Keywords: nerve regeneration, acrylamide, hippocampus, neurons, developmental toxicity, growth associated protein 43, synaptophysin, weaning rats, dentate gyrus, protein, developmental neurobiology, neural regeneration
Abstract
Although numerous studies have examined the neurotoxicity of acrylamide in adult animals, the effects on neuronal development in the embryonic and lactational periods are largely unknown. Thus, we examined the toxicity of acrylamide on neuronal development in the hippocampus of fetal rats during pregnancy. Sprague-Dawley rats were mated with male rats at a 1:1 ratio. Rats were administered 0, 5, 10 or 20 mg/kg acrylamide intragastrically from embryonic days 6–21. The gait scores were examined in pregnant rats in each group to analyze maternal toxicity. Eight weaning rats from each group were also euthanized on postnatal day 21 for follow-up studies. Nissl staining was used to observe histological change in the hippocampus. Immunohistochemistry was conducted to observe the condition of neurites, including dendrites and axons. Western blot assay was used to measure the expression levels of the specific nerve axon membrane protein, growth associated protein 43, and the presynaptic vesicle membrane specific protein, synaptophysin. The gait scores of gravid rats significantly increased, suggesting that acrylamide induced maternal motor dysfunction. The number of neurons, as well as expression of growth associated protein 43 and synaptophysin, was reduced with increasing acrylamide dose in postnatal day 21 weaning rats. These data suggest that acrylamide exerts dose-dependent toxic effects on the growth and development of hippocampal neurons of weaning rats.
Introduction
Acrylamide (ACR) is a water-soluble vinyl monomer used to synthesize polyacrylamide, which has broad applications in the petrochemical, water treatment, paper making, textile manufacturing and scientific research fields (Exon, 2006; Doerge et al., 2008). Although polyacrylamide is not considered toxic, ACR compounds can often contain traces of toxic monomers (Lipworth et al., 2012). For example, monoacrylamide was reported to exhibit neurotoxicity, reproductive toxicity and carcinogenicity in various animal species (Lehning et al., 2003; Sen et al., 2015). This has drawn extensive global interest as ACR was detected during the processing of starchy food treated at a temperature > 120°C (Lingnert, et al, 2002). The average daily intake of ACR for adults is approximately 0.5 μg/kg body weight (WHO, 2002; Sansano et al., 2017). Interestingly, children may have two to three times more ACR than adults, as their relative intake may be increased by increased snacking and lower body weights (Konings et al., 2003; Svensson et al., 2003; Garey et al., 2005). Given this increasing risk of ACR exposure in children, it is important to assess the potential toxic effects of ACR on nervous system development.
In adult studies, ACR exposure is known to cause axonal neuropathy, which can affect both the central and peripheral nervous systems, and is associated with ataxia, weight loss and skeletal muscle weakness. The main pathological characteristics of ACR exposure involve distal axon swelling and degeneration. However, the effects of ACR exposure during embryonic mammalian development remain unclear (Exon, 2006; Manuela et al., 2013).
The neural stem cells in the hippocampal dentate gyrus of the adult mammalian brain retain the ability to produce and incorporate new neurons, termed adult neurogenesis. The process of hippocampal neurogenesis includes the proliferation of stem cells, differentiation of progenitor cells, migration of newborn neurons, synaptic growth and axon formation of pyramidal cells in the cornus ammonis 3 zone (Zhao et al., 2006; Toni et al., 2007, 2008). Growth associated protein 43 (GAP-43) is regarded as a key factor related to neural regeneration (Benowitz et al., 1997). Mcphail et al. (2004) reported that GAP-43 expression resulted from the recombination of proteins, and was strongly associated with axon regeneration and functional reconstruction. As a specific molecular marker of synaptic remodeling, synaptophysin (SYP), a glycoprotein distributed on the membrane of synaptic vesicles, is strongly associated with neurotransmitter release, synaptic vesicle recycling and synaptogenesis (Derksen et al., 2007; Kwon et al., 2011). SYP is important for synaptic formation and synaptic maintenance, and its content reflects the number of synapses (Navratil et al., 2009). As such, changes in SYP expression may reflect changes in nerve transmission. In the present study, we investigated the toxic effects of ACR exposure on neuronal development in the hippocampus of weaning rats by measuring GAP-43 and SYP expression.
Materials and Methods
Animals and experimental design
Thirty-two male and 32 female specific-pathogen-free Sprague-Dawley rats aged 5 weeks and weighing 150–180 g were provided by the Guangdong Medical Laboratory Animal Center, China (license No. SCXK (Yue) 2008-0002). All rats were maintained under controlled conditions at 24 ± 1°C and a relative humidity of 55 ± 5% in a 12-hour light/dark cycle, and were allowed free access to chow and water.
The study protocol was approved by the Animal Ethics Committee of Guangdong Pharmaceutical University of China (approval No. GDPULAC2012117). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986). All efforts were made to minimize animal suffering and reduce the number of animals.
The female rats were mated 1:1 with males after acclimation for 1 week. We checked daily for vaginal plugs, the presence of which indicated day 0 of pregnancy. Thirty-two pregnant rats were randomly divided into four groups (n = 8 per group) before drug administration. Rats in each group were treated by intragastric administration. Control group animals received 0.9% saline, while rats in the 5, 10 and 20 mg/kg ACR groups were treated with 5, 10 and 20 mg/kg ACR (analytical grade, 99.9%; Yongda Inc., Tianjin, China), respectively. Intragastric administration was repeated daily for 15 days from pregnancy days 6–21 (the neural tube is generated from the 6th day of pregnancy, while rats are born on the 21st day). The optimal dose of ACR was chosen based on a previous study, with modifications, which described developmental gait disorders induced by exposure to similar ACR concentrations in female rats (Takahashi et al., 2008; Ma et al., 2011; Yao et al., 2014). After the dams gave birth, eight pups were randomly selected from each group for follow-up experiments. The general situation of gravid rats and offspring was closely monitored, and the body weight was recorded weekly during pregnancy and in offspring. At postnatal day 21, the weaning rats were euthanized under general anesthesia, and in each group, the brains were either fixed and paraffin-embedded for immunohistochemistry (n = 8 per group) or collected for western blot assay (n = 8 per group).
Gait scores
In each pregnant rat, the gait scores were examined weekly for 5 weeks (from the 6th day of pregnancy), following a previously described method (Noble et al., 2005; Ogawa et al., 2012; Prasad and Muralidhara, 2013). In brief, rats were placed individually on an empty flat surface and observed for 3 minutes to assign subjective gait scores divided into four levels, as follows: Level 1: the rat was active and not affected (score 1); level 2: the rat was slightly affected and characterized by weakness, mild ataxia and foot splay (score 2); level 3: the rat was moderately affected and characterized by reduced activity and obvious foot splay with limb spread during ambulation (score 3); level 4: the rat was severely affected and displayed reduced activity, obvious foot splay with limb spread during ambulation, inability to support body weight, dragging of the hind-limbs and inability to rear (score 4).
Nissl staining of hippocampal neurons
Brain tissues from postnatal day 21 weaning rats were fixed in neutral formalin, dehydrated in graded ethanol and embedded in paraffin. Paraffin-embedded brain coronal sections (5 μm thick) were dewaxed with xylene and rehydrated in graded ethanol. The sections were washed three times with distilled water, and then stained with 1% toluidine blue at 60°C for 40 minutes, or with Cresyl violet at 60°C for 30 seconds. The stained sections were dehydrated in graded ethanol solutions, permeabilized with xylene, mounted with neutral balsam (Yiyang Inc., Shanghai, China) and photographed with a Zeiss microscope (Baden Wurttemberg, Germany). Optical density values of positive staining were determined and the positive expression area fraction in full field image was calculated. These procedures were performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry
Paraffin-embedded brain coronal sections (5 μm thick) from postnatal day 21 weaning rats were also used for immunohistochemistry. The sections with intact hippocampi were dewaxed with xylene and rehydrated in graded ethanol solutions, followed by heat-mediated antigen retrieval using 0.01 M citrate buffer (trisodium citrate dihydrate, citric acid, pH 6.0) in a microwave at 95°C. Endogenous peroxidase activity was quenched by incubation in 3% hydrogen peroxide in phosphate buffer saline (PBS) for 30 minutes, and sections were washed in PBS for 5 minutes. Sections were incubated for 1 hour in blocking solution (10% albumin from bovine serum) at room temperature, and then incubated overnight with rabbit SYP polyclonal antibody (A6344; ABclonal Inc., Boston, MA, USA) or rabbit GAP-43 polyclonal antibody (A6376; ABclonal Inc.) in blocking solution at 4°C (using PBS as negative control). Sections were then washed in PBS and incubated with horseradish peroxidase AffiniPure goat anti-rabbit IgG (Earthox LLC, San Francisco, CA, USA) for 40 minutes at 37°C. Sections were washed with PBS, incubated for 2 minutes in a solution of 0.02% diaminobenzidine, rinsed in distilled water, counterstained with hematoxylin for 1 minute, mounted with neutral balsam and then photographed with a Zeiss microscope (Baden Wurttemberg, Germany). The optical density value of positive expression was measured by ImageJ software, and the positive expression area fraction in the full field image was calculated. All procedures were performed in three different sections for each animal, and the mean value was used for analysis.
Western blot assay
For western blot assay, the hippocampal tissues from postnatal day 1 rat brains were isolated and homogenized in ice-cold radioimmune precipitation assay lysis buffer (Beyotime Inc., Jiangsu, China). The homogenates were centrifuged at 12,000 revolutions per minute for 15 minutes at 4°C, and the supernatants then collected for protein concentration using the BCA-100 protein assay kit (KeyGen BioTECH, Nanjing, China). An equal concentration of protein (20 μg) from each sample was boiled for 10 minutes in 5 × sodium dodecyl sulfate buffer (Jetway Biotech, Guangzhou, China), and then loaded onto 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5% non-fat milk powder for 60 minutes at 37°C, and then incubated overnight at 4°C with rabbit SYP polyclonal antibody (1:1,000 dilution; A6344; ABclonal Inc.) or rabbit GAP-43 polyclonal antibody (1:1,000 dilution; A6376; ABclonal Inc.).
After incubation with horseradish peroxidase AffiniPure goat anti-rabbit IgG (Earthox LLC) in a recommended dilution of 1:100,000, the relative levels of protein expression were detected with the Sper ECL Assay Kit (Earthox LLC) using rabbit anti-β-tubulin (AC008; ABclonal Inc.) as an internal control. For data analysis, quantification of relative protein levels was presented as gray values, and the ratio of target protein in each group to β-tubulin was measured. The ratio of target protein to β-tubulin in the control group was set as a reference, and this ratio was compared with the control group. Image analysis was performed with ImageJ software.
Statistical analysis
All analyses were performed using SPSS 23.0 statistical software (IBM, Armonk, NY, USA). All data are presented as the mean ± SEM (n = 8 per group). One-way analysis of variance followed by Tukey post hoc test was used for statistical analysis. Statistical significance was set to α = 0.05.
Results
Effects of body weight and gait scores induced by ACR
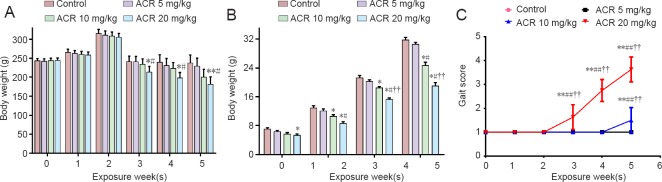
As shown in Figure 1A, there was no difference in body weight between the groups at the beginning of ACR exposure (P > 0.05). However, there was a significant decrease in the mean body weight in the ACR 20 mg/kg group at week 3 compared with the control group (P < 0.05). The mean body weight in the ACR 20 mg/kg group was significantly decreased from 3 weeks until the end of the experiment (P < 0.01). At the end of the experiment, the average weight of the rats in the ACR 10 mg/kg group was decreased by 15.7% compared with the control group, while the body weight of rats in the ACR 20 mg/kg group was reduced by 23.7% (P < 0.01). A similar phenomenon occurred in weaning rats (P < 0.01) (Figure 1B).
Figure 1.
Body weight and gait scores changes induced by acrylamide (ACR) exposure.
Gait scores were examined in pregnant rats from all groups from the 6th day of pregnancy for 5 weeks. (A, B) Body weight of gravid and weaning rats decreased with increasing exposure time (mean ± SD, n = 8, one-way analysis of variance followed by Tukey post hoc test). *P < 0.05, **P < 0.01, vs. control group; #P < 0.05, vs. ACR 5 mg/kg group; ††P < 0.01, vs. ACR 10 mg/kg group. (C) Gait scores of gravid rats were increased with ACR exposure. The locomotion of gravid rats was impaired by ACR exposure (mean ± SD, n = 8, one-way analysis of variance followed by Tukey post hoc test). **P < 0.01, vs. control group; ##P < 0.01, vs. ACR 5 mg/kg group; ††P < 0.01, vs. ACR 10 mg/kg group. Higher gait scores represent increased motor function impairment.
There was no significant difference in gait scores between the control group and the ACR 5 mg/kg group at any time (P > 0.05). However, compared with controls, gait scores were significantly increased in the ACR 10 mg/kg group (1.50 ± 0.53; P < 0.01) in the 5th week. Furthermore, in the ACR 20 mg/kg group, gait scores were significantly increased in the 3rd week of intragastric treatment (1.63 ± 0.52; P < 0.01), and progressively increased with time (5th week: 3.63 ± 0.52; P < 0.01 vs. control group) (Figure 1C).
Results of Nissl staining on hippocampal neurons
The Nissl body is a specific chromatophilic substance found within the cytoplasm of neuronal dendrites. The Nissl bodies generally have a fixed form, although their morphology can change or even disappear with brain injury. Thus, the presence and distribution of neurons and their pathological changes can be identified by the morphology of Nissl bodies (Pullen, 1990; Niu et al., 2015). The arrangement of neural cone cells which were one of the components of the cerebral cortex in the hippocampal dentate gyrus was compact with cells for 4 or 5 layers. Furthermore, the neuronal cell bodies were large, with pale and uniform staining of the cytoplasm. The nuclei were also large and round, and all cells showed deep dye staining (Li et al., 2015). As shown in Figure 2, following ARC treatment, hippocampal neurons showed a disordered arrangement, with fewer layers and less cells compared with the control group. Neurons also showed a ’fuzzy’ body edge with reduced cellular integrity, mild cell body staining and loss of part of the Nissl bodies. The severity of these changes increased with increasing ACR concentration.
Figure 2.
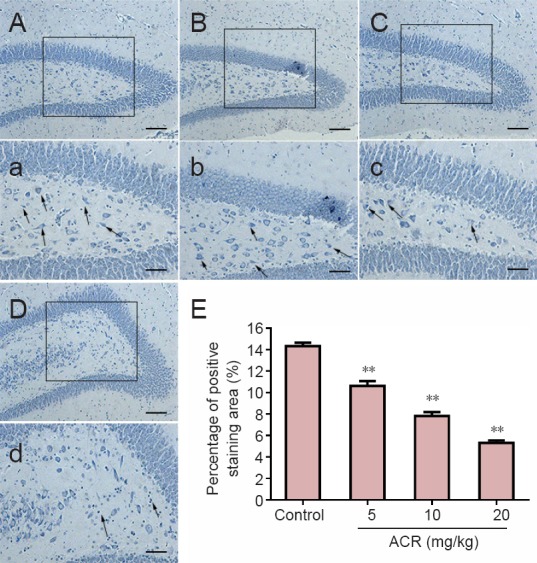
Nissl staining of hippocampal neurons of postnatal day 21 weaning rats.
(A–D) Nissl staining of control and acrylamide (ACR) 5, 10 and 20 mg/kg groups. Scale bars: 100 μm. (a–d) Higher magnification for boxes in A–D. Scale bars: 10 μm. The number of Nissl bodies decreased with increasing ACR concentration. (E) Percentage of positively stained area (%) in each field. **P < 0.01, vs. control group (mean ± SD, n = 8, one-way analysis of variance followed by Tukey post hoc test).
Dose-dependent changes in hippocampal GAP-43 expression
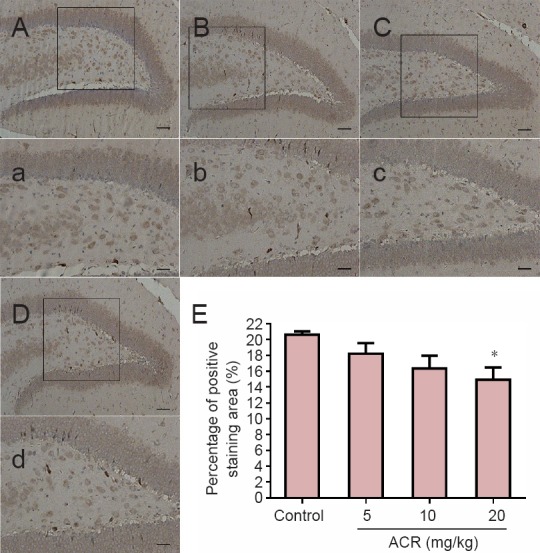
GAP-43 is a phosphorylated protein specifically expressed on the neuronal cell membrane, and is important for adjusting neuronal responses to axonal guidance signaling in neuronal development, as well as axon growth and structure. As such, GAP-43 is a widely used molecular marker of neuronal development (Chirwa et al., 2005). There was no difference in the volume of GAP-43-immunoreactive cells between the control group and ACR 5 mg/kg group in the hippocampal dentate gyrus at postnatal day 21 (Figure 3). However, GAP-43 levels were significantly decreased in the ACR 10 mg/kg group (P < 0.05), and even further reduced in the ACR 20 mg/kg group (P < 0.01 vs. ACR 10 mg/kg group). Western blot assay results showed a similar trend (Figure 4). GAP-43 expression in ACR 20 mg/kg group was decreased significantly (P < 0.01 vs. control group).
Figure 3.
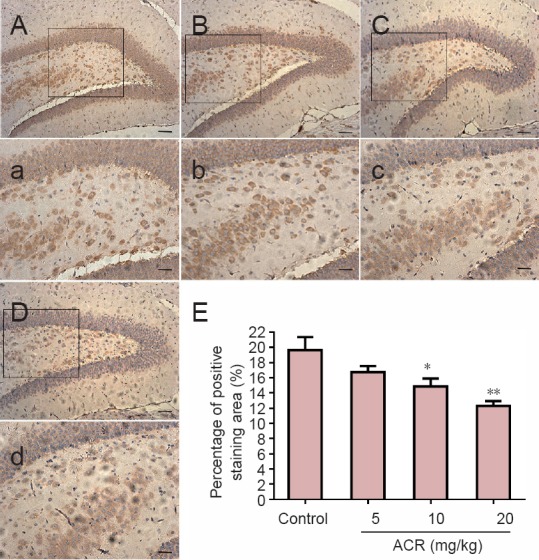
Percentage of positively stained area for growth associated protein 43 (GAP-43) in hippocampal neurons of postnatal day 21 weaning rats.
(A–D) Immunoreactivity for GAP-43 in the control and acrylamide (ACR) 5, 10 and 20 mg/kg groups. Scale bars: 100 μm. (a–d) Higher magnification for boxes in A–D. Scale bars: 10 μm. Positively stained cells (brown dots) showing normal morphology and function of neurons in the control group. Note that neurons in the ACR groups showed lighter staining and reduced GAP-43 numbers. The histogram in (E) shows the percentage of positively stained area (%). *P < 0.05, **P < 0.01, vs. control group (mean ± SD, n = 8, one-way analysis of variance followed by Tukey post hoc test).
Figure 4.
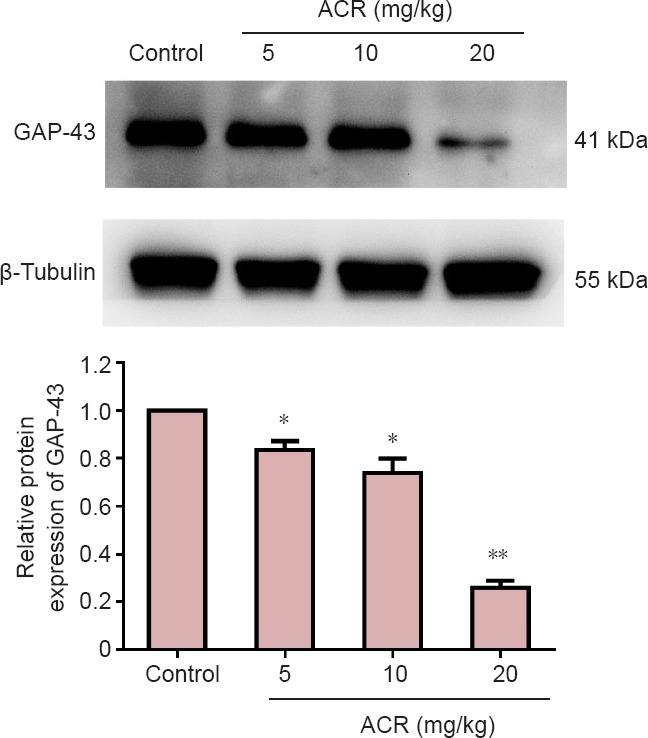
Western blot assay of growth associated protein 43 (GAP-43) in the hippocampal dentate gyrus of postnatal day 21 weaning rats.
The gray value ratio of GAP-43 to β-tubulin in each group was measured. The ratio in the control group was set as a reference, and then all experimental ratios were compared with the control group. GAP-43 expression was decreased following acrylamide (ACR) administration. *P < 0.05, **P < 0.01, vs. control group (mean ± SD, n = 8, one-way analysis of variance followed by Tukey post hoc test).
ACR inhibited SYP expression in the hippocampal dentate gyrus
SYP is a calcium-binding glycoprotein (molecular weight of 38 kDa) that forms an abundant integral membrane protein constituent of neural synaptic vesicles (Evans et al., 2005; Rossetti et al., 2016). In control animals, SYP immunoreactivity was mainly found in the dentate gyrus granular layer of the synapses, but not in the nucleus. Image analysis showed that compared with the control group, there was a significant reduction in SYP-immunoreactive neurons in the hippocampal dentate gyrus in the ACR 20 mg/kg group (P < 0.05), but not in other groups (Figure 5). Western blot assay results showed a similar trend (Figure 6). SYP expression in ACR 20 mg/kg group was decreased significantly (P < 0.01 vs. control group).
Figure 5.
Percentage of synaptophysin (SYP)-immunoreactive area of hippocampal neurons in postnatal day 21 weaning rats.
(A–D) Immunoreactivity of SYP in the control and acrylamide (ACR) 5, 10 and 20 mg/kg groups. Scale bars: 100 μm. (a–d) Higher magnification for boxes in A–D. Scale bars: 10 μm. Brown points represent positive staining, demonstrating that ACR had a mild toxic effect on SYP volume. (E) Percentage of positively stained SYP area in postnatal day 21 weaning rats in hippocampal neurons. *P < 0.05, vs. control group (mean ± SD, n = 8, one-way analysis of variance followed by Tukey post hoc test).
Figure 6.
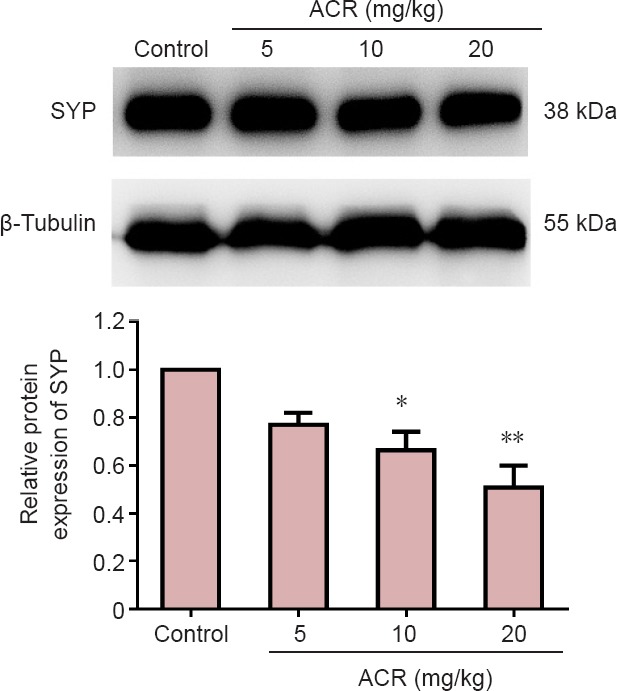
Western blot assay for synaptophysin (SYP) in the hippocampal dentate gyrus of postnatal day 21 weaning rats.
The gray value ratio of SYP to β-tubulin in each group was measured. The ratio in the control group was set as a reference, and all experimental ratios were compared with the control group. SYP expression was decreased following acrylamide (ACR) exposure. *P < 0.05, **P < 0.01, vs. control group (mean ± SD, n = 8, one-way analysis of variance followed by Tukey post hoc test).
Discussion
ACR neurotoxicity has been previously reported in adult animal studies (Lopachin et al., 2012; Tian et al., 2015), which leads to axonal lesions in the central and peripheral nervous systems, with associated weight loss, skeletal muscle weakness and ataxia. The pathological features also include peripheral nerve axonal swelling and degeneration (Shi et al., 2012; He et al., 2017). In the present study, there were no obvious pathological changes in gravid rats in the ACR 5 mg/kg group, while mild ataxia appeared in the ACR 10 mg/kg group and typical ataxia and hind limb weakness were observed in the ACR 20 mg/kg group. These findings are consistent with the clinical symptoms in patients with ACR toxicity and the pathology observed to mature neurons.
Nissl bodies are a characteristic structure in newborn neurons. Nissl bodies are mainly involved in protein synthesis, and are essential for advanced brain activity, including learning and memory (Niu et al., 2008; Cheng et al., 2010). The expression of Nissl bodies is strongly associated with neuronal function. Neurons constantly utilize proteins during excitatory transmission, which requires new protein synthesis by Nissl bodies to prevent protein depletion. In the present study, we found a decrease in the number of Nissl bodies, and they showed light staining, after ACR exposure, reflecting partial inhibition of neuronal protein synthesis. These findings also suggest that the toxic effects of ACR on neuronal development may be associated with reduced neuronal proliferation. Thus, a decrease in the number of new neurons and decreased protein synthesis may have an overall detrimental effect on newborn brain function.
To verify this hypothesis, we tested two related markers, GAP-43 and SYP. Given that the expression level of GAP-43 is related to neuronal growth (Lai et al., 2011), we used GAP-43 protein expression to assess the function of hippocampal dentate gyrus neurons. Numerous studies have also shown that GAP-43 protein is important for promoting axonal elongation and maintaining axonal morphology. Furthermore, GAP-43 protein was reported to be widely distributed at the tip of the synapse, but rarely in dendrites, suggesting that GAP-43 may modulate the transmission of neural signals. The axonal length of PC12 cells was also positively correlated with GAP-43 mRNA expression (Baetge et al., 1991; Benowitz et al., 1997), supporting a role in axonal growth. In the present study, the decreased expression of GAP-43 following ACR exposure suggests an impairment in neuronal development, including effects on axonal growth and synaptic inhibition.
SYP is a marker of synaptogenesis during embryonic developmental. SYP is widely used as a specific marker of the presynaptic membrane, and for detection of the density and distribution of synapses (Cabalka et al., 1990; Dahlqvist et al., 2004). Previous studies have also reported that increased SYP levels are associated with increased synaptic development (Leclerc et al., 1989; Liu et al., 2016). In the present study, long-term exposure to ACR may have caused reduced axonal and dendritic growth in developing hippocampal neurons, with associated functional changes. Indeed, a decrease in SYP expression may reflect a decrease in nerve transmission, which may have a detrimental effect on cognitive and limb functions (Robinson et al., 2011).
Accumulating evidence also suggests that the proliferation and differentiation of neurons during hippocampal neurogenesis can be altered by deficits in GAP-43 and SYP during development (Groves et al., 2005; Xiao et al., 2015; Sakharkar et al., 2016). Neurites, including axons and dendrites, are critical for the morphological and functional development of immature neurons. Abnormal expression of GAP-43 and SYP were previously reported to reflect central nervous system dysfunction during early neuronal development (Wang et al., 2014; Williams et al., 2016). In turn, this may result in biochemical alterations in neural metabolism and axonal transport, as previously reported following ARC exposure (Honig and Rosenberg, 2000; LoPachin et al., 2004), which may be aggravated by decreased GAP-43 expression.
In summary, we found that fetal ACR exposure during pregnancy was associated with reduced expression of GAP-43 and SYP in the hippocampus of postnatal day 21 offspring, which may be associated with inhibition of neuronal proliferation and differentiation and synaptic function.
Acknowledgments
The authors would like to thank Guo-ying Li from Guangdong Medical Association of China for study design and De-hui Yang from Guangdong Pharmaceutical University of China for the assistance in collecting data.
Footnotes
Conflicts of interest: None declared.
Research ethics: The study protocol was approved by the Animal Ethics Committee of Guangdong Pharmaceutical University of China (approval No. gdpulac 2012117). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Elizabeth Hernández-Echeagaray, Universidad Nacional Autonoma de Mexico Unidad de Biomedicina, Tlalnepantla, Mexico.
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
Funding: This study was supported by the Guangdong Provincial Department of Science and Technology in China, No. 2016A020225007.
References
- Baetge EE, Hammang JP. Neurite outgrowth in pc12 cells deficient in gap-43. Neuron. 1991;6:21–30. doi: 10.1016/0896-6273(91)90118-j. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. Gap-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Cabalka LM, Ritchie TC, Coulter JD. Immunolocalization and quantitation of a novel nerve terminal protein in spinal cord development. J Comp Neurol. 1990;295:83–91. doi: 10.1002/cne.902950108. [DOI] [PubMed] [Google Scholar]
- Cheng O, Ostrowski RP, Liu W, Zhang JH. Activation of liver x receptor reduces global ischemia brain injury by reduction of nuclear factor-κb. Neuroscience. 2010;166:1101. doi: 10.1016/j.neuroscience.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirwa S, Aduonum A, Pizarro J, Reasor J, Kawai Y, Gonzalez M, Mcadory BS, Onaivi E, Barea-Rodriguez EJ. Dopaminergic DA1 signaling couples growth-associated protein-43 and long-term potentiation in guinea pig hippocampus. Brain Res Bull. 2005;64:433–440. doi: 10.1016/j.brainresbull.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Dahlqvist P, Rönnbäck A, Bergström SA, Söderström I, Olsson T. Environmental enrichment reverses learning impairment in the morris water maze after focal cerebral ischemia in rats. Eur J Neurosci. 2004;19:2288–2298. doi: 10.1111/j.0953-816X.2004.03248.x. [DOI] [PubMed] [Google Scholar]
- Derksen MJ, Ward NL, Hartle KD, Ivanco TL. Map2 and synaptophysin protein expression following motor learning suggests dynamic regulation and distinct alterations coinciding with synaptogenesis. Neurobiol Learn Mem. 2007;87:404–415. doi: 10.1016/j.nlm.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Young JF, Chen JJ, Dinovi MJ, Henry SH. Using dietary exposure and physiologically based pharmacokinetic/pharmacodynamic modeling in human risk extrapolations for acrylamide toxicity. J Agr Food Chem. 2008;56:6031–6038. doi: 10.1021/jf073042g. [DOI] [PubMed] [Google Scholar]
- Evans GJO, Cousin MA. Tyrosine phosphorylation of synaptophysin in synaptic vesicle recycling. Biochem Soc Trans. 2005;33:1350–1353. doi: 10.1042/BST20051350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exon JH. A review of the toxicology of acrylamide. J Toxicol Env Heal B. 2006;9:397. doi: 10.1080/10937400600681430. [DOI] [PubMed] [Google Scholar]
- Freeman LW, Wright TW. Experimental observations of concussion and contusion of the spinal cord. Ann Surg. 1953;137:433–443. doi: 10.1097/00000658-195304000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey J, Ferguson SA, Paule MG. Developmental and behavioral effects of acrylamide in Fischer 344 rats. Neurotoxicol Teratol. 2005;27:553. doi: 10.1016/j.ntt.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Groves NJ, Thj B. The impact of vitamin d deficiency on neurogenesis in the adult brain. Neural Regen Res. 2017;12:393–394. doi: 10.4103/1673-5374.202936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Tan D, Mi Y, Zhou Q, Ji S. Epigallocatechin-3-gallate attenuates cerebral cortex damage and promotes brain regeneration in acrylamide-treated rats. Food Funct. 2017;8:2275–2282. doi: 10.1039/c6fo01823h. [DOI] [PubMed] [Google Scholar]
- Honig LS, Rosenberg RN. Apoptosis and neurologic disease. Am J Med. 2000;108:317–330. doi: 10.1016/s0002-9343(00)00291-6. [DOI] [PubMed] [Google Scholar]
- Konings EJ, Baars AJ, van Klaveren JD, Spanjer MC, Rensen PM, Hiemstra M, van Kooij JA, Peters PW. Acrylamide exposure from foods of the Dutch population and an assessment of the consequent risks. Food Chem Toxicol. 2003;41:1569–1579. doi: 10.1016/s0278-6915(03)00187-x. [DOI] [PubMed] [Google Scholar]
- Kwon SE, Chapman ER. Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron. 2011;70:847. doi: 10.1016/j.neuron.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai HC, Wu MJ, Chen PY, Sheu TT, Chiu SP, Lin MH, Ho CT, Yen JH. Neurotrophic effect of citrus 5-hydroxy-3,6,7,8,3’,4’-hexamethoxyflavone: promotion of neurite outgrowth via cAMP/PKA/CREB pathway in PC12 cells. PLoS One. 2011;6:e28280. doi: 10.1371/journal.pone.0028280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc N, Beesley PW, Brown I, Colonnier M, Gurd JW, Paladino T, Hawkes R. Synaptophysin expression during synaptogenesis in the rat cerebellar cortex. J Comp Neurol. 1989;280:197–212. doi: 10.1002/cne.902800204. [DOI] [PubMed] [Google Scholar]
- Lehning EJ, Balaban CDRoss JF, Lopachin RM. Acrylamide neuropathy. III. Spatiotemporal characteristics of nerve cell damage in forebrain. Neurotoxicology. 2003;24:125–136. doi: 10.1016/s0161-813x(02)00155-9. [DOI] [PubMed] [Google Scholar]
- Li J, Wen PY, Li WW, Zhou J. Upregulation effects of tanshinone iia on the expressions of neun, nissl body, and iκb and downregulation effects on the expressions of gfap and nf-κb in the brain tissues of rat models of alzheimer's disease. Neuroreport. 2015;26:758–766. doi: 10.1097/WNR.0000000000000419. [DOI] [PubMed] [Google Scholar]
- Lingnert H, Grivas S, Jägerstad M, Skog K, Törnqvist M, Šman P. Acrylamide in food: mechanisms of formation and influencing factors during heating of foods. Food Nutr Res. 2002;46:159–172. [Google Scholar]
- Lipworth L, Sonderman JS, Tarone RE, Mclaughlin JK. Review of epidemiologic studies of dietary acrylamide intake and the risk of cancer. Eur J Cancer Prev. 2012;21:375. doi: 10.1097/CEJ.0b013e3283529b64. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Yang C, Zhang Y, Su RY, Chen JL, Jiao MM, Chen HF, Zheng N, Luo S, Chen YB, Quan SJ, Wang Q. Neuroprotective effect of β-asarone against alzheimer's disease: regulation of synaptic plasticity by increased expression of SYP and glur1. Drug Des Devel Ther. 2016;10:1461–1469. doi: 10.2147/DDDT.S93559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin RM, Gavin T. Molecular mechanism of acrylamide neurotoxicity: lessons learned from organic chemistry. Environ Health Perspect. 2012;120:1650–1657. doi: 10.1289/ehp.1205432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin RM, Schwarcz AI, Gaughan CL, Mansukhani S, Das S. In vivo and in vitro effects of acrylamide on synaptosomal neurotransmitter uptake and release. Neurotoxicology. 2004;25:349–363. doi: 10.1016/S0161-813X(03)00149-9. [DOI] [PubMed] [Google Scholar]
- Ma Y, Shi J, Zheng M, Liu J, Tian S, He X, Zhang D, Li G, Zhu J. Toxicological effects of acrylamide on the reproductive system of weaning male rats. Toxicol Ind Health. 2011;27:617. doi: 10.1177/0748233710394235. [DOI] [PubMed] [Google Scholar]
- Manuela P, Giulia M, Valentina P, Luisa V, Marco V, Mariano M. Neurotoxicity of acrylamide in exposed workers. Int J Environ Res Public Health. 2013;10:3843–3854. doi: 10.3390/ijerph10093843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcphail LT, Fernandes KJ, Chan CC, Vanderluit JL, Tetzlaff W. Axonal reinjury reveals the survival and re-expression of regeneration-associated genes in chronically axotomized adult mouse motoneurons. Exp Neurol. 2004;188:331–340. doi: 10.1016/j.expneurol.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Navratil V, de Chassey B, Meyniel L, Delmotte S, Gautier C, André P, Lotteau V, Rabourdin-Combe C. Virhostnet: a knowledge base for the management and the analysis of proteome-wide virus–host interaction networks. Nucleic Acids Res. 2009;37:D661–668. doi: 10.1093/nar/gkn794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Li C, Wu H, Feng X, Su Q, Li S, Zhang L, Yew DT, Cho EY, Sha O. Propidium iodide (pi) stains nissl bodies and may serve as a quick marker for total neuronal cell count. Acta Histochem. 2015;117:182–187. doi: 10.1016/j.acthis.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Niu R, Sun Z, Wang J, Cheng Z, Wang J, China S. Effects of fluoride and lead on locomotor behavior and expression of nissl body in brain of adult rats. Fluoride. 2008;41:276–282. [Google Scholar]
- Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, Lafrancois J, Feinstein B. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa B, Wang L, Ohishi T, Taniai E, Akane H, Suzuki K, Mitsumori K, Shibutani M. Reversible aberration of neurogenesis targeting late-stage progenitor cells in the hippocampal dentate gyrus of rat offspring after maternal exposure to acrylamide. Arch Toxicol. 2012;86:779–790. doi: 10.1007/s00204-012-0801-y. [DOI] [PubMed] [Google Scholar]
- Prasad SN, Muralidhara Neuroprotective Efficacy of eugenol and isoeugenol in acrylamide-induced neuropathy in rats: behavioral and biochemical evidence. Neurochem Res. 2013;38:330–345. doi: 10.1007/s11064-012-0924-9. [DOI] [PubMed] [Google Scholar]
- Pullen AH. Morphometric evidence from c-synapses for phased nissl body response in alpha-motoneurones retrogradely intoxicated with diphtheria toxin. Brain Res. 1990;509:8–16. doi: 10.1016/0006-8993(90)90302-r. [DOI] [PubMed] [Google Scholar]
- Robinson PA. Neural field theory of synaptic plasticity. J Theor biol. 2011;285:156–163. doi: 10.1016/j.jtbi.2011.06.023. [DOI] [PubMed] [Google Scholar]
- Rossetti MF, Varayoud J, Lazzarino GP, Luque EH, Ramos JG. Pregnancy and lactation differentially modify the transcriptional regulation of steroidogenic enzymes through DNA methylation mechanisms in the hippocampus of aged rats. Mol Cell Endocrinol. 2016;429:73–83. doi: 10.1016/j.mce.2016.03.037. [DOI] [PubMed] [Google Scholar]
- Sakharkar AJ, Vetreno RP, Zhang H, Kokare DM, Crews FT, Pandey SC. A role for histone acetylation mechanisms in adolescent alcohol exposure-induced deficits in hippocampal brain-derived neurotrophic factor expression and neurogenesis markers in adulthood. Brain Struct Funct. 2016;221:1–13. doi: 10.1007/s00429-016-1196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansano M, Heredia A, Peinado I, Andrés A. Dietary acrylamide: what happens during digestion. Food Chem. 2017;237:58–64. doi: 10.1016/j.foodchem.2017.05.104. [DOI] [PubMed] [Google Scholar]
- Sen E, Tunali Y, Erkan M. Testicular development of male mice offsprings exposed to acrylamide and alcohol during the gestation and lactation period. Hum Exp Toxicol. 2015;34:401–414. doi: 10.1177/0960327114542883. [DOI] [PubMed] [Google Scholar]
- Shi J, Ma Y, Zheng M, Ruan Z, Liu J, Tian S, Zhang D, He X, Li G. Effect of sub-acute exposure to acrylamide on GABAergic neurons and astrocytes in weaning rat cerebellum. Toxicol Ind Health. 2012;28:10. doi: 10.1177/0748233711401264. [DOI] [PubMed] [Google Scholar]
- Svensson K, Abramsson L, Becker W, Glynn A, Hellenäs KE, Lind Y, Rosén J. Dietary intake of acrylamide in Sweden. Food Chem Toxicol. 2003;41:1581–1586. doi: 10.1016/s0278-6915(03)00188-1. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Shibutani M, Inoue K, Fujimoto H, Hirose M, Nishikawa A. Pathological assessment of the nervous and male reproductive systems of rat offspring exposed maternally to acrylamide during the gestation and lactation periods-a preliminary study. J Toxicol Sci. 2008;33:11–24. doi: 10.2131/jts.33.11. [DOI] [PubMed] [Google Scholar]
- Tian SM, Ma YX, Shi J, Lou T Y, Liu SS, Li GY. Acrylamide neurotoxicity on the cerebrum of weaning rats. Neural Regen Res. 2015;10:938–943. doi: 10.4103/1673-5374.158357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang R, Xu S, Lakshmana MK. Ranbp9 overexpression accelerates loss of pre and postsynaptic proteins in the AP Delta E9 transgenic mouse brain. PLoS One. 2014;9:e85484. doi: 10.1371/journal.pone.0085484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S, Chen L, Savignac HM, Tzortzis G, Anthony DC, Burnet PW. Neonatal prebiotic supplementation increases the levels of synaptophysin, glun2a-subunits and bdnf proteins in the adult rat hippocampus. Synapse. 2016;70:121–124. doi: 10.1002/syn.21880. [DOI] [PubMed] [Google Scholar]
- WHO. Health implications of acrylamide in food. Geneva, Switzerland: FAO/WHO; 2002. [Google Scholar]
- Xiao F, Xu JM, Jiang XH. Cx3 chemokine receptor 1 deficiency leads to reduced dendritic complexity and delayed maturation of newborn neurons in the adult mouse hippocampus. Neural Regen Res. 2015;10:772–777. doi: 10.4103/1673-5374.156979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Yan L, Yao L, GuanW, Zeng F, Cao F. Acrylamide exposure impairs blood-cerebrospinal fluid barrier function. Neural Regen Res. 2014;9:555–560. doi: 10.4103/1673-5374.130080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Teng EM, SR, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]