Keywords: nerve regeneration, erythropoietin, hypothermia, hypoxic-ischemic encephalopathy, neonate, tau protein, biomarkers, prognosis, neuroprotection, neural regeneration
Abstract
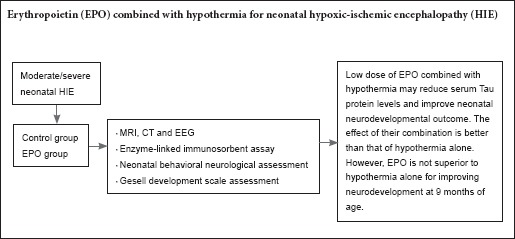
Although hypothermia therapy is effective to treat neonatal hypoxic-ischemic encephalopathy, many neonatal patients die or suffer from severe neurological dysfunction. Erythropoietin is considered one of the most promising neuroprotective agents. We hypothesized that erythropoietin combined with hypothermia will improve efficacy of neonatal hypoxic-ischemic encephalopathy treatment. In this study, 41 neonates with moderate/severe hypoxic-ischemic encephalopathy were randomly divided into a control group (hypothermia alone for 72 hours, n = 20) and erythropoietin group (hypothermia + erythropoietin 200 IU/kg for 10 days, n = 21). Our results show that compared with the control group, serum tau protein levels were lower and neonatal behavioral neurological assessment scores higher in the erythropoietin group at 8 and 12 days. However, neurodevelopmental outcome was similar between the two groups at 9 months of age. These findings suggest that erythropoietin combined with hypothermia reduces serum tau protein levels and improves neonatal behavioral neurology outcome but does not affect long-term neurodevelopmental outcome.
Introduction
Hypoxic-ischemic encephalopathy (HIE) is a significant cause of neonatal brain injury. Approximately one million newborns die from HIE worldwide each year. Although the incidence of moderate/severe neonatal encephalopathy is 0.5–1 per 1,000 in developed countries (Levene et al., 1986), it is much higher in developing countries (Kurinczuk et al., 2010). Altogether, 15–20% of infants with HIE will die, while 25% of those surviving will suffer from severe short-term or long-term motor or sensory dysfunction, cognitive impairment, hypoevolutism, seizures, mental retardation, and vision and hearing impairments (Vannucci and Perlman, 1997; Dammann et al., 2001; Barnett et al., 2002; Glass and Ferriero, 2007; Verklan, 2009). Because the pathogenesis of neonatal HIE is not very clear, there is no effective therapeutic method (Thoresen et al., 2013). In recent years, through a large number of animal experiments, studies on the pathophysiology of neonatal HIE have made encouraging progress and have provided a therapeutic window for neuroprotection or intervention. Clinical studies show that moderate/severe neonatal HIE can be treated by hypothermia within 6 hours after birth (Thoresen and Whitelaw, 2005). Moreover, benefits of hypothermia have been shown for neuroprotection, improving neurodevelopment, and reducing death and disability (Gluckman et al., 2005; Shankaran et al., 2005; Buonocore et al., 2012; Tagin et al., 2012; Jacobs et al., 2013; Azzopardi et al., 2014; Muller and Marks, 2014; Zubcevic et al., 2015; Ahearne et al., 2016; Zheng and Gao, 2016). Nevertheless, there is still 44–53% of neonates that die or are left with moderate to severe neurological dysfunction (Edwards et al., 2010; Wu and Gonzalez, 2015). Therefore, application of neuroprotective agents is necessary to prevent brain injury, promote nerve recovery, and reduce mortality and incidence of neurodevelopment.
Erythropoietin (EPO) is an acidic glycoprotein composed of 165 amino acids, with its biological effect achieved by combination with a specific receptor (EPO-R). EPO is produced by fetal liver and adult kidney, and its functions are to stimulate bone marrow hematopoietic stem cells, promote proliferation, differentiation, and maturation of erythrocyte progenitor cells, and regulate oxygen supply to the body (Reissmann and Udupa, 1972; Saito and Tojo, 1987; Sasaki et al., 2000; Kim et al., 2010; Malik et al., 2013; Lv et al., 2015b). Recombinant human EPO (rhEPO) is widely used in clinical treatment of various types of anemia, brain injury, subarachnoid hemorrhage, and intracerebral hemorrhage, and has exhibited remarkable curative effects since 1989 (Velly et al., 2010). Clinical trials show that EPO is a safe and effective neuroprotective agent, which is absent of serious adverse effects (McPherson and Juul, 2010). The protective mechanism of EPO on nerves is not completely understood. However, in recent years, central nervous system injury has been treated with exogenous EPO, which has become a ’hot’ research topic. In particular, human cases of neonatal HIE have been treated with EPO, although large-scale trials to determine whether EPO can further improve the prognosis of neonatal HIE treated with hypothermia have yet to be performed (Wu et al., 2012). Tau protein is a specific marker protein of central neurons, and brain damage can cause an increase in tau protein (Sussmuth et al., 2001; Hu et al., 2012; Schiefecker et al., 2016; Wang et al., 2016; Banks et al., 2017). The aim of this study was to detect changes in serum tau protein before and after treatment with EPO combined with hypothermia in neonates with moderate/severe HIE. Our intention was to investigate the relationship between EPO and neonatal behavioral neurology and neurodevelopmental outcome at 9 months of age to provide clinical data for EPO treatment of neonatal HIE.
Subjects and Methods
Subjects
Forty-one neonates with moderate/severe HIE were enrolled from the Neonatal Intensive Care Unit, Maternal and Child Health Care Hospital of Handan City, China from August 2014 to August 2015.
Inclusion criteria: Patients presenting with all of the following criteria were considered for study inclusion: (1) moderate/severe HIE in accordance with the diagnostic criteria of neonatal HIE formulated by the Chinese Medical Association (Group of Neonatology et al., 2005); (2) gestational age ≥ 37 weeks and weight ≥ 2,500 g; (3) severe asphyxia at birth, and Apgar score (Edwards et al., 2010) ≤ 3 at 1 minute or ≤ 5 at 5 minutes; (4) umbilical artery blood pH ≤ 7.0 at birth; and (5) abnormal neurological signs in the first 24 hours of life (e.g., drowsiness or coma, convulsions, abnormal muscle tone, original reflex weak or absent, irregular breathing or pupil size, and light reflection anomaly), or neonates with HIE and abnormal electroencephalogram recordings and brain abnormalities confirmed by computed tomography scan or magnetic resonance imaging.
Exclusion criteria: patients with one or more of the following conditions were excluded from this study: (1) severe intracranial hemorrhage; (2) congenital malformations or inherited metabolic disease; (3) severe infectious disease; (4) maternal history of drug abuse; (5) severe anemia, with hemoglobin < 120 g/L; (6) automatically refused treatment and with incomplete follow-up. All neonates with HIE were admitted to the Neonatal Intensive Care Unit. Their intracranial pressure was reduced, and they received respiratory support, convulsive control, maintenance of blood perfusion to the brain and body, maintenance of blood sugar at normal high levels, hypothermia or neuroprotective drugs, and other comprehensive treatments.
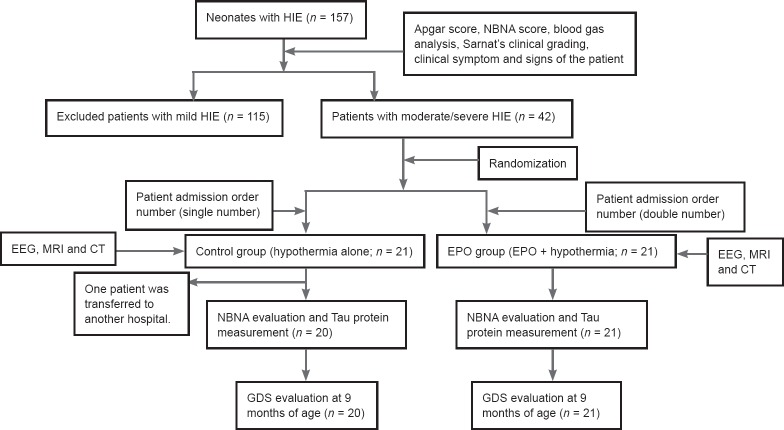
Neonates with HIE were randomly divided into a control group (hypothermia alone, n = 20) and EPO group (EPO and hypothermia, n = 21) according to their order of admission. This study was conducted in accordance with the principles of the Declaration of Helsinki, and approved by the Medical Ethics Committee of Handan Maternal and Child Health Care Hospital of Hebei Province of China (approval No. 2014-1). This paper was prepared in accordance with the CONSORT checklist. All patients and their families participated voluntarily and were fully informed about the experimental process. They provided signed informed consent based on the condition that they fully understood the procedure. Tests of blood, liver function, renal function, blood glucose, and blood pressure were also performed for all patients after admission (Figure 1).
Figure 1.
Flow chart of patient screening after admission.
HIE: Hypoxic-ischemic encephalopathy; NBNA: neonatal behavioral neurological assessment; EEG: electroencephalogram; MRI: magnetic resonance imaging; CT: computed tomography; EPO: erythropoietin; GDS: Gesell development scale.
Treatment
Forty-one neonates with HIE were treated at the Neonatal Intensive Care Unit after admission.
Control group: Conventional treatment was administered, including respiratory support, fluid infusion, anti-convulsive medication, reduction of intracranial pressure, maintenance of blood perfusion, maintenance of blood glucose, correction of acidosis, and correction of electrolyte balance. Neonates in the control group were administered brain hypothermia using a HGT-2000 therapeutic instrument (Zhuhai Hokai Medical Equipment, Zhuhai, China). The nasopharyngeal temperature probe from the temperature control instrument was wet and placed into the nasopharynx of the patient as it gradually cooled. Nasopharyngeal temperature of the skull's base temperature was maintained at 33.5–34.0°C. Hypothermia treatment was stopped after 72 hours. Patients were allowed to rewarm naturally, and rectal temperature returned to ≥ 36°C within 6 hours. All patients were administered far infrared radiation temperature when necessary. Rectal temperature was not allowed to exceed 0.5°C every 2 hours during recovery.
EPO group: Based on treatment in the control group, neonates in the EPO group were injected with rhEPO (Chinese hamster ovary cell) (North China Pharmaceutical Jin Tan Biotechnology, Shijiazhuang, China; batch No. GYZZ S20000026; rule batch No. 201405YC14; specifications: 3,000 IU/1 mL IU) from the second day of hospitalization. rhEPO (200 IU/kg soluble in 10% glucose solution) was intravenously administered, once a day, for 10 consecutive days. Blood tests (e.g., net woven erythrocytes, hematocrit), liver function, renal function, blood glucose, blood pressure, and adverse reactions were recorded during treatment.
Clinical grading of neonatal HIE (criteria for assessing moderate and severe HIE)
Neonatal HIE was diagnosed according to the classification of Sarnat and Sarnat (Sarnat and Sarnat, 1976). If there were no seizures in neonates from birth to 72 hours, yet muscle tone continued to decline or presented a highly excited state, HIE was defined as “mild". If the infant was lethargic and had hypotonia, weak primitive reflexes, and seizures, HIE was defined as “moderate". If the infant suffered frequent seizures, apnea, flaccid weakness, or coma, HIE was defined as “severe".
Serum tau protein measurement
Blood samples were taken from the radial vein at 24 hours after birth (before EPO treatment) and 4, 8, and 12 days after EPO treatment. Blood samples were placed in a test tube (specifications: 5 mL, No A06277602; Hebi City Zhong Xing Medical Supplies, Hebi, China), maintained without stirring for 30 minutes at room temperature, and then centrifuged in a L-400 low-speed automatic balancing centrifuge (Hunan Xiangyi Laboratory Instrument Development, Changsha, Hunan Province, China) at 3,000 r/min for 15 minutes.
Serum was removed into another test tube and placed in a freezer at −70°C until use. To avoid any influence on the results, blood transfusions to the neonates were avoided before collecting blood samples. Serum tau protein was detected using an enzyme-linked immunosorbent assay. The kit was provided by the Shanghai HuDing Biological Science and Technology Co., Ltd. (R & D Systems, Minneapolis, MN, USA; tau protein detection range: 30–1,200 pg/mL). Before serum was analyzed, samples were removed from −70°C to room temperature, and then placed on an oscillator (FWZ-1; Guangzhou Fenghua Bioengineering Co., Ltd., Guangzhou, China) for 1 hour at 1,200 r/min, in accordance with the manufacturer's instructions.
Neurological assessment
Neonatal behavioral neurological assessment (NBNA): According to Bao et al.'s method (1991), neonates with HIE were assessed at 2, 7, 14, and 28 days after treatment. NBNA contains five clusters: behavior (6 items); passive tone (4 items); active tone (4 items); primary reflexes (3 items); and general assessment (3 items). Each item has three scales (0, 1, and 2). Twenty items have a maximum of 40 points. NBNA score < 35 points indicates brain injury; 28–32 points indicates severe brain injury; and 33–35 points indicates mild brain injury.
Gesell development scale (GDS): Neurodevelopmental examination was performed at 9 months of age, according to GDS (Roe, 1977; Lin et al., 1986; Li et al., 2014). GDS includes gross motor, fine motor, adaptability, language, and personal social skills, which measure development quotient (DQ). Evaluation criteria: DQ ≥ 85 points is defined as good neurodevelopment; 75–85 points is defined as a boundary situation; and < 75 points is defined as neurodevelopmental retardation.
NBNA and GDS were evaluated by two trained professionals.
Statistical analysis
Measurement data are expressed as the mean ± SD, and group comparisons were performed using Student's t-test and paired t-test. Categorical data were expressed as number and percentage, and group comparisons were performed with Fisher's test or chi-square test. Correlation between serum tau protein levels and NBNA score was tested by Spearman's rank-order correlation analysis. All calculations and test were analyzed using SPSS 11.5 software (SPSS, Chicago, IL, USA). A P-value < 0.05 was considered statistically significant.
Results
Characteristics of HIE neonates
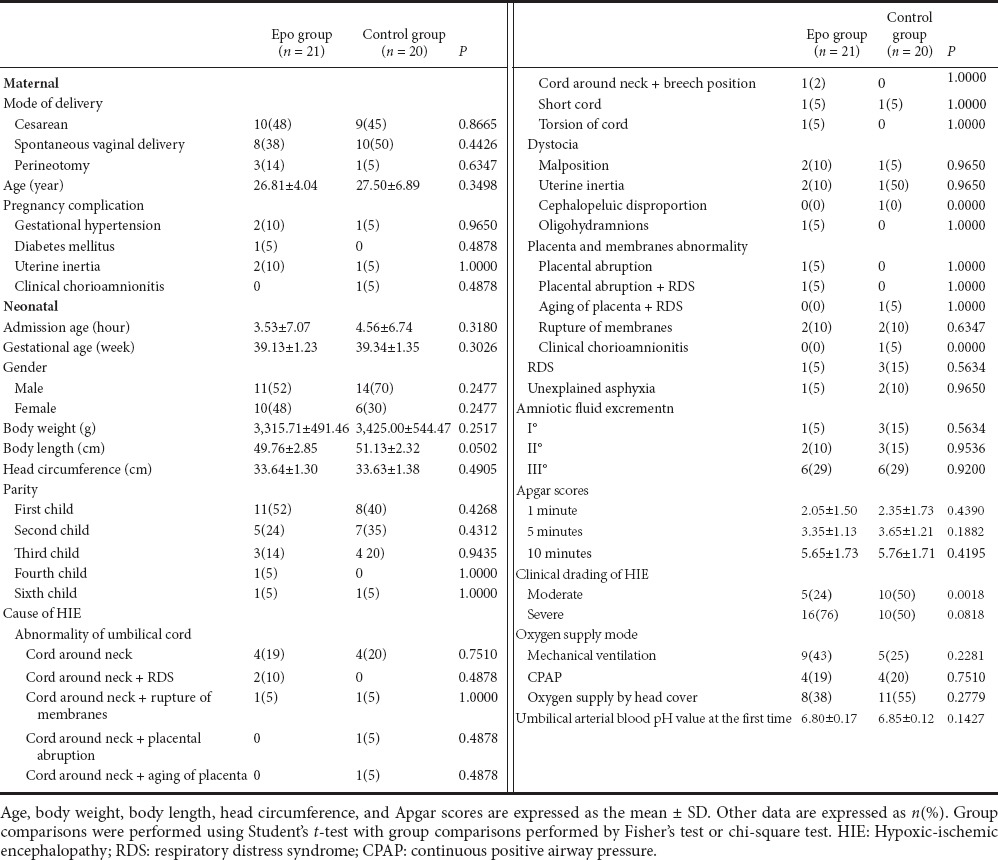
Characteristics of the subjects and their mothers are shown in Table 1. The parameters of gestational age, gender, weight, height, head circumference, mode of delivery, and maternal parameters were not statistically significant between the HIE and control groups (P > 0.05).
Table 1.
Characteristics and clinical parameters of mothers and neonates at admission
Comparison of serum tau protein levels
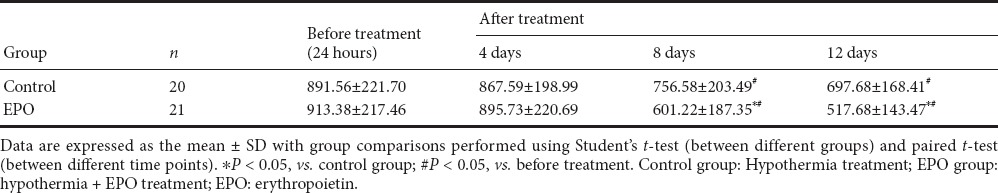
Before treatment, serum tau protein levels were not significantly different between the EPO and control groups (t = 0.3181, P > 0.05). After treatment, serum tau protein levels at 8 and 12 days were significantly lower in the EPO group than in the control group (8 days: t = 2.5450, 12 days: t =2.4119, respectively, P < 0.05; Table 2). Further analysis showed that serum tau protein levels demonstrated a gradual downward trend after treatment in both groups. In particular, at 8 and 12 days, tau protein levels were significantly lower after treatment compared with before treatment (EPO group: t = 4.9837, 6.9603, respectively, P < 0.05; control group: t = 2.0059, 3.1143, respectively, P < 0.05; Figure 1).
Table 2.
Serum tau levels (pg/mL) before and after treatment
Comparison of NBNA scores
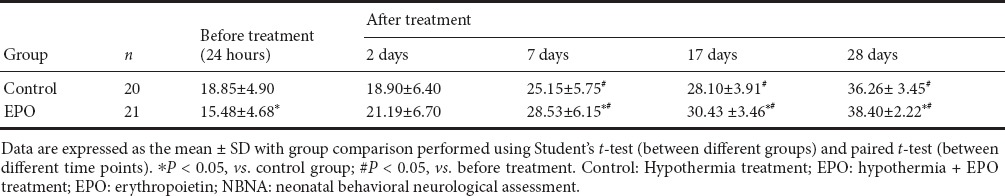
Before treatment, NBNA scores were significantly lower in the EPO group than in the control group (t = 1.9825, P < 0.05). After treatment, NBNA scores were significantly higher in the EPO group than in the control group at 7, 14, and 28 days (t = 1.8156, 2.3661, 1.7726, respectively, P < 0.05; Table 3). Further analysis showed that NBNA scores were significantly higher at 7, 14, and 28 days after treatment compared with before treatment in both groups (EPO group: t = 7.2382, 11.6591, 14.8660, respectively, P < 0.05; control group: t = 5.3332, 8.0463, 15.1480, respectively, P < 0.05).
Table 3.
NBNA scores before and after treatment
Correlation analysis between serum tau protein levels and NBNA scores
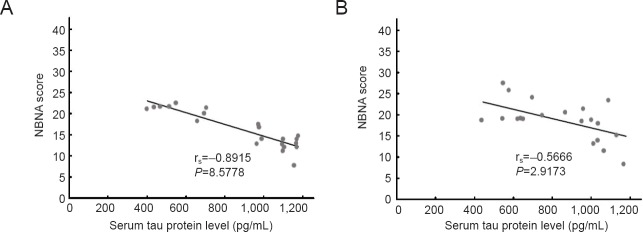
Before treatment, serum tau levels were significantly negatively correlated with NBNA scores in neonates with HIE in the EPO group (rs = −0.8915, P < 0.01). Serum tau levels were also significantly negatively correlated with NBNA scores in neonates with HIE in the control group (rs = −0.5666, P < 0.01).
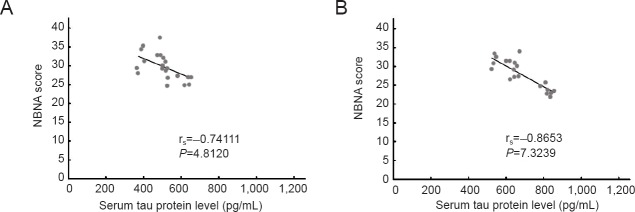
After treatment, serum tau protein levels at 12 days were significantly negatively correlated with NBNA scores at 14 days (EPO group: rs = −0.74111, P < 0.01; Control group: rs = −0.8653, P < 0.01; Figures 2, 3).
Figure 2.
Correlation analysis between serum tau protein levels and NBNA scores before treatment.
(A) EPO group (rs = −0.8915, P < 0.01); (B) control group (rs = −0.5666, P < 0.01). Correlation between serum tau protein levels and NBNA scores was tested by Spearman's rank-order correlation analysis. EPO: Erythropoietin.
Figure 3.
Correlation analysis between serum tau protein levels at 12 days and NBNA scores at 14 days after treatment.
(A) EPO group (rs = −0.74111, P < 0.01); (B) control group (rs = −0.8653, P < 0.01). Correlation between serum tau protein levels and NBNA scores was tested by Spearman's rank-order correlation analysis. EPO: Erythropoietin.
Neurodevelopmental outcome at 9 months of age
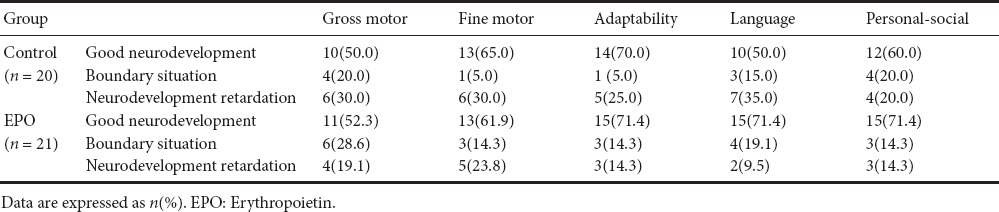
Neurodevelopmental outcome at 9 months of age was evaluated using the Gesell scale. The results are shown in Table 4. Neurodevelopmental outcome at 9 months after birth showed normal developmental rates of gross motor skills accounted for 52.3%, fine motor skills 61.9%, adaptation ability 71.4%, language 71.4%, and personal-social skills 71.4% of the EPO group. These values were not significantly different compared with the control group (all P > 0.05).
Table 4.
Neurodevelopmental outcome at 9 months of age by Gesell's assessment
Discussion
EPO is a neuroprotective agent and neurotrophic factor that has gained attention in the treatment of neonates with HIE. However, use of EPO is still controversial for improving short-term and long-term prognoses of neonatal HIE. In this study, 21 neonates with HIE were treated with a low dose of EPO (200 IU/kg). The effects of EPO on serum tau protein levels and neurological outcome were analyzed.
Brain injury biomarkers in serum or cerebrospinal fluid can be used to determine the severity and prognosis of neonatal HIE (Lv et al., 2015a; Graham et al., 2016). These markers are even considered as treatment effect indices in hypothermia or drug intervention measures. Tau protein, a microtubule protein of central neurons, is detached from the microtubule and enters into cerebrospinal fluid and blood after neuronal damage, and consequently is a marker of brain damage. Tau protein is a specific component of neurons with strong specificity. Normally, tau protein in brain tissue is phosphorylated and dephosphorylated in two forms, which are in a state of equilibrium. Tau protein over-phosphorylation can reduce binding ability to microtubules, resulting in separation of tau protein from microtubules, which causes neuronal damage and neurological dysfunction. Animal experiments show significantly increased tau protein after ischemic brain injury (Song et al., 2013). Clinical trials have also shown that tau protein levels are strongly associated with disease severity and infarct volume after adult cerebral ischemia (Bielewicz et al., 2011). Therefore, serum tau protein levels can directly reflect the severity of neuronal damage (Liliang et al., 2010; Randall et al., 2013). Our previous study found significantly higher serum tau protein levels in neonates with HIE than healthy neonates, as well as significantly higher levels in neonates with severe HIE than neonates with moderate HIE. To date, the effect of EPO on serum tau protein is not well known. Our results show that serum tau protein levels increase rapidly after the onset of hypoxia-ischemia. After treatment with EPO, serum tau protein levels showed a gradual declining trend, and were significantly lower from 8 to 12 days in the EPO group compared with the control group, suggesting that EPO prevents neuronal damage and promotes neuronal repair during early neonatal HIE onset. Dynamic detection of serum tau levels can help understanding of the pathological changes and disease development state in neonates with HIE, and also evaluate EPO efficacy. Protective effects and mechanisms of EPO on brain tissue after hypoxia-ischemia may be achieved in the following ways. (1) Promotion of neuronal differentiation and regeneration: animal experiments show that hypoxia can induce EPO expression. Further, EPO appears to regulate the occurrence of neural stem cells in the forebrain and promote formation of neural precursor cells. Altogether, these results suggest that EPO is involved in neural regeneration after hypoxia (Shingo et al., 2001). (2) Inhibition of neuronal apoptosis: Ma et al. (2013) showed that EPO can inhibit apoptosis of fetal rat brain cells after intrauterine hypoxic-ischemic injury. A rat model of hypoxic injury also showed that neuronal apoptosis was significantly decreased by rhEPO (Yamada et al., 2011). (3) Reducing and regulating the inflammatory response: animal experiments show that EPO can delay the increase of IL-1 beta in neonatal rats with ischemic brain injury, reduce leukocyte infiltration, and exert a neuroprotective function (Sun et al., 2005). (4) Anti-oxidative stress: EPO can enhance the activity of superoxide dismutase, glutathione peroxidase, and catalase in brain tissue. It also reduces oxidative damage of oxygen free radicals to nerve cells (Malhotra et al., 2004). (5) Anti-glutamate excitatory toxicity: in vitro experiments show that EPO reduces Ca2+-induced glutamate release from cultured cerebellar granule neurons and protects neurons (Kawakami et al., 2001).
Indeed, since entering the era of hypothermia, many lives of neonates with HIE have been saved. Accordingly, mortality by hypothermia and disability rates has been significantly reduced. Nevertheless, neonates with HIE are still at risk. To improve the therapeutic effect of neonatal HIE, EPO is used in combination with hypothermia in the field of experimental research. So far, the effect of EPO on neurodevelopmental outcome of neonates with HIE has mainly been obtained from animal experiments. Because of different times and dosages of EPO for treating neonatal HIE, there has been variable results (Kumral et al., 2004; Demers et al., 2005; Spandou et al., 2005; Iwai et al., 2007; Kellert et al., 2007; van der Kooij et al., 2009; Wu et al., 2016). No significant benefit was observed from treatment with either hypothermia or hypothermia-EPO combination therapy (Fang et al., 2013). This study also showed a significant neuroprotective effect of hypothermia on neonatal HIE rats. Further, EPO only slightly improved sensory function of neonatal rats with HIE, and did not restore brain tissue morphology (Fan et al., 2013). However, a prospective randomized clinical trial showed that rEPO reduced the risk of death or moderate/severe disability in HIE neonates without hypothermia, but had no such effect on severe HIE. Death or severe disability occurred in 35 cases (43.8%) of 80 patients in the control group, and 18 cases (24.6%) of 73 patients in the EPO group at 18 months of follow-up (Zhu et al., 2009). Elmahdy et al. (2010) demonstrated that EPO treatment improved background electroencephalography recordings and reduced serum nitric oxide concentration in 30 cases of neonatal HIE. Neonates in the HIE-EPO group had fewer neurological and developmental abnormalities after 6 months of age (Elmahdy et al., 2010). EPO improved fine motor skills, language skills, and perceptual development (Wang et al., 2011).
NBNA is a neurological examination that comprehensively evaluates neonatal behavior. It is widely used for early detection of neonatal brain injury-induced behavioral neurological anomalies. Moreover, GDS comprehensively reflects maturity of neonatal nervous movement and development of intelligence. Indeed, it objectively reflects the maturity of nervous movement, and mental and psychological development in children, and is also a diagnostic tool for evaluation of neurological motor damage and mental disorders. Therefore, we used these two methods to investigate neural developmental state in the neonatal period and infants at 9 months of age, respectively. In this study, we used rhEPO (200 IU/kg) for 10 consecutive days. Our results show significantly higher NBNA scores after treatment than before treatment in both the EPO group and control group. However, NBNA scores at 7, 14, and 28 days after treatment were significantly higher in the EPO group than control group. This indicates that EPO combined with hypothermia is superior to hypothermia alone in improving behavioral neurological outcomes in early neonatal HIE. With regard the effect of EPO on long-term neurodevelopment in neonates with HIE, we performed a follow-up at 9 months of age. Our results show that normal development rates of gross motor skills, fine motor skills, adaptive ability, language ability, and personal-social skills were higher in the EPO group than control group, but were not significantly different. These findings suggest that EPO combined with hypothermia is not superior to hypothermia alone in improving neurodevelopmental outcome of neonates with HIE at 9 months of age. This may be due to the low dose of EPO or whether the patient performed rehabilitation training after discharge.
It is worth noting that in addition to degree of HIE severity, long-term prognosis of neonates with moderate/severe HIE is also associated with whether the patient is treated with hypothermia and a neuroprotective drug during hospitalization and rehabilitation training after discharge. At the time of follow-up, we found the same clinical indicators and status in many infants at discharge. There were significant differences in neurodevelopmental outcome at 9 months of age. Rehabilitation training after discharge was performed in infants with good neurodevelopmental outcome. In contrast, rehabilitation training was not performed in infants with poor neurodevelopmental outcome. The status of infants receiving rehabilitation training for a longer time was better than those receiving training for a short time. Moreover, the status of infants receiving early rehabilitation training was better than those receiving late rehabilitation training. These results indicate that rehabilitation training can promote recovery of neurological function in infants with HIE, and its mechanism needs further study. Therefore, it is beneficial to reduce the incidence of neurodevelopmental defects in infants with HIE, which should be strengthened during rehabilitation training after discharge.
EPO for the treatment of neonatal brain injury has been used in clinical phase II testing. As far as our clinical observations are concerned, a low dose of EPO used in the early stages of neonatal HIE can decrease serum tau levels and prevent disease development, as well as improve neonatal early behavioral neurology outcome. EPO combined with hypothermia in neonates with HIE has a synergistic effect. Because the long-term neurodevelopmental outcome of patients is affected by many factors, such as rehabilitation training and training duration, the related factors need to be further studied. In addition, it is of importance to determine the optimal dosage of EPO in treatment of neonatal HIE.
Footnotes
Conflicts of interest: None declared.
Research ethics: The study was approved by the Medical Ethics Committee of Handan Maternal and Child Health Care Hospital of Hebei Province of China (approval No. 2014-1) and followed the principles of the Declaration of Helsinki. All guardians provided written informed consent for publication of associated data and accompanying images.
Declaration of parental/guardian consent: The authors certify that they have obtained all appropriate parental/guardian consent forms. In the form, parents/guardians have given their consent for their children's images and other clinical information to be reported in the journal. The parents/guardians understand that their children's names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Copyedited by James R, Frenchman B, Yu J, Li CH, Qiu Y, Song LP, Zhao M
Funding: This study was supported by a grant from the Health and Family Planning Commission of Hebei Province of China, No. 20150033; a grant from the Science and Technology Research and Development Project of Handan City of Hebei Province of China, No. 152810879-6.
References
- Ahearne CE, Boylan GB, Murray DM. Short and long term prognosis in perinatal asphyxia: An update. World J Clin Pediatr. 2016;5:67–74. doi: 10.5409/wjcp.v5.i1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, Goodwin J, Halliday HL, Juszczak E, Kapellou O, Levene M, Linsell L, Omar O, Thoresen M, Tusor N, Whitelaw A, Edwards AD TOBY Study Group. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371:140–149. doi: 10.1056/NEJMoa1315788. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kovac A, Majerova P, Bullock KM, Shi M, Zhang J. Tau Proteins Cross the Blood-Brain Barrier. J Alzheimers Dis. 2017;55:411–419. doi: 10.3233/JAD-160542. [DOI] [PubMed] [Google Scholar]
- Bao XL, Yu RJ, Li ZS, Zhang BL. Twenty-item behavioral neurological assessment for normal newborns in 12 cities of China. Chin Med J (Engl) 1991;104:742–746. [PubMed] [Google Scholar]
- Barnett A, Mercuri E, Rutherford M, Haataja L, Frisone MF, Henderson S, Cowan F, Dubowitz L. Neurological and perceptual-motor outcome at 5-6 years of age in children with neonatal encephalopathy: relationship with neonatal brain MRI. Neuropediatrics. 2002;33:242–248. doi: 10.1055/s-2002-36737. [DOI] [PubMed] [Google Scholar]
- Bielewicz J, Kurzepa J, Czekajska-Chehab E, Stelmasiak Z, Bartosik-Psujek H. Does serum Tau protein predict the outcome of patients with ischemic stroke. J Mol Neurosci. 2011;43:241–245. doi: 10.1007/s12031-010-9403-4. [DOI] [PubMed] [Google Scholar]
- Buonocore G, Perrone S, Turrisi G, Kramer BW, Balduini W. New pharmacological approaches in infants with hypoxic-ischemic encephalopathy. Curr Pharm Des. 2012;18:3086–3100. [PubMed] [Google Scholar]
- Dammann O, Durum S, Leviton A. Do white cells matter in white matter damage. Trends Neurosci. 2001;24:320–324. doi: 10.1016/s0166-2236(00)01811-7. [DOI] [PubMed] [Google Scholar]
- Demers EJ, McPherson RJ, Juul SE. Erythropoietin protects dopaminergic neurons and improves neurobehavioral outcomes in juvenile rats after neonatal hypoxia-ischemia. Pediatr Res. 2005;58:297–301. doi: 10.1203/01.PDR.0000169971.64558.5A. [DOI] [PubMed] [Google Scholar]
- Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmahdy H, El-Mashad AR, El-Bahrawy H, El-Gohary T, El-Barbary A, Aly H. Human recombinant erythropoietin in asphyxia neonatorum: pilot trial. Pediatrics. 2010;125:e1135–1142. doi: 10.1542/peds.2009-2268. [DOI] [PubMed] [Google Scholar]
- Fan X, van Bel F, van der Kooij MA, Heijnen CJ, Groenendaal F. Hypothermia and erythropoietin for neuroprotection after neonatal brain damage. Pediatr Res. 2013;73:18–23. doi: 10.1038/pr.2012.139. [DOI] [PubMed] [Google Scholar]
- Fang AY, Gonzalez FF, Sheldon RA, Ferriero DM. Effects of combination therapy using hypothermia and erythropoietin in a rat model of neonatal hypoxia-ischemia. Pediatr Res. 2013;73:12–17. doi: 10.1038/pr.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass HC, Ferriero DM. Treatment of hypoxic-ischemic encephalopathy in newborns. Curr Treat Options Neurol. 2007;9:414–423. doi: 10.1007/s11940-007-0043-0. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- Graham EM, Burd I, Everett AD, Northington FJ. Blood Biomarkers for Evaluation of Perinatal Encephalopathy. Front Pharmacol. 2016;7:196. doi: 10.3389/fphar.2016.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group of Neonatology, Chinese Pediatric Society, Chinese Medical Association (2005) Diagnostic criteria for neonatal hypoxic-ischemic encephalopathy. Zhonghua Er Ke Za Zhi. 43:584. [PubMed] [Google Scholar]
- Hu HT, Xiao F, Yan YQ, Wen SQ, Zhang L. The prognostic value of serum tau in patients with intracerebral hemorrhage. Clin Biochem. 2012;45:1320–1324. doi: 10.1016/j.clinbiochem.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38:2795–2803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M, Sekiguchi M, Sato K, Kozaki S, Takahashi M. Erythropoietin receptor-mediated inhibition of exocytotic glutamate release confers neuroprotection during chemical ischemia. J Biol Chem. 2001;276:39469–39475. doi: 10.1074/jbc.M105832200. [DOI] [PubMed] [Google Scholar]
- Kellert BA, McPherson RJ, Juul SE. A comparison of high-dose recombinant erythropoietin treatment regimens in brain-injured neonatal rats. Pediatr Res. 2007;61:451–455. doi: 10.1203/pdr.0b013e3180332cec. [DOI] [PubMed] [Google Scholar]
- Kim MS, Seo YK, Park HJ, Lee KH, Lee KH, Choi EJ, Kim JK, Chung HL, Kim WT. The neuroprotective effect of recombinant human erythropoietin via an antiapoptotic mechanism on hypoxic-ischemic brain injury in neonatal rats. Korean J Pediatr. 2010;53:898–908. doi: 10.3345/kjp.2010.53.10.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumral A, Uysal N, Tugyan K, Sonmez A, Yilmaz O, Gokmen N, Kiray M, Genc S, Duman N, Koroglu TF, Ozkan H, Genc K. Erythropoietin improves long-term spatial memory deficits and brain injury following neonatal hypoxia-ischemia in rats. Behav Brain Res. 2004;153:77–86. doi: 10.1016/j.bbr.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:329–338. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Levene MI, Sands C, Grindulis H, Moore JR. Comparison of two methods of predicting outcome in perinatal asphyxia. Lancet. 1986;1:67–69. doi: 10.1016/s0140-6736(86)90718-x. [DOI] [PubMed] [Google Scholar]
- Li J, Bo T, Chen TQ, Kuang XN, Yu Z, Zhang L, Zeng YD. Neurobehavioral development in preterm infants: a retrospective study of 181 cases. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16:696–700. [PubMed] [Google Scholar]
- Liliang PC, Liang CL, Lu K, Wang KW, Weng HC, Hsieh CH, Tsai YD, Chen HJ. Relationship between injury severity and serum tau protein levels in traumatic brain injured rats. Resuscitation. 2010;81:1205–1208. doi: 10.1016/j.resuscitation.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Lin CJ, Li JP, Zhang XL. Handbook of Infant Development. Beijing: Beijing Child Health Care Institute; 1986. [Google Scholar]
- Lv H, Wang Q, Wu S, Yang L, Ren P, Yang Y, Gao J, Li L. Neonatal hypoxic ischemic encephalopathy-related biomarkers in serum and cerebrospinal fluid. Clin Chim Acta. 2015a;450:282–297. doi: 10.1016/j.cca.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Lv W, Li WY, Xu XY, Jiang H, Bang OY. Bone marrow mesenchymal stem cells transplantation promotes the release of endogenous erythropoietin after ischemic stroke. Neural Regen Res. 2015b;10:1265–1270. doi: 10.4103/1673-5374.162759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YS, Zhou J, Liu H, Du Y, Lin XM. Effect of recombinant human erythropoietin on apoptosis of neural cells in fetal rats after intrauterine hypoxic-ischemic injury. Sichuan Da Xue Xue Bao Yi Xue Ban. 2013;44:31–35. [PubMed] [Google Scholar]
- Malhotra S, Nijhawan S, Rosenbaum DM. Erythropoietin (epoetin) as a protective factor for the brain. Curr Atheroscler Rep. 2004;6:301–306. doi: 10.1007/s11883-004-0062-1. [DOI] [PubMed] [Google Scholar]
- Malik J, Kim AR, Tyre KA, Cherukuri AR, Palis J. Erythropoietin critically regulates the terminal maturation of murine and human primitive erythroblasts. Haematologica. 2013;98:1778–1787. doi: 10.3324/haematol.2013.087361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson RJ, Juul SE. Erythropoietin for infants with hypoxic-ischemic encephalopathy. Curr Opin Pediatr. 2010;22:139–145. doi: 10.1097/MOP.0b013e328336eb57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AJ, Marks JD. Hypoxic ischemic brain injury: Potential therapeutic interventions for the future. Neoreviews. 2014;15:e177–e186. doi: 10.1542/neo.15-5-e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall J, Mortberg E, Provuncher GK, Fournier DR, Duffy DC, Rubertsson S, Blennow K, Zetterberg H, Wilson DH. Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: results of a pilot study. Resuscitation. 2013;84:351–356. doi: 10.1016/j.resuscitation.2012.07.027. [DOI] [PubMed] [Google Scholar]
- Reissmann KR, Udupa KB. Effect of erythropoietin on proliferation of erythropoietin-responsive cells. Cell Tissue Kinet. 1972;5:481–489. doi: 10.1111/j.1365-2184.1972.tb00386.x. [DOI] [PubMed] [Google Scholar]
- Roe KV. Correlations between Gesell scores in infancy and performance on verbal and non-verbal tests in early childhood. Percept Mot Skills. 1977;45:1131–1134. doi: 10.2466/pms.1977.45.3f.1131. [DOI] [PubMed] [Google Scholar]
- Saito T, Tojo A. Binding of erythropoietin to receptors on fetal mouse liver cells. Nihon Ketsueki Gakkai Zasshi. 1987;50:1565–1577. [PubMed] [Google Scholar]
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- Sasaki R, Masuda S, Nagao M. Erythropoietin: multiple physiological functions and regulation of biosynthesis. Biosci Biotechnol Biochem. 2000;64:1775–1793. doi: 10.1271/bbb.64.1775. [DOI] [PubMed] [Google Scholar]
- Schiefecker AJ, Dietmann A, Beer R, Pfausler B, Lackner P, Kofler M, Fischer M, Broessner G, Sohm F, Mulino M, Thome C, Humpel C, Schmutzhard E, Helbok R. Neuroinflammation is Associated with brain extracellular TAU-protein release after spontaneous subarachnoid hemorrhage. Curr Drug Targets. 2016 doi: 10.2174/1389450117666160201111804. doi: 10.2174/1389450117666160201111804. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Shi JP. The methods of sample size estimation in clinical study. Zhongguo Linchuang Kangfu. 2003;7:1569–1571. [Google Scholar]
- Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21:9733–9743. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Ao Q, Wang Z, Liu W, Niu Y, Shen Q, Zuo H, Zhang X, Gong Y. Phosphorylation of tau protein over time in rats subjected to transient brain ischemia. Neural Regen Res. 2013;8:3173–3182. doi: 10.3969/j.issn.1673-5374.2013.34.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandou E, Papadopoulou Z, Soubasi V, Karkavelas G, Simeonidou C, Pazaiti A, Guiba-Tziampiri O. Erythropoietin prevents long-term sensorimotor deficits and brain injury following neonatal hypoxia-ischemia in rats. Brain Res. 2005;1045:22–30. doi: 10.1016/j.brainres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Sun Y, Calvert JW, Zhang JH. Neonatal hypoxia/ischemia is associated with decreased inflammatory mediators after erythropoietin administration. Stroke. 2005;36:1672–1678. doi: 10.1161/01.STR.0000173406.04891.8c. [DOI] [PubMed] [Google Scholar]
- Sussmuth SD, Reiber H, Tumani H. Tau protein in cerebrospinal fluid (CSF): a blood-CSF barrier related evaluation in patients with various neurological diseases. Neurosci Lett. 2001;300:95–98. doi: 10.1016/s0304-3940(01)01556-7. [DOI] [PubMed] [Google Scholar]
- Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166:558–566. doi: 10.1001/archpediatrics.2011.1772. [DOI] [PubMed] [Google Scholar]
- Thoresen M, Whitelaw A. Therapeutic hypothermia for hypoxic-ischaemic encephalopathy in the newborn infant. Curr Opin Neurol. 2005;18:111–116. doi: 10.1097/01.wco.0000162850.44897.c6. [DOI] [PubMed] [Google Scholar]
- Thoresen M, Tooley J, Liu X, Jary S, Fleming P, Luyt K, Jain A, Cairns P, Harding D, Sabir H. Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology. 2013;104:228–233. doi: 10.1159/000353948. [DOI] [PubMed] [Google Scholar]
- van der Kooij MA, Groenendaal F, Kavelaars A, Heijnen CJ, van Bel F. Combination of deferoxamine and erythropoietin: therapy for hypoxia-ischemia-induced brain injury in the neonatal rat? Neurosci Lett. 2009;451:109–113. doi: 10.1016/j.neulet.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Perlman JM. Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics. 1997;100:1004–1014. doi: 10.1542/peds.100.6.1004. [DOI] [PubMed] [Google Scholar]
- Velly L, Pellegrini L, Guillet B, Bruder N, Pisano P. Erythropoietin 2nd cerebral protection after acute injuries: a double-edged sword. Pharmacol Ther. 2010;128:445–459. doi: 10.1016/j.pharmthera.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Verklan MT. The chilling details: hypoxic-ischemic encephalopathy. J Perinat Neonatal Nurs. 2009;23:59–68. doi: 10.1097/01.JPN.0000346221.48202.7e. quiz 69-70. [DOI] [PubMed] [Google Scholar]
- Wang J, Li J, Han L, Guo S, Wang L, Xiong Z, Chen Z, Chen W, Liang J. Serum tau protein as a potential biomarker in the assessment of traumatic brain injury. Exp Ther Med. 2016;11:1147–1151. doi: 10.3892/etm.2016.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Pan KL, Zhao XL, Qiang H, Cheng SQ. Therapeutic effects of erythropoietin on hypoxic-ischemic encephalopathy in neonates. Zhongguo Dang Dai Er Ke Za Zhi. 2011;13:855–858. [PubMed] [Google Scholar]
- Wu YW, Gonzalez FF. Erythropoietin: a novel therapy for hypoxic-ischaemic encephalopathy? Dev Med Child Neurol. 2015;57(Suppl 3):34–39. doi: 10.1111/dmcn.12730. [DOI] [PubMed] [Google Scholar]
- Wu YW, Bauer LA, Ballard RA, Ferriero DM, Glidden DV, Mayock DE, Chang T, Durand DJ, Song D, Bonifacio SL, Gonzalez FF, Glass HC, Juul SE. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics. 2012;130:683–691. doi: 10.1542/peds.2012-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YW, Mathur AM, Chang T, McKinstry RC, Mulkey SB, Mayock DE, Van Meurs KP, Rogers EE, Gonzalez FF, Comstock BA, Juul SE, Msall ME, Bonifacio SL, Glass HC, Massaro AN, Dong L, Tan KW, Heagerty PJ, Ballard RA. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: a phase II trial. Pediatrics. 2016:137. doi: 10.1542/peds.2016-0191. [DOI] [PubMed] [Google Scholar]
- Yamada M, Burke C, Colditz P, Johnson DW, Gobe GC. Erythropoietin protects against apoptosis and increases expression of non-neuronal cell markers in the hypoxia-injured developing brain. J Pathol. 2011;224:101–109. doi: 10.1002/path.2862. [DOI] [PubMed] [Google Scholar]
- Zheng RJ, Gao YH. Effect of mild hypothermia on nerve regeneration microenvironment of infarcted area in rat models of cerebral infarction. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:4013–4019. [Google Scholar]
- Zhu C, Kang W, Xu F, Cheng X, Zhang Z, Jia L, Ji L, Guo X, Xiong H, Simbruner G, Blomgren K, Wang X. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:e218–226. doi: 10.1542/peds.2008-3553. [DOI] [PubMed] [Google Scholar]
- Zubcevic S, Heljic S, Catibusic F, Uzicanin S, Sadikovic M, Krdzalic B. Neurodevelopmental follow up after therapeutic hypothermia for perinatal asphyxia. Med Arch. 2015;69:362–366. doi: 10.5455/medarh.2015.69.362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]