these criteria, we classified subjects into the ADHD (n = 90) and TD groups (n = 94). Each group was further divided into child, adolescent, and adult groups based on age. Subjects under 10 years of age were considered children, and subjects between 10 and 19 years of age were classified as adolescents. Finally, 27 ADHD children, 32 ADHD adolescents, 31 ADHD adults, 31 TD children, 32 TD adolescents, and 31 TD adults were included in the study. Comparison of the sex ratio did not yield significant differences among the groups. Detailed participant information is given in Table 1.
Table 1.
Demographic data of children, adolescents, and adults in the ADHD and TD groups
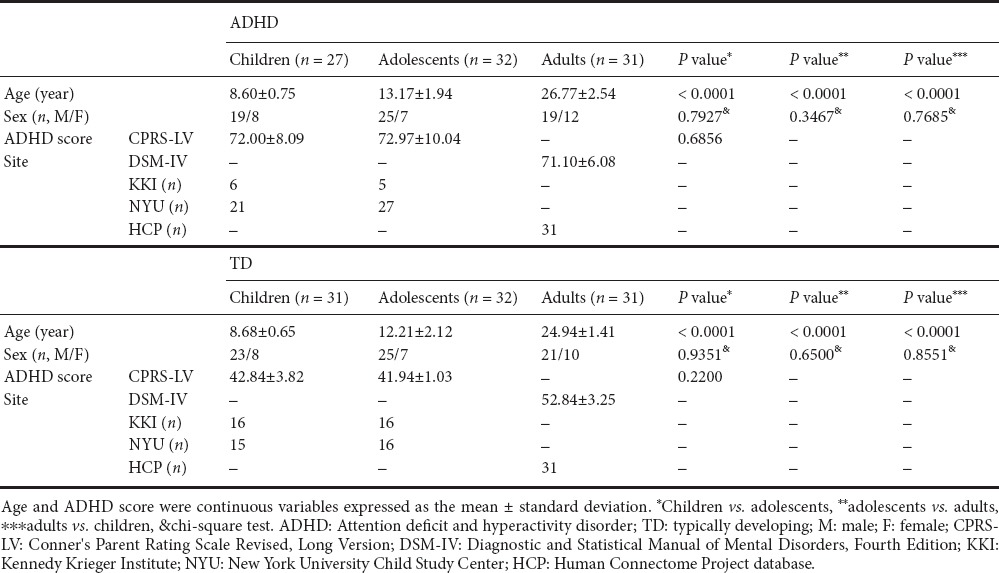
Although the ADHD-200 database consists of eight data collection sites, data from only two sites were retained after adopting the criteria mentioned above: the Kennedy Krieger Institute (KKI) and New York University Child Study Center (NYU). The T1-weighted structural data from the KKI were acquired with the following imaging parameters: repetition time (TR) = 8.0 ms; echo time (TE) = 3.7 ms; field of view (FOV) = 256 × 256 mm2; and voxel resolution = 1.0 × 1.0 × 1.0 mm3. The rs-fMRI functional data from the KKI were acquired with the following imaging parameters: TR = 2,500 ms; TE = 30 ms; FOV = 256 × 256 mm2; number of slices = 72; and voxel resolution = 2.67 × 2.67 × 3.0 mm3. The T1-weighted structural data from NYU were acquired with the following imaging parameters: TR = 2,530 ms; TE = 3.25 ms; FOV = 256 × 256 mm2; and voxel resolution = 1.3 × 1.0 × 1.3 mm3. The rs-fMRI functional data from NYU were acquired with the following imaging parameters: TR = 2,000 ms; TE = 15 ms; FOV = 240 × 192 mm2; number of slices = 33; and voxel resolution = 3.0 × 3.0 × 4.0 mm3. The T1-weighted structural data from the HCP were acquired with the following imaging parameters: TR = 2,400 ms; TE = 2.14 ms; FOV = 224 × 224 mm2; and voxel resolution = 0.7 × 0.7 × 0.7 mm3. Finally, the rs-fMRI functional data from the HCP were acquired with the following imaging parameters: TR = 720 ms; TE = 33.1 ms; FOV = 208 × 180 mm2; number of slices = 72; and voxel resolution = 2.0 × 2.0 × 2.0 mm3. The TD-child group included 16 subjects from the KKI site and 15 subjects from the NYU site. The TD-adolescent group included 16 subjects from the KKI site and 16 subjects from the NYU site. The TD-adult group included 31 subjects from the HCP site. The ADHD-child group included 6 subjects from the KKI site and 21 subjects from the NYU site. The ADHD-adolescent group included 5 subjects from the KKI site and 27 subjects from the NYU site. The ADHD-adult group included 31 subjects from the HCP site.
Imaging preprocessing
The neuroimaging data from the ADHD-200 and HCP were preprocessed using AFNI and FSL software (Cox, 1996; Jenkinson et al., 2012). These preprocessing steps consisted of structural and functional preprocessing. The structural preprocessing included the following steps: performing a de-oblique procedure; reorienting into a right posterior inferior (RPI) orientation; skull-stripping; registering the skull-stripped anatomical image onto the template space at 3 × 3 × 3 mm3 resolution; segmentation into cerebral-spinal fluid (CSF), white matter (WM), and gray matter (GM); and constructing WM and CSF masks by binarizing probability masks using a 0.99 threshold. The functional preprocessing included the following steps: performing a de-oblique procedure; reorienting into a RPI orientation; removal of the first 6 echo planar imaging (EPI) volumes; motion correcting EPI volumes; frame scrubbing based on frame-wise displacement (FD) to exclude frames with FD greater than 0.5 mm (Power et al., 2012); slice timing correction; registration of the mean EPI image onto the corresponding anatomic image; masking the dataset to exclude non-brain tissue; mapping of the fMRI data and mean image onto the template space at 3 × 3 × 3 mm3 resolution; extraction of WM and CSF time-courses from EPI volumes; regressing out WM, CSF, and motion time courses as well as a low-order polynomial signal; band-pass (0.009 Hz< f < 0.08 Hz) filtering of time courses; and blurring the data using a 6 mm full width half maximum (FWHM) Gaussian filter.
Network construction
To construct the functional network from the images, connectivity analysis was performed with regions of interest (ROIs) specified by the Brainnetome Atlas. The Brainnetome Atlas is a structural atlas that consists of 246 regions (Fan et al., 2016). Connectivity information was assessed with a graph structure using nodes and edges. The nodes were 246 ROIs derived from the Brainnetome Atlas. Pearson correlation values of the time series between two nodes were used as edges. The edge values were filled into the matrix as elements and the matrix was referred to as the correlation matrix. We adopted the weighted and un-directional network model. Soft thresholding was used to prevent binarizing of the correlation matrix using the following equation (1).
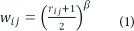
The rij term denotes the edge value between the node i and j (Mumford et al., 2010; Schwarz and McGonigle, 2011). The β value was set to 6 to ensure scale-free topology (Mumford et al., 2010).
Connectivity measures
We used degree centrality (DC) to assess the regional connectivity of brain networks (Lohmann et al., 2010; Fransson et al., 2011). The DC value for a node i is defined as the number of links connected directly to the node (Rubinov and Sporns, 2010). We used MATLAB (version 2016; Mathworks Inc., Natic, MA, USA) to compute the DC values (The Mathworks Inc., 2016).
Multi-site effect
Since our neuroimaging data was acquired from different sites, we adopted a dummy coding regression model to remove multi-site effects from the DC values using the following equation (2).
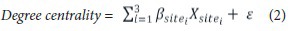
The Xsitei term is the dummy vector denoting different data collection sites, βi is the regression coefficient of the ith dummy vector, and ε is the residual DC value (Hardy, 1993). The regression model removed the multi-site effects and the residuals were used for further analyses.
Statistical analysis
We used MATLAB for statistical analysis (version 2016; Mathworks Inc.). The two-way analysis of variance (ANOVA) was used to explore differences in age-related DC patterns between the ADHD and TD groups (Fujikoshi, 1993). The DC values were set as the dependent variables, and disease status (ADHD or TD) and age group (child, adolescent, or adult) were set as the independent variables. The significance of the interaction effects of disease and age group was quantified using P values (P < 0.001). We adopted an uncorrected P value of 0.001 due to the exploratory nature of our study. We applied a stringent P value threshold of 0.001 compared to the conventional 0.05 since our study was an exploratory study investigating 246 regions covering the whole brain. We also reported results using a relaxed P value of 0.05. Chi-square tests were applied to assess differences in sex among comparison groups.
Results
Motion scrubbing
We calculated the FD for each volume from the rs-fMRI data. Two children from the TD group had part of the frames scrubbed. Figure 1 shows the FD of these two subjects. We removed 13 frames from one child in the TD group and 5 frames from the other child in the TD group.
Figure 1.
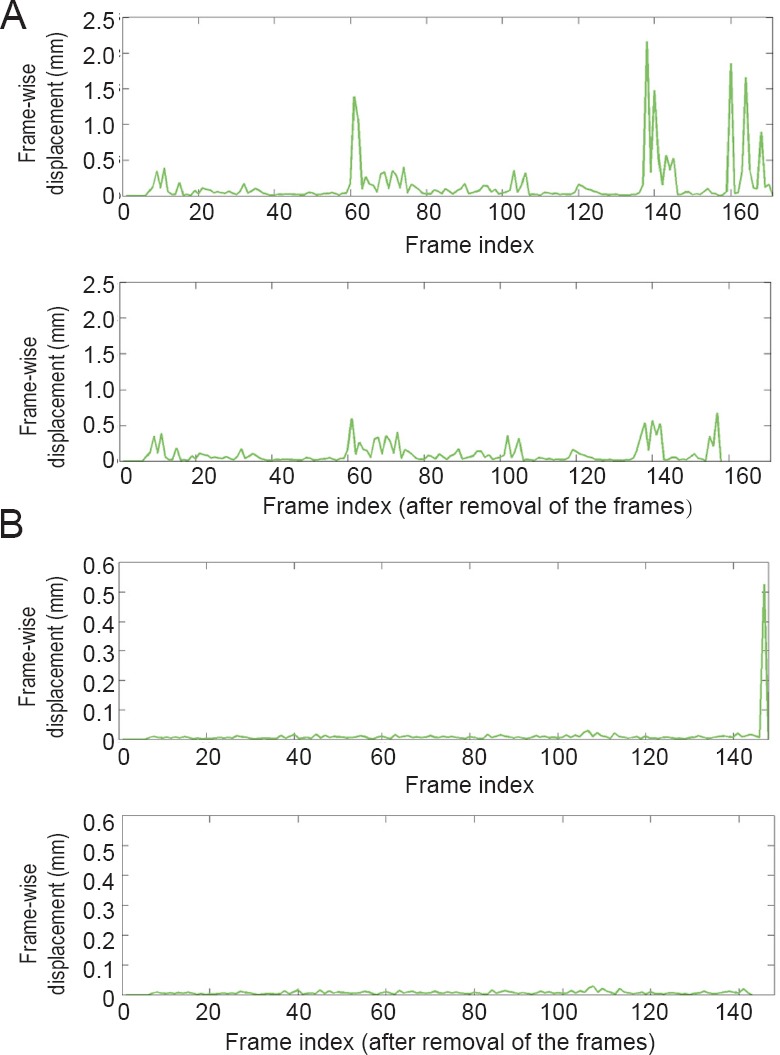
The plot of the FD values for two children from TD group whose frames were partly censored.
(A) The FD of one subject (13 frames removed). (B) The FD of another subject (5 frames removed). The lower sub-figures are the results after removing the frames. FD: Frame-wise displacement; TD: typically developing.
Connectivity differences
We performed a two-way ANOVA to determine the brain regions that showed significant interaction effects of disease status and age. The left superior frontal gyrus, left inferior frontal gyrus, right inferior frontal gyrus, right precentral gyrus, left superior temporal gyrus, left middle temporal gyrus, right postcentral gyrus, left insular gyrus, left medioventral occipital cortex, right medioventral occipital cortex, left amygdala, and left basal ganglia showed significant interaction effects (P < 0.05) (Table 2). Figure 2a shows the locations and their P values of the identified regions. Among the identified regions, the left middle temporal gyrus showed the most significant interaction effects (P < 0.001; Figure 2b). Further post-hoc tests were not conducted because there were no significant main effects of disease status and age.
Table 2.
Identified regions with significant interaction effects of age-by-status
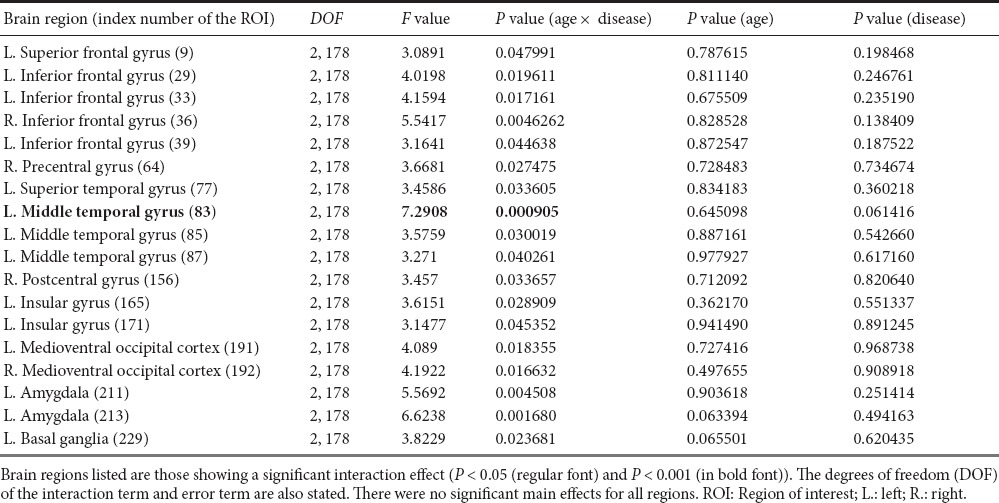
Figure 2.
Brain regions that showed a significant age-by-status interaction by the two-way analysis of variance (ANOVA) test using degree centrality (DC).
The P values from analysis of variance (ANOVA) are visualized using the color bar (from red to yellow) in the middle of the plot. (A) The left superior frontal gyrus, left inferior frontal gyrus, right inferior frontal gyrus, right precentral gyrus, left superior temporal gyrus, left middle temporal gyrus, right postcentral gyrus, left insular gyrus, left medioventral occipital cortex, right medioventral occipital cortex, left amygdala, and left basal ganglia showed a significant effect of interaction (P < 0.05). (B) The left middle temporal gyrus region showed a significant effect of interaction (P < 0.001). The ADHD and TD groups showed different patterns based on age as inferred by the two-way ANOVA. ADHD: Attention deficit and hyperactivity disorder; TD: typically developing; L: left: R: right.
Age-related patterns
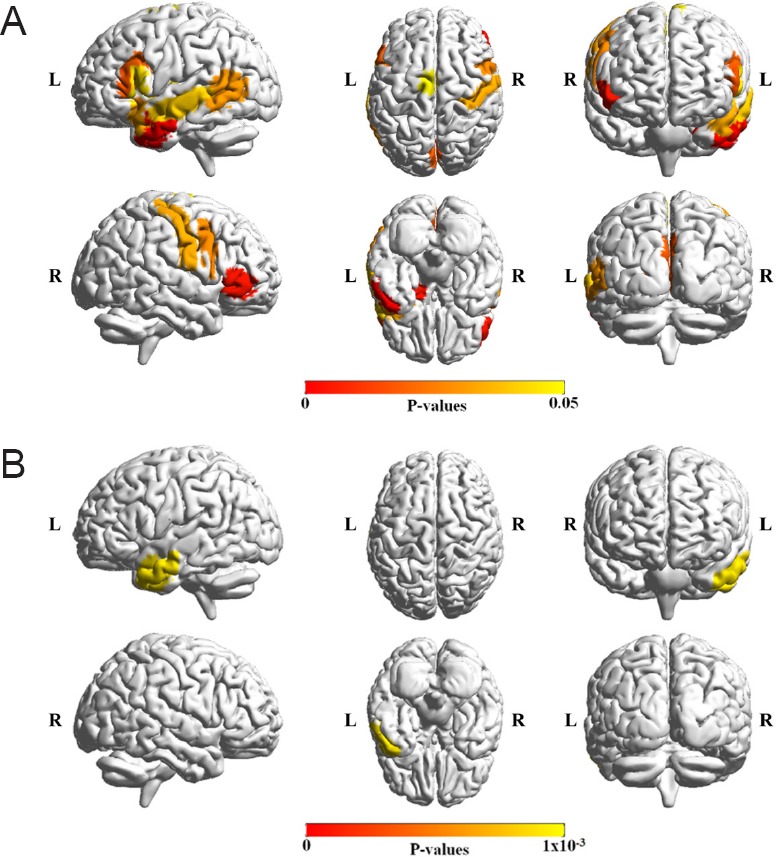
We show regions that have significant age-by-status interaction for each age group in Figure 3.
Figure 3.
Age-related degree centrality patterns of the identified regions.
The mean and standard error of degree centrality for brain regions that showed significant age-by-status interactions for each age group from Table 2 (P < 0.05). The green plot denotes the mean degree centrality values of the TD groups and the blue plot denotes the mean degree centrality values of the ADHD groups. L.: Left: R.: right; TD: typically developing; ADHD: attention deficit and hyperactivity disorder.
Discussion
The main purpose of this study was to determine if there were age-related network differences between ADHD patients and TD subjects. We divided the subjects into six groups based on disease status (i.e., ADHD or TD) and age (i.e., children, adolescents, and adults) to form comparison groups. From the two-way ANOVA results, we found significant interaction effects of disease status and age. Since this was an exploratory study investigating hundreds of brain regions, we relaxed the constraint of the P value and found significant interaction effects of disease status and age based on functional connectivity.
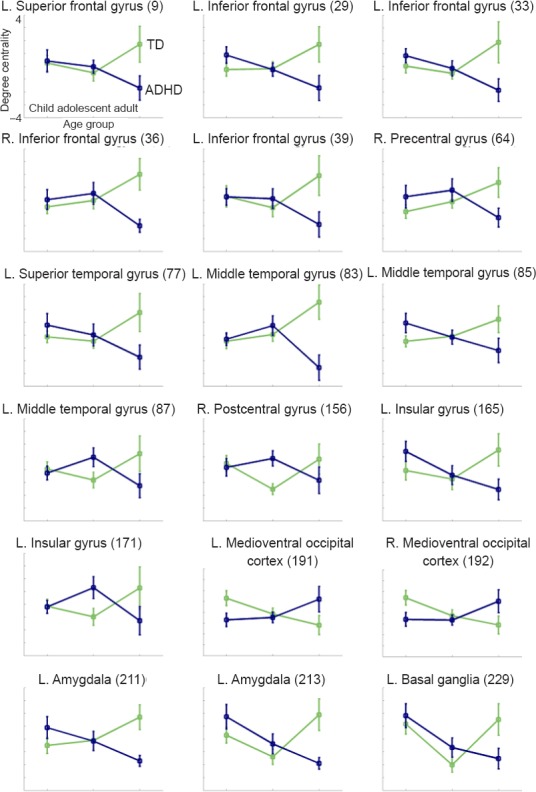
Among the identified regions, the left inferior frontal gyrus, the left middle temporal gyrus, which showed the most significant interaction effects, and the left insular gyrus were known to be related to cognitive function (Vandenberghe et al., 1996; Goel and Dolan, 2001; Swick et al., 2008; Fan et al., 2016). Swick et al. (2008) reported that subjects with damage in the left inferior frontal gyrus and left insula region had higher error rates than controls in a response inhibition task. The response inhibition task is known as a major task that can discriminate between ADHD and TD subjects (Nigg, 1999; Epstein et al., 2001; Tamm et al., 2004). Tamm et al. (2004) also found significant differences in brain activation in the middle temporal gyrus between subjects with ADHD and TD subjects in a behavioral response inhibition task. Figure 4B shows the locations of these regions. There is a noticeable overlap between the region previously reported in the literature and the region we found as shown in Figure 4A. The cognitive system plays an important role in typical development from childhood through adolescence to adulthood (Blakemore and Choudhury, 2006). Thus, aberrant development in the cognitive brain regions that we identified between ADHD and TD groups implies that impairment in cognitive function might be associated with age-related brain network changes in ADHD patients.
Figure 4.
Comparison between identified regions and known regions related to cognitive functions.
(A) Regions (orange mask) that showed a significant age-by-status interaction by the two-way analysis of variance (ANOVA). (B) Regions (blue mask) related to cognitive function from previous studies (Vandenberghe et al., 1996; Goel and Dolan, 2001; Swick et al., 2008; Fan et al., 2016). L: Left; R: right.
In this study, we used multi-center neuroimaging data to obtain a sufficient number of samples. Although the differences in imaging parameters were relatively small, this could lead to different amounts of noise and distortions in the data, making a fair comparison difficult. The high-resolution data from the HCP were resampled and pre-processed to low-resolution ADHD-200 data so that data can be fairly compared. We applied the common image processing steps performed on the low quality (i.e., low resolution ADHD-200) spatial reference space so that high quality (i.e., high resolution HCP) data was effectively rendered to low quality data. Such approaches have been successfully applied in other studies (Fennema-Notestine et al., 2007; Di Martino et al., 2014; Bellec et al., 2017). We visually confirmed similar image qualities by computing the average of T1-weighted structural data for each subgroup as shown in Figure 5 and they all appeared similar in the low resolution common space. Furthermore, we used the correlation of rs-fMRI time series between two different brain regions as the main feature in this study. Each region contained over hundreds of voxels, hence the regional average time series might reduce the potential differences in image quality. Finally, we also performed a multi-site regression using the dummy-coding to remove the potential multi-site effects from the centrality measurement. The dummy-coding regression approach has been applied in other studies comparing data from different sites (Hardy, 1993; Sanfilipo et al., 2004).
Figure 5.
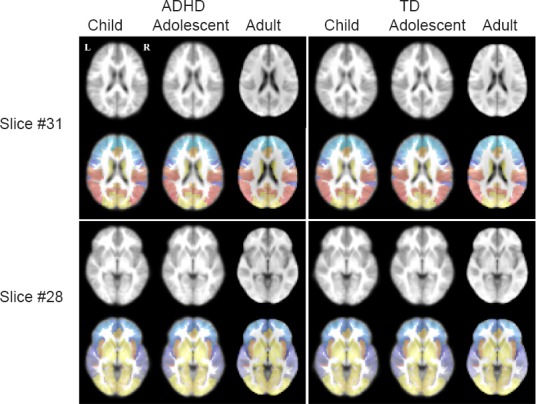
The average T1 structural images of each subgroup after we applied the common anatomical preprocessing steps.
Results of two axial slices are shown. The first and third rows show average T1 images and the second and fourth rows show overlaid region of interest (ROI) information from the Brainnetome atlas. All six subgroups showed visually similar average T1 images and the overlaid ROIs matched well with known structural boundaries. ADHD: attention deficit and hyperactivity disorder; TD: typically developing.
We used Brainnetome atlas to specify the ROIs for child, adolescent, and adult groups. The Brainnetome atlas was derived from adults and thus application to adults is natural. We investigated if a single atlas could specify the ROIs for various age groups. We registered T1 anatomical images onto a common space for each age group and then compared averaged images with one another. The average images for each age group appeared quite similar and those for each group were compared with overlaid ROIs from the atlas and they seemed reasonable as well.
Our study adopted an uncorrected P value of 0.001 for statistical significance. We had limited samples but explored hundreds of regions and thus we chose to adopt an uncorrected P value. Use of an uncorrected P value is rather common in many exploratory studies involving the whole brain (Konishi et al., 1999; Anand et al., 2005).
Our study has some limitations. First, rs-fMRI was the only modality used. Using multi-modal imaging data might provide complementary information that could better describe the differences between the ADHD and TD groups. Second, the sample size might be insufficient due to the limitation of available cases from the online databases. A future study performed on a larger cohort is necessary to confirm our findings with higher statistical power. Third, we could not compute the correlation between DC and ADHD scores, because we used two types of ADHD related scores coming from two research databases. Finally, we did not consider longitudinal data for our study, as we are not aware of any openly accessible research database housing longitudinal ADHD neuroimaging data. Thus, we performed our study in a cross-sectional fashion with comparison of different age groups within the ADHD and TD groups. Ideally, a future study should consider longitudinal cases so that age-related brain network differences in ADHD could be better assessed.
In summary, our study suggested a possible statistical link between ADHD disease status and the brain network for three age groups. The main finding of our study was that connectivity differences in cognitive system could be biomarkers for distinguishing ADHD and TD subjects. It has been shown that behavioral patterns and psychopathology of ADHD patients for different developmental stages are strongly related to impairments of the cognitive system (Blakemore and Choudhury, 2006; Singh et al., 2015; Huang et al., 2016). Huang et al. (2016) found that the impairment of inhibition function which is involved in the cognitive system was significantly different between child ADHD and TD groups, and the difference decreased when subjects develop from children to adolescents. This study suggested that the developmental differences in cognitive functions should be considered to better understand the psychiatric symptoms of ADHD patients. Our results found that the cognitive dysfunction might be associated with age-related brain network changes in ADHD patients, and hence, thus our results might provide complementary information for understanding developmental ADHD psychopathology. ADHD is one of the many brain disorders affected by neuroplasticity and thus our study might be loosely related to neuroplasticity and neural regeneration research (Gevensleben et al., 2014; Cowley et al., 2016; Liu et al., 2017; Van Doren et al., 2017)
Additional file: Open peer review report 1 (36.8KB, pdf) .
“Neurological and neuropsychological consequences of electrical and lightning shock: review and theories of causation”
Acknowledgments
Data were provided by the Neuro Bureau, the ADHD 200 consortium, and Virginia Tech's ARC. Data were also provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and KamilUgurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research and by the McDonnel Center for Systems Neuroscience at Washington University.
Footnotes
Conflicts of interests: None declared.
Research ethics: The Institutional Review Board (IRB) of Sungkyunkwan University approved our study (#2015-09-007). Our study was performed in full accordance with local IRB guidelines and the principles of the Declaration of Helsinki, and informed consent was obtained from all subjects.
Declaration of participant consent: The authors certify that they have obtained all appropriate consent forms of participant or their guardians. In the form, participants or guardians have given their consent for participants’ images and other clinical information to be reported in the journal. Participants or guardians understand that participants’ names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Hao Chen, Shanghai 6th Peoples Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, China.
Copyedited by Li CH, Song LP, Zhao M
Funding: This work was supported by the Institute for Basic Science [grant No. IBS-R015-D1] and the National Research Foundation of Korea (grant No. NRF-2016R1A2B4008545).
References
- ADHD-200 Consortium (2012) The ADHD-200 Consortium: a model to advance the translational potential of neuroimaging in clinical neuroscience. Front Syst Neurosci. 6:62. doi: 10.3389/fnsys.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4th ed American PsychiatricAssociation (1994) Diagnostic and Statistical Manual of Mental Disorders (DSM IV) [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Activity and connectivity of brain mood regulating circuit in depression: A functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Bellec P, Chu C, Chouinard-Decorte F, Benhajali Y, Margulies DS, Craddock RC. The neuro bureau ADHD-200 preprocessed repository. Neuroimage. 2017;144(Pt B):275–286. doi: 10.1016/j.neuroimage.2016.06.034. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Bresnahan SM, Barry RJ. Specificity of quantitative EEG analysis in adults with attention deficit hyperactivity disorder. Psychiatry Res. 2002;112:133–144. doi: 10.1016/s0165-1781(02)00190-7. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Bullmore E, Sporns O, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley B, Holmström É, Juurmaa K, Kovarskis L, Krause CM. Computer enabled neuroplasticity treatment: a clinical trial of a novel design for neurofeedback therapy in adult ADHD. Front Hum Neurosci. 2016;10:205. doi: 10.3389/fnhum.2016.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Yan CG, Li Q, Denio E, Castellanos FX, Alaerts K, Anderson JS, Assaf M, Bookheimer SY, Dapretto M, Deen B, Delmonte S, Dinstein I, Ertl-Wagner B, Fair DA, Gallagher L, Kennedy DP, Keown CL, Keysers C, Lainhart JE, et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry. 2014;19:659–667. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos Siqueira A, Biazoli Junior CE, Comfort WE, Rohde LA, Sato JR. Abnormal functional resting-state networks in ADHD: graph theory and pattern recognition analysis of fMRI data. Biomed Res Int. 2014;2014:380531. doi: 10.1155/2014/380531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Johnson DE, Varia IM, Conners CK. Neuropsychological assessment of response inhibition in adults with ADHD. J Clin Exp Neuropsychol. 2001;23:362–371. doi: 10.1076/jcen.23.3.362.1186. [DOI] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, Yang Z, Chu C, Xie S, Laird AR, Fox PT, Eickhoff SB, Yu C, Jiang T. The human brainnetome atlas: a new brain atlas based on Connectional Architecture. Cereb Cortex. 2016;26:3508–3526. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C, Gamst AC, Quinn BT, Pacheco J, Jernigan TL, Thal L, Buckner R, Killiany R, Blacker D, Dale AM, Fischl B, Dickerson B, Gollub RL. Feasibility of multi-site clinical structural neuroimaging studies of aging using legacy data. Neuroinformatics. 2007;5:235–245. doi: 10.1007/s12021-007-9003-9. [DOI] [PubMed] [Google Scholar]
- Ferreira LK, Busatto GF. Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev. 2013;37:384–400. doi: 10.1016/j.neubiorev.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Fransson P, Aden U, Blennow M, Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex. 2011;21:145–154. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- Fujikoshi Y. Two-way ANOVA models with unbalanced data. Discrete Math. 1993;116:315–334. [Google Scholar]
- Gevensleben H, Kleemeyer M, Rothenberger LG, Studer P, Flaig-Röhr A, Moll GH, Rothenberger A, Heinrich H. Neurofeedback in ADHD: Further pieces of the puzzle. Brain Topogr. 2014;27:20–32. doi: 10.1007/s10548-013-0285-y. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. The functional anatomy of humor: segregating cognitive and affective components. Nat Neurosci. 2001;4:237–238. doi: 10.1038/85076. [DOI] [PubMed] [Google Scholar]
- Hardy MA. Regression with dummy variables. Sage Univ Pap Ser Quant Appl Soc Sci. 1993 DOI: http://dx.doi.org/10.4135/9781412985628. [Google Scholar]
- Huang F, Sun L, Qian Y, Liu L, Ma QG, Yang L, Cheng J, Cao QJ, Su Y, Gao Q, Wu ZM, Li HM, Qian QJ, Wang YF. Cognitive function of children and adolescents with attention deficit hyperactivity disorder and learning difficulties: a developmental perspective. Chin Med J (Engl) 2016;129:1922–1928. doi: 10.4103/0366-6999.187861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtig T, Ebeling H, Taanila A, Miettunen J, Smalley SL, McGough JJ, Loo SK, Järvelin MR, Moilanen IK. ADHD symptoms and subtypes: relationship between childhood and adolescent symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46:1605–1613. doi: 10.1097/chi.0b013e318157517a. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Knouse LE, Safren SA. Current status of cognitive behavioral therapy for adult attention-deficit hyperactivity disorder. Psychiatr Clin North Am. 2010;33:497–509. doi: 10.1016/j.psc.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122(Pt 5):981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp. 2010;31:904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZX, Lishak V, Tannock R, Woltering S. Effects of working memory training on neural correlates of Go/Nogo response control in adults with ADHD: A randomized controlled trial. Neuropsychologia. 2017;95:54–72. doi: 10.1016/j.neuropsychologia.2016.11.023. [DOI] [PubMed] [Google Scholar]
- Lohmann G, Margulies DS, Horstmann A, Pleger B, Lepsien J, Goldhahn D, Schloegl H, Stumvoll M, Villringer A, Turner R. Eigenvector centrality mapping for analyzing connectivity patterns in fMRI data of the human brain. PLoS One. 2010;5:e10232. doi: 10.1371/journal.pone.0010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JA, Horvath S, Oldham MC, Langfelder P, Geschwind DH, Poldrack RA. Detecting network modules in fMRI time series: a weighted network analysis approach. Neuroimage. 2010;52:1465–1476. doi: 10.1016/j.neuroimage.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. The ADHD response-inhibition deficit as measured by the stop task: replication with DSM-IV combined type, extension, and qualification. J Abnorm Child Psychol. 1999;27:393–402. doi: 10.1023/a:1021980002473. [DOI] [PubMed] [Google Scholar]
- Park BY, Hong J, Lee SH, Park H. Functional connectivity of child and adolescent attention deficit hyperactivity disorder patients: Correlation with IQ. Front Hum Neurosci. 2016;10:565. doi: 10.3389/fnhum.2016.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostain AL, Ramsay JR. A combined treatment approach for adults with ADHD--results of an open study of 43 patients. J Atten Disord. 2006;10:150–159. doi: 10.1177/1087054706288110. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sanfilipo MP, Benedict RH, Zivadinov R, Bakshi R. Correction for intracranial volume in analysis of whole brain atrophy in multiple sclerosis: the proportion vs. residual method. Neuroimage. 2004;22:1732–1743. doi: 10.1016/j.neuroimage.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Schneider M, Retz W, Coogan A, Thome J, Rösler M. Anatomical and functional brain imaging in adult attention-deficit/hyperactivity disorder (ADHD)--a neurological view. Eur Arch Psychiatry Clin Neurosci. 2006;256(Suppl 1):i32–41. doi: 10.1007/s00406-006-1005-3. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, McGonigle J. Negative edges and soft thresholding in complex network analysis of resting state functional connectivity data. Neuroimage. 2011;55:1132–1146. doi: 10.1016/j.neuroimage.2010.12.047. [DOI] [PubMed] [Google Scholar]
- Segen JC. Concise Dictionary of Modern Medicine. Mc-Graw-Hill; 2006. Available at: https://books.google.co.kr/books?id=vVNqAAAAMAAJ . [Google Scholar]
- Singh A, Yeh CJ, Verma N, Das AK. Overview of attention deficit hyperactivity disorder in young children. Health Psychol Res. 2015;3:2115. doi: 10.4081/hpr.2015.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV, Marks DJ, Mitchell KJ, Wasserstein J, Kofman MD. Development of a new psychosocial treatment for adult ADHD. J Atten Disord. 2008;11:728–736. doi: 10.1177/1087054707305100. [DOI] [PubMed] [Google Scholar]
- Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management. Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, Ganiats TG, Kaplanek B, Meyer B, Perrin J, Pierce K, Reiff M, Stein MT, Visser S. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and, adolescents. Pediatrics. 2011;128:1007–1022. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Ringel J, Reiss AL. Event-related FMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- The Mathworks Inc. MATLAB - MathWorks [WWW Document] 2016. doi:2016-11-26. www.mathworks.com/products/matlab .
- Tian L, Jiang T, Liang M, Zang Y, He Y, Sui M, Wang Y. Enhanced resting-state brain activities in ADHD patients: a fMRI study. Brain Dev. 2008;30:342–348. doi: 10.1016/j.braindev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Kraemer M, Abdel-Hamid M, Schimmelmann BG, Hebebrand J, Daum I, Wiltfang J, Kis B. Social cognition in attention-deficit hyperactivity disorder (ADHD) Neurosci Biobehav Rev. 2010;34:734–743. doi: 10.1016/j.neubiorev.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Van Doren J, Heinrich H, Bezold M, Reuter N, Kratz O, Horndasch S, Berking M, Ros T, Gevensleben H, Moll GH, Studer P. Theta/beta neurofeedback in children with ADHD: Feasibility of a short-term setting and plasticity effects. Int J Psychophysiol. 2017;112:80–88. doi: 10.1016/j.ijpsycho.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K WU-Minn HCP Consortium. The WU-Minn Human Connectome Project: an overview. Neuroimage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RS. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Wilens T.E, McDermott S.P, Biederman J, Abrantes A, Hahesy A, Spencer T.J. Cognitive therapy in the treatment of adults with ADHD: A systematic chart review of 26 cases. J Cogn Psychother. 1999;13:215–226. [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
“Neurological and neuropsychological consequences of electrical and lightning shock: review and theories of causation”


