Keywords: nerve regeneration, peripheral nerve injury, end-to-side neurorrhaphy, motor-sensory specificity, rat, dorsal root ganglion, motor neuron, axon, cutaneous antebrachii medialis nerve, ulnar nerve, acetylcholinesterase staining, retrograde neuron tracing, neural regeneration
Abstract
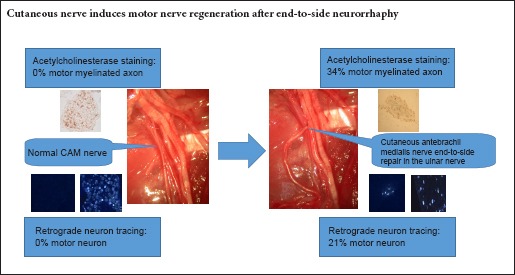
End-to-side neurorrhaphy is an option in the treatment of the long segment defects of a nerve. It involves suturing the distal stump of the disconnected nerve (recipient nerve) to the side of the intimate adjacent nerve (donor nerve). However, the motor-sensory specificity after end-to-side neurorrhaphy remains unclear. This study sought to evaluate whether cutaneous sensory nerve regeneration induces motor nerves after end-to-side neurorrhaphy. Thirty rats were randomized into three groups: (1) end-to-side neurorrhaphy using the ulnar nerve (mixed sensory and motor) as the donor nerve and the cutaneous antebrachii medialis nerve as the recipient nerve; (2) the sham group: ulnar nerve and cutaneous antebrachii medialis nerve were just exposed; and (3) the transected nerve group: cutaneous antebrachii medialis nerve was transected and the stumps were turned over and tied. At 5 months, acetylcholinesterase staining results showed that 34% ± 16% of the myelinated axons were stained in the end-to-side group, and none of the myelinated axons were stained in either the sham or transected nerve groups. Retrograde fluorescent tracing of spinal motor neurons and dorsal root ganglion showed the proportion of motor neurons from the cutaneous antebrachii medialis nerve of the end-to-side group was 21% ± 5%. In contrast, no motor neurons from the cutaneous antebrachii medialis nerve of the sham group and transected nerve group were found in the spinal cord segment. These results confirmed that motor neuron regeneration occurred after cutaneous nerve end-to-side neurorrhaphy.
Introduction
Peripheral nerve injury is a common result of trauma (Reyes et al., 2005; Chen et al., 2007; Sullivan et al., 2016). The incidence of peripheral nerve injury in patients with multiple injuries was 2–2.8% (Selecki et al., 1982; Noble et al., 1998), and this injury may cause severe disability. End-to-side neurorrhaphy is an alternative procedure for some nerve injury repairs (Lutz et al., 2000; Hanna and Dempsey, 2013; Isaacs, 2013; Gao et al., 2015; Li et al., 2015). It consists of suturing the distal stump of the disconnected nerve (recipient nerve) to the side of the intimate adjacent nerve (donor nerve) (Viterbo et al., 1998; Al-Qattan, 2001; Yan et al., 2002; Kakibuchi et al., 2004; Baltzer et al., 2016). This technique was used a long time ago, but was recently revived by Viterbo et al. (1992) in an experimental study of rats. Although the value of end-to-side nerve repair is still controversial, experimental evidence suggests that it promotes axonal growth and nerve regeneration in the receptors of motor or sensory nerves (Yuksel et al., 1999; De Sa et al., 2004; Hayashi et al., 2004; Haastert et al., 2010; Gao et al., 2012; Zheng et al., 2012; Hanna and Dempsey, 2013; Kettle et al., 2013; Civi et al., 2017).
We have concentrated our investigations on the motor-sensory specificity after end-to-side neurorrhaphy. In previous studies, we found that the motor or sensory specificity was not clear when a mixed nerve was the recipient nerve (Yu et al., 2011, 2013). In this study, we used a rat ulnar nerve (mixed sensory and motor) as the donor nerve and the cutaneous antebrachii medialis nerve as the recipient sensory nerve. The aim of this study was to assess whether a cutaneous nerve can induce motor nerve regeneration after end-to-side neurorrhaphy, and explore the sensory/motor specificity after end-to-side suturing.
Materials and Methods
Animals and surgery procedures
Thirty adult male Sprague-Dawley rats (~200 g, 8 weeks old) were housed and fed for 5 months at the Experimental Animal Center of Wenzhou Medical University of China (animal license No. SYXK (Zhe) 2010-0150).
Study protocols were approved by the Animal Ethics Committee of Wenzhou Medical University of China (approval No. 201601-005). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, revised 1986).
Rats were randomly divided into end-to-side group (n = 12), sham group (n = 12) and the transected nerve group (n = 6). Surgery was performed as described previously (Yu et al., 2011). Briefly, rats were anesthetized by intraperitoneal injection of ketamine (40 mg/kg, Shanghai Ziyuan Pharmaceutical Co., Ltd., Shanghai, China) and atropine (0.04 mg/kg, Shanghai Ziyuan Pharmaceutical Co., Ltd.). The right cutaneous antebrachii medialis nerve and ulnar nerves were exposed. For rats in the end-to-side group, we cut a window (diameter, 1 mm) in the ulnar nerve epineurium. The cutaneous antebrachii medialis nerve was transected at the root level, and the distal stump was then sutured to the ulnar nerve medial window with 10/0 non-invasive monofilament polyamide (Ethicon, San Angelo, TX, USA) to form an end-to-side junction. The proximal stump of the cutaneous antebrachii medialis nerve was ligated tightly and turned back to prevent spontaneous innervation (Figure 1). In the sham group, we exposed the cutaneous antebrachii medialis nerve and ulnar nerve, and then closed the skin. In the transected nerve group, we cut the cutaneous antebrachii medialis nerve, ligated the two ends, and turned them back to prevent spontaneous innervation. After regaining consciousness, the rats were housed separately in boxes in an approved animal house facility.
Figure 1.
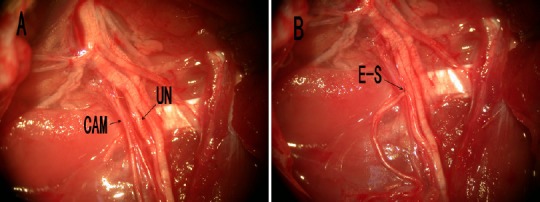
Surgical operation of the end-to-side neurorrhaphy.
(A) Anatomical view of the operated limb after careful dissection of the muscles and supplying nerves. (B) End-to-side neurorrhaphy (E-S) of the distal cutaneous antebrachii medialis nerve (CAM) to the ulnar nerve (UN).
Acetylcholinesterase staining
At 5 months after surgery (Yu et al., 2011), 1-cm segments were removed from the cutaneous antebrachii medialis nerve, distal to the junction (all groups, n = 6) and immediately fixed in 6% phosphate-buffered formaldehyde at 4°C for 1 hour, and then washed several times in the buffer. The nerves were embedded in 15% gelatin at 50°C for 1 hour, allowed to cool at 4°C and then sliced into cross-sections (15 μm) using a cryostat. The sections were mounted on slides and allowed to dry at room temperature. The slices were incubated for 24 hours at 4°C in Karnovsky and Roots medium containing 12.5 mg acetylthiocholine iodide (Shanghai Haoran Biological Technology Co., Ltd., Shanghai, China), 15.8 mL 0.1 M phosphate buffer (pH 6.5), 1.2 mL 0.1 M sodium citrate, 2.5 mL of 30 mM copper sulfate and 2.5 mL of distilled water. The staining control was tested by eliminating the addition of acetylthiocholine iodide from the incubation medium or by adding 10 M of eserine to the incubation medium. The sections were photographed with a Leica DM3000 microscope (Leica, Wetzlar, Germany) and myelinated fibers were counted and the percentage of stained myelinated fibers calculated.
Application of neuron tracer
Five months after surgery, the fluorescence retrograde axon tracer Fluoro-Gold (Sigma, St. Louis, MO, USA) was used to demonstrate axonal growth after end-to-side neurorrhaphy (McBride et al., 1990; Byers and Lin, 2003; Chiu et al., 2008; Chen et al., 2012). As described above, the animals were re-anesthetized, and 1 μL 3% Fluoro-Gold dyes were injected into the cutaneous antebrachii medialis nerve (end-to-side and sham groups, n = 6) using a Hamilton syringe. Retrograde neuron tracing was not used in the transected nerve group because the nerve had been cut off and could not transport the tracer retrogradely. To avoid contamination of adjacent tissues or leakage of dyes, the surrounding tissue was covered with filter paper infused by Vaseline (Sigma) and the filter paper was removed after injection. The wound was closed with 5/0 sutures. The retrograde transport of the dyes took 6 days.
Retrogradely labeled neurons
Animals survived for 6 days and were sacrificed by pentobarbital overdose. The rats were then perfused through the heart with 500 mL of warm Ringer solution + heparin 1,000 IE/kg/rat, followed by 500 mL of ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer. The spinal cord segments (C5-T1) and the corresponding dorsal root ganglion (DRG) were removed and placed in 30% sucrose/4% paraformaldehyde solution for 24 hours. Continuous frozen sections (50 μm for motor neurons in the spinal cord segment and 40 μm for DRG neurons) were cut with a cryostat and mounted on slides. Specimens were observed under a fluorescence microscope (DMLB2) with a filter (Leica G/R; Leica, Wetzlar, Germany) to observe Fluoro-Gold fluorescence staining. Neurons marked with yellow-white fluorescence with clearly visible nuclei were counted in the ventral horn of the spinal cord segment (C5-T1) and the corresponding DRG.
Statistical analysis
All data are consistent with the normal distribution and presented as the mean ± SD. One-way analysis of variance followed by the least significant difference post hoc test were used to analyze the data statistically with STATA 7.0 software (StataCorp, TX, USA). A value of P less than 0.05 was considered statistically significant.
Results
Acetylcholinesterase stain
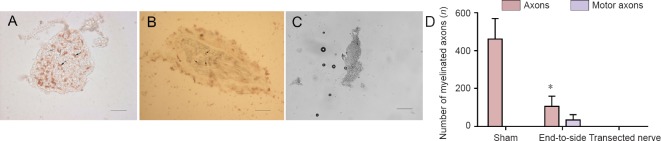
In the cutaneous antebrachii medialis nerve of the end-to-side group, there were 106 ± 54 myelinated axons but only 34 ± 28 myelinated axons (34 ± 16%) that showed acetylcholine esterase activity. In the sham group, of the 461.3 ± 108.3 myelinated axons, none were stained. The differences between groups were statistically significant (P < 0.05). There were a great many unmyelinated fibers that stained for acetylcholinesterase in the sham group. There were no axons in the nerve of the transected nerve group (Figure 2).
Figure 2.
Cutaneous antebrachii medialis nerve motor axons stained for acetylcholinesterase utilizing Karnovsky and Roots’ histochemical method, viewed through a Leica DM3000 microscope.
(A) Cutaneous antebrachii medialis nerve in the sham group: the stained unmyelinated fibers (black arrow) and the unstained myelinated fibers (white arrow). (B) Cutaneous antebrachii medialis nerve in end-to-side group: the stained myelinated fibers (black arrow). (C) Cutaneous antebrachii medialis nerve in the transected nerve group. Scale bars: 20 μm. (D) Quantification of myelinated axons: Number of myelinated nerve cross-sections in sham nerves and distal to the end-to-side anastomotic sites. Black represents the total number of myelinated nerves, and gray represents the number of motor nerves (stained for AChE). *P < 0.05, vs. sham group. All data are presented as the mean ± SD (n = 6; one-way analysis of variance followed by the least significant difference post hoc test).
Retrograde neuron tracing
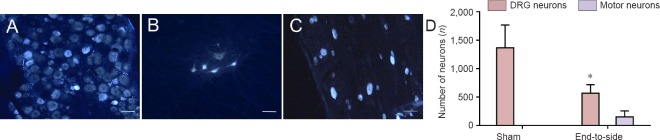
The retrogradely Fluoro-Gold labeled neurons were detected by yellow-white fluorescence on transverse sections through both the spinal cord segments and DRG (Naumann et al., 2000; Puigdellivol-Sanchez et al., 2002). In the sham group, no motor neuron for the cutaneous antebrachii medialis nerve was found in the spinal cord segment. In contrast, the percentage of motor neurons for the cutaneous antebrachii medialis nerve of the end-to-side group was 21 ± 5%. Statistically significant differences were found in the percentage of motor neurons labeled by yellow-white fluorescence (Figure 3). The percentage of Fluoro-Gold-positive DRG neurons was 100% in the sham group and 79 ± 5% in the end-to-side group.
Figure 3.
Regeneration of motor neuron cells in nerve tissue proximal to the site of end-to-side neurorrhaphy.
(A–C) Cryostat sections, stained with Fluoro-Gold, through the DRG (A, the sham group; C, end-to-side group) and corresponding ipsilateral spinal cord segment (B, end-to-side group) containing labeled neurons. (A, C) Sensory neurons of the DRG; (B) motor neuron of the spinal cord segment. Scale bars: 100 μm. (D) Total number of labeled motor neurons of the corresponding spinal cord segment and DRG sensory neurons for the cutaneous antebrachii medialis nerve in the end-to-side group and the sham group. *P < 0.05, vs. sham group. All data are presented as the mean ± SD (n = 6; one-way analysis of variance followed by the least significant difference post hoc test). DRG: Dorsal root ganglion.
Discussion
This study was conducted to explore whether a sensory cutaneous nerve can induce motor nerve regeneration after end-to-side neurorrhaphy. The results contribute to a better understanding of the motor-sensory specificity after end-to-side neurorrhaphy. The use of acetylcholinesterase stain as well as retrograde neuron tracing provides information on the origin of regenerating axons.
In the present study, motor and sensory nerve fibers were differentiated by Karnovsky and Roots’ histochemical method (Engel et al., 1980; He and Zhong, 1988). In the sham group, the myelinated nerve fibers were unstained, but a great many unmyelinated fibers were stained for acetylcholine esterase in amongst the unstained myelinated fibers. With end-to-side neurorrhaphy, we found significantly increased numbers of myelinated axons showing acetylcholinesterase activity. A total of 34% of the myelinated fibers of the recipient cutaneous antebrachii medialis nerve were stained, and percentages were similar to the donor ulnar nerve demonstrated by our previous report (Yu et al., 2011). The results confirmed that the regenerated axons showed the same acetylcholinesterase activity as the donor ulnar nerve after end-to-side neurorrhaphy.
Retrograde neuron tracing demonstrated that no motor neurons for the cutaneous antebrachii medialis nerve were found in the spinal cord segment in the sham group. In contrast, the proportion of motor neurons for the cutaneous antebrachii medialis nerve of the end-to-side group was 21%. The result is consistent with the acetylcholinesterase stain findings. The Fluoro-Gold tracer was injected into the nerve and would be incorporated into the axon when the axon was damaged during injection (Prodanov and Feirabend, 2008). As a result, the cell population labeled and quantified at DRGs and ventral horns would be underestimated.
End-to-side nerve repair is an alternative to the treatment of certain nerve damage, especially for surgeons confronted with a defect of a long segment of nerve (Koh et al., 2002; Kovacic et al., 2003, 2012; Barbour et al., 2012; Celis-Aguilar et al., 2013; Civi et al., 2017). Experimental studies published by Viterbo et al. (1992, 1994) and Lundborg et al. (1994) are generally considered to represent the rediscovery and development of the concept of end-to-side neurorrhaphy. They used the rat model to demonstrate that the denervated nerve stem connected to the donor nerve in an end-to-side manner stimulates the collateral sprouting of the axons from the intact donor nerve.
Consistent with our findings, Kalliainen et al. (1999) reported a good motor recovery after end-to-side suture. In their model, the distal stump of the peroneal nerve in the rat was sutured to the side of the tibial nerve. Despite the lower muscle mass and the higher percentage of denervated fibers, these authors did not observe any significant differences between the end-to-end and end-to-side groups associated with force contraction.
A good motor recovery after peripheral nerve injury requires the precise regeneration of the motor axons to its original target tissue and structure (Tessier-Lavigne and Goodman, 1996; Hoke et al., 2006; Wright et al., 2014). Brushart et al. (1988, 1998, 2005) found that motor axons regenerating after transection of mixed nerve preferentially reinnervate distal muscle branches and termed it preferential motor reinnervation. Madison et al. (1996) suggest that preferential motor reinnervation is potentially regulated by distal nerve pathways, terminal organs and motor neurons. The motor-sensory specificity after end-to-side neurorrhaphy is currently unclear. Our experiments show that cutaneous nerve can induce motor nerve regeneration after end-to-side neurorrhaphy and confirmed that motor-sensory specificity following end-to-side neurorrhaphy is not significant.
There are some limitations to our study. We did not test the efficiency and functional recovery of motor and sensory fibers. We also need to do further experiments with end-to-side neurorrhaphy of a motor nerve recipient as a control experiment and perform tests of function.
In conclusion, end-to-side nerve repair of a sensory nerve gives rise to successful motor nerve reinnervation. We also show no marked motor-sensory specificity after end-to-side neurorrhaphy of a sensory nerve recipient.
Footnotes
Conflicts of interest: None declared.
Research ethics: The study protocol was approved by Animal Ethics Committee of Wenzhou Medical University of China (approval No. 201601-005). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
References
- Al-Qattan MM. Terminolateral neurorrhaphy: review of experimental and clinical studies. J Reconstr Microsurg. 2001;17:99–108. doi: 10.1055/s-2001-12698. [DOI] [PubMed] [Google Scholar]
- Baltzer H, Woo A, Oh C, Moran SL. Comparison of Ulnar Intrinsic function following supercharge end-to-side anterior interosseous-to-ulnar motor nerve transfer: a matched cohort study of proximal ulnar nerve injury patients. Plastic Reconstrucsurg. 2016;138:1264–1272. doi: 10.1097/PRS.0000000000002747. [DOI] [PubMed] [Google Scholar]
- Barbour J, Yee A, Kahn LC, Mackinnon SE. Supercharged end-to-side anterior interosseous to ulnar motor nerve transfer for intrinsic musculature reinnervation. J Hand Surgery. 2012;37:2150–2159. doi: 10.1016/j.jhsa.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Brushart TM. Preferential reinnervation of motor nerves by regenerating motor axons. J Neurosci. 1988;8:1026–1031. doi: 10.1523/JNEUROSCI.08-03-01026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM, Gerber J, Kessens P, Chen YG, Royall RM. Contributions of pathway and neuron to preferential motor reinnervation. J Neurosci. 1998;18:8674–8681. doi: 10.1523/JNEUROSCI.18-21-08674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM, Jari R, Verge V, Rohde C, Gordon T. Electrical stimulation restores the specificity of sensory axon regeneration. Exp Neurol. 2005;194:221–229. doi: 10.1016/j.expneurol.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Byers MR, Lin KJ. Patterns of fluoro-gold entry into rat molar enamel, dentin, and pulp. J Dent Res. 2003;82:312–317. doi: 10.1177/154405910308200414. [DOI] [PubMed] [Google Scholar]
- Celis-Aguilar E, Lassaletta L, Roda JM, Gavilan J. End-to-Side interposed donor grafting as a facial nerve reinforcement technique after vestibular schwannoma surgery. Ann Otol Rhinol Laryngol. 2013;122:520–523. doi: 10.1177/000348941312200807. [DOI] [PubMed] [Google Scholar]
- Chen L, Hu M, Zhang L, Liu S, Luo J, Deng T, Tao Y. Motor fiber organization in the extratemporal trunk of the facial nerve in rats: a retrograde Fluoro-Gold study. Exp Ther Med. 2012;4:844–848. doi: 10.3892/etm.2012.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- Chiu K, Lau WM, Yeung SC, Chang RC, So KF. Retrograde labeling of retinal ganglion cells by application of fluoro-gold on the surface of superior colliculus. J Vis Exp. 2008 doi: 10.3791/819. doi: 10.3791/819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civi S, Durdag E, Aytar MH, Kardes O, Kaymaz F, Aykol S. Usefulness of end-to-side bridging anastomosis of sural nerve to tibial nerve: an experimental research. J Korean Neurosurg Soc. 2017;60:417–423. doi: 10.3340/jkns.2016.1010.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sa JM, Mazzer N, Barbieri CH, Barreira AA. The end-to-side peripheral nerve repair. Functional and morphometric study using the peroneal nerve of rats. J Neurosci Methods. 2004;136:45–53. doi: 10.1016/j.jneumeth.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Engel J, Ganel A, Melamed R, Rimon S, Farine I. Choline acetyltransferase for differentiation between human motor and sensory nerve fibers. Ann Plastic Surg. 1980;4:376–380. doi: 10.1097/00000637-198005000-00004. [DOI] [PubMed] [Google Scholar]
- Gao W, Liu Q, Li S, Zhang J, Li Y. End-to-side neurorrhaphy for nerve repair and function rehabilitation. J Surg Res. 2015;197:427–435. doi: 10.1016/j.jss.2015.03.100. [DOI] [PubMed] [Google Scholar]
- Gao WS, Dong CJ, Li SQ, Kunwar KJ, Li B. Re-innervation of the bladder through end-to-side neurorrhaphy of autonomic nerve and somatic nerve in rats. J Neurotrauma. 2012;29:1704–1713. doi: 10.1089/neu.2011.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haastert K, Joswig H, Jaschke KA, Samii M, Grothe C. Nerve repair by end-to-side nerve coaptation: histologic and morphometric evaluation of axonal origin in a rat sciatic nerve model. Neurosurgery. 2010;66:567–576. doi: 10.1227/01.NEU.0000365768.78251.8C. [DOI] [PubMed] [Google Scholar]
- Hanna A, Dempsey R. Nerve conduits used for end-to-side grafting after nerve injury. Neurosurgery. 2013;72:N15–16. doi: 10.1227/01.neu.0000426212.67089.72. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Yanai A, Komuro Y, Nishida M, Inoue M, Seki T. Collateral sprouting occurs following end-to-side neurorrhaphy. Plast Reconstr Surg. 2004;114:129–137. doi: 10.1097/01.prs.0000129075.96217.92. [DOI] [PubMed] [Google Scholar]
- He YS, Zhong SZ. Acetylcholinesterase: a histochemical identification of motor and sensory fascicles in human peripheral nerve and its use during operation. Plast Reconstr Surg. 1988;82:125–132. [PubMed] [Google Scholar]
- Hoke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs J. Supercharged end-to-side nerve transfer: too soon for “prime time". J Hand Surg. 2013;38:617–618. doi: 10.1016/j.jhsa.2012.12.041. [DOI] [PubMed] [Google Scholar]
- Kakibuchi M, Tuji K, Fukuda K, Terada T, Yamada N, Matsuda K, Kawai K, Sakagami M. End-to-side nerve graft for facial nerve reconstruction. Ann Plastic Surg. 2004;53:496–500. doi: 10.1097/01.sap.0000116283.76872.de. [DOI] [PubMed] [Google Scholar]
- Kalliainen LK, Cederna PS, Kuzon WM., Jr Mechanical function of muscle reinnervated by end-to-side neurorrhaphy. Plast Reconstr Surg. 1999;103:1919–1927. doi: 10.1097/00006534-199906000-00017. [DOI] [PubMed] [Google Scholar]
- Kettle SJ, Starritt NE, Glasby MA, Hems TE. End-to-side nerve repair in a large animal model: how does it compare with conventional methods of nerve repair. J Hand Surg Eur. 2013;38:192–202. doi: 10.1177/1753193412445119. [DOI] [PubMed] [Google Scholar]
- Koh KS, Kim J, Kim CJ, Kwun BD, Kim SY. Hypoglossal-facial crossover in facial-nerve palsy: pure end-to-side anastomosis technique. Br J Plastic Surg. 2002;55:25–31. doi: 10.1054/bjps.2001.3727. [DOI] [PubMed] [Google Scholar]
- Kovacic U, Sketelj J, Bajrovic FF. Sex-related difference in collateral sprouting of nociceptive axons after peripheral nerve injury in the rat. Exp Neurol. 2003;184:479–488. doi: 10.1016/s0014-4886(03)00269-3. [DOI] [PubMed] [Google Scholar]
- Kovacic U, Zele T, Tomsic M, Sketelj J, Bajrovic FF. Influence of breaching the connective sheaths of the donor nerve on its myelinated sensory axons and on their sprouting into the end-to-side coapted nerve in the rat. J Neurotrauma. 2012;29:2805–2815. doi: 10.1089/neu.2011.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhang P, Yin X, Jiang B. Early nerve protection with anterior interosseous nerve in modified end-to-side neurorrhaphy repairs high ulnar nerve injury: a hypothesis of a novel surgical technique. Artif Cells Nanomed Biotechnol. 2015;43:103–105. doi: 10.3109/21691401.2013.848873. [DOI] [PubMed] [Google Scholar]
- Lundborg G, Zhao Q, Kanje M, Danielsen N, Kerns JM. Can sensory and motor collateral sprouting be induced from intact peripheral nerve by end-to-side anastomosis? J Hand Surg. 1994;19:277–282. doi: 10.1016/0266-7681(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Lutz BS, Ma SF, Chuang DC, Wei FC. Role of the target in endto-side neurorrhaphy: reinnervation of a single muscle vs. multiple muscles. J Reconstr Microsurg. 2000;16:443–448. doi: 10.1055/s-2006-947151. [DOI] [PubMed] [Google Scholar]
- Madison DL, Kruger WH, Kim T, Pfeiffer SE. Differential expression of rab3 isoforms in oligodendrocytes and astrocytes. J Neurosci Res. 1996;45:258–268. doi: 10.1002/(SICI)1097-4547(19960801)45:3<258::AID-JNR7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- McBride RL, Feringa ER, Garver MK, Williams JK., Jr Retrograde transport of fluoro-gold in corticospinal and rubrospinal neurons 10 and 20 weeks after T-9 spinal cord transection. Exp Neurol. 1990;108:83–85. doi: 10.1016/0014-4886(90)90011-g. [DOI] [PubMed] [Google Scholar]
- Naumann T, Hartig W, Frotscher M. Retrograde tracing with Fluoro-Gold: different methods of tracer detection at the ultrastructural level and neurodegenerative changes of back-filled neurons in long-term studies. J Neurosci Methods. 2000;103:11–21. doi: 10.1016/s0165-0270(00)00292-2. [DOI] [PubMed] [Google Scholar]
- Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116–122. doi: 10.1097/00005373-199807000-00025. [DOI] [PubMed] [Google Scholar]
- Prodanov D, Feirabend HK. Automated characterization of nerve fibers labeled fluorescently: determination of size, class and spatial distribution. Brain Res. 2008;1233:35–50. doi: 10.1016/j.brainres.2008.07.049. [DOI] [PubMed] [Google Scholar]
- Puigdellivol-Sanchez A, Valero-Cabre A, Prats-Galino A, Navarro X, Molander C. On the use of fast blue, fluoro-gold and diamidino yellow for retrograde tracing after peripheral nerve injury: uptake, fading, dye interactions, and toxicity. J Neurosci Methods. 2002;115:115–127. doi: 10.1016/s0165-0270(01)00532-5. [DOI] [PubMed] [Google Scholar]
- Reyes O, Sosa I, Kuffler DP. Promoting neurological recovery following a traumatic peripheral nerve injury. P R Health Sci J. 2005;24:215–223. [PubMed] [Google Scholar]
- Selecki BR, Ring IT, Simpson DA, Vanderfield GK, Sewell MF. Trauma to the central and peripheral nervous systems. Part II: a statistical profile of surgical treatment New South Wales 1977. Aust N Z J Surg. 1982;52:111–116. doi: 10.1111/j.1445-2197.1982.tb06081.x. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Dailey T, Duncan K, Abel N, Borlongan CV. Peripheral nerve injury: stem cell therapy and peripheral nerve transfer. Int J Mol Sci. 2016;17:2101. doi: 10.3390/ijms17122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Viterbo F, Trindade JC, Hoshino K, Mazzoni Neto A. Latero-terminal neurorrhaphy without removal of the epineural sheath. Experimental study in rats. Rev Paul Med. 1992;110:267–275. [PubMed] [Google Scholar]
- Viterbo F, Trindade JC, Hoshino K, Mazzoni Neto A. End-to-side neurorrhaphy with removal of the epineurial sheath: an experimental study in rats. Plast Reconstr Surg. 1994;94:1038–1047. doi: 10.1097/00006534-199412000-00019. [DOI] [PubMed] [Google Scholar]
- Viterbo F, Teixeira E, Hoshino K, Padovani CR. End-to-side neurorrhaphy with and without perineurium. Sao Paulo Med J. 1998;116:1808–1814. doi: 10.1590/s1516-31801998000500005. [DOI] [PubMed] [Google Scholar]
- Wright MC, Mi R, Connor E, Reed N, Vyas A, Alspalter M, Coppola G, Geschwind DH, Brushart TM, Hoke A. Novel roles for osteopontin and clusterin in peripheral motor and sensory axon regeneration. J Neurosci. 2014;34:1689–1700. doi: 10.1523/JNEUROSCI.3822-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan JG, Matloub HS, Sanger JR, Zhang LL, Riley DA, Jaradeh SS. A modified end-to-side method for peripheral nerve repair: large epineurial window helicoid technique versus small epineurial window standard end-to-side technique. J Hand Surg. 2002;27:484–492. doi: 10.1053/jhsu.2002.32953. [DOI] [PubMed] [Google Scholar]
- Yu Q, Lin ZK, Ding J, Wang T, Chi YL, Gao WY. Functional motor nerve regeneration without motor-sensory specificity following end-to-side neurorrhaphy: an experimental study. J Hand Surg. 2011;36:2010–2016. doi: 10.1016/j.jhsa.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Yu Q, Chen C, Zhang X, Lv L, Lin K, Chi Y, Gao W. Quantitative assessment of the motor-sensory specificity of the motor and primary sensory neurons after the end-to-side neurorrhaphy. J Reconstr Microsurg. 2013;29:579–586. doi: 10.1055/s-0033-1348036. [DOI] [PubMed] [Google Scholar]
- Yuksel F, Karacaoglu E, Guler MM. Nerve regeneration through side-to-side neurorrhaphy sites in a rat model: a new concept in peripheral nerve surgery. Plast Reconstr Surg. 1999;104:2092–2099. doi: 10.1097/00006534-199912000-00022. [DOI] [PubMed] [Google Scholar]
- Zheng MX, Xu WD, Shen YD, Xu JG, Gu YD. Reconstruction of elbow flexion by end-to-side neurorrhaphy in phrenic nerve transfer. Plast Reconstr Surg. 2012;129:573e–575e. doi: 10.1097/PRS.0b013e3182419c00. [DOI] [PubMed] [Google Scholar]