Abstract
Remyelination plays a key role in functional recovery of axons after spinal cord injury. Glial cells are the most abundant cells in the central nervous system. When spinal cord injury occurs, many glial cells at the lesion site are immediately activated, and different cells differentially affect inflammatory reactions after injury. In this review, we aim to discuss the core role of oligodendrocyte precursor cells and crosstalk with the rest of glia and their subcategories in the remyelination process. Activated astrocytes influence proliferation, differentiation, and maturation of oligodendrocyte precursor cells, while activated microglia alter remyelination by regulating the inflammatory reaction after spinal cord injury. Understanding the interaction between oligodendrocyte precursor cells and the rest of glia is necessary when designing a therapeutic plan of remyelination after spinal cord injury.
Keywords: nerve regeneration, spinal cord injury, remyelination, oligodendrocyte precursor cells, astrocytes, oligodendrocytes, microglia, glial scar, demyelination, myelin, central nervous system, neural regeneration
Introduction
Spinal cord injury (SCI) is common and involves widespread damage to the central nervous system (CNS). SCI often leads to severe neurological symptoms such as varying degree of paralysis, paresthesia, urinary obstruction, and other progressive neurological abnormalities. SCI also involves social loss: data on western countries show that governments spend $40,000–$180,000 for each patient depending on the site of injury. Patients lose their jobs and receive medical treatment, rehabilitation, and maintenance, and each patient costs the country millions of dollars (Ning et al., 2012). In the 1920s, SCI cases increased from 6.7 to 60 per million in some regions of China (Ning et al., 2012).
The pathological process of SCI can be divided into two stages: primary injury and secondary injury. Primary injury occurs immediately after the initial injury, and its pathological processes include demyelination of the spinal cord and necrosis of neurons and axons (Yu et al., 2016). Secondary injury occurs throughout the disease, and its pathological processes include demyelination, axonal and neuronal necrosis, nervous tissue ischemia and edema, oxidative stress, inflammatory reaction, and glial scar formation (Balentine, 1978; Kwo et al., 1989; Wrathall et al., 1996; Azbill et al., 1997; Ray et al., 2016). Among these pathological reactions, demyelination occurs immediately after injury, and is induced by oligodendrocyte necrosis after mechanical damage. At the stage of secondary injury, because of extensive apoptosis and autophagy of oligodendrocytes, axons that have not been damaged or are slightly damaged become necrotic owing to demyelination (Almad et al., 2011).
Myelin can be regenerated. When demyelinating lesions occur, newly generated oligodendrocytes can repair or reconstruct damaged myelin. Regeneration of myelin, with oligodendrocyte generation as the main physiological process, can last up to three months after SCI. A recent study found that most oligodendrocytes required for remyelination after demyelination are derived from oligodendrocyte precursor cells (OPCs) and neural progenitor cells. OPCs can be labeled by neural/glial antigen 2 (NG2) or platelet-derived growth factor (PDGF) receptor alpha, and show very active proliferation in the CNS (Alizadeh et al., 2015). Previously, OPCs were discovered to have a role in repairing myelin (Hackett and Lee, 2016). Moreover, OPCs have been called the fourth glial cells, in addition to astrocytes, microglia, and oligodendrocytes. OPCs become mature oligodendrocytes through migration, proliferation, differentiation, and maturation, and subsequently repair injured myelin. Nevertheless, the amount of new myelin is unable to cover all exposed axons, and the remyelination rate cannot keep up with the speed of demyelination. A negative myelin balance increases the number of naked axons, and thereby results in disability, degeneration, and sensory and motor disorders in residual nerves. In the CNS, the interaction between various glial cells and neurons is consistently demonstrated. Further, many of the physiological and pathological responses are strongly associated with intercellular biological signaling pathways. Increasing evidence shows that cellular interactions play a significant role in demyelination and remyelination (Domingues et al., 2016). After central nerve injury, various glial cells directly or indirectly damage myelin. Simultaneously, these glial cells also affect myelin regeneration. Here, the aim of this review is to summarize latest research results and discuss the effect of glial cells on remyelination in nervous tissue after SCI.
Myelin and Demyelination
Myelin is composed of cytoplasm and the membrane of oligodendrocytes and Schwann cells. Myelin wraps around axons forming a special sheath-like structure. In the nervous system, the resistance of myelin is high, which reduces the capacitance of ensheathed axons. Consequently, myelin provides the structural basis for saltatory conduction of nerve signals (Nave and Werner, 2014). Myelin also provides nutritional support for ensheathed axons (Li and Leung, 2015). Only CNS oligodendrocytes generate myelin. Moreover, oligodendrocytes are associated with nerve signal transduction. A previous study suggested that myelin damage leads to abnormal neurological behavior (Love, 2006). Demyelination occurs immediately after SCI. The mechanism of demyelination remains unclear, but one likely reason is death of oligodendrocytes induced by various factors (Nave and Trapp, 2008). These factors include tumor necrosis factor-alpha- and interleukin-1 beta-mediated inflammatory reactions, glucose–adenosine triphosphate-mediated cytotoxicity, edema, and various free radical-induced ischemia/reperfusion injury (Almad et al., 2011; Plemel et al., 2014). A previous study demonstrated that a single oligodendrocyte can be involved with 30–80 axons, with each connection wrapping into an internode (O’Rourke et al., 2014). Thus, accidental death of each oligodendrocyte can cause a series of demyelination (Chong et al., 2012; Young et al., 2013). Physiologically, there is a special signaling pathway between myelin and the axon, with one reason for demyelination being that the axon–oligodendrocyte signaling pathway is damaged after axonal injury (Alizadeh et al., 2015). In the absence of axonal nutritional support, oligodendrocyte degeneration rapidly occurs, resulting in demyelinating lesions (Lappe-Siefke et al., 2003).
OPCs and Remyelination
OPCs are small cells of bipolar or tripolar structure, which can be found in the white and gray matter of the CNS. The number of OPCs is greater in the white matter than gray matter (Dawson et al., 2003; Dincman et al., 2012). As precursor cells, a new view of OPC outcome has recently been developed from in vivo and in vivo studies. Purified rat OPCs can dedifferentiate into neural stem cells and then differentiate into neurons, oligodendrocytes, and type I and II astrocytes (Kondo and Raff, 2000; Belachew et al., 2003; Nunes et al., 2003). In contrast, Tognatta et al. (2017) meticulously labeled differentiating OPCs in mice with 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP)-Cre, but obtained insufficient evidence of OPC differentiation to neurons. OPCs can be specifically labeled by PDGF receptor alpha and NG2 proteoglycans (Tripathi and McTigue, 2007; Barnabe-Heider et al., 2010). OPCs can directly differentiate into oligodendrocytes without cell division (Hughes et al., 2013), but do not express NG2 after differentiating into oligodendrocytes.
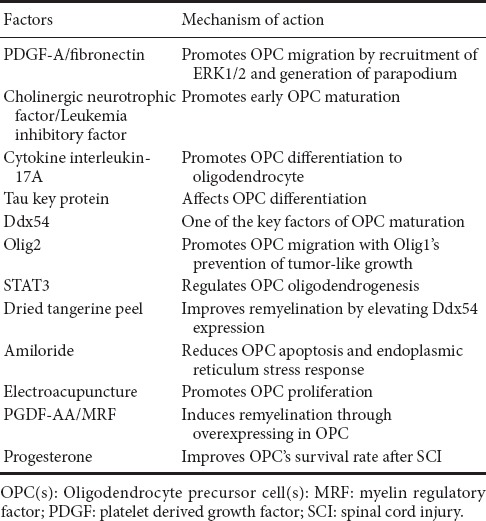
After demyelination following SCI, remyelination occurs spontaneously on residual axons (Salgado-Ceballos et al., 1998; Zawadzka et al., 2010). During remyelination, some lost oligodendrocytes are replaced by OPCs. After injury, OPCs migrate to the injury site and rapidly proliferate. From the day of injury to day 7, the number of OPCs persistently increases, with high levels maintained within one month. Under permissible conditions, these OPCs differentiate into oligodendrocytes outside myelin (Franklin and Ffrench-Constant, 2008; Hesp et al., 2015). This process can be regulated by various signaling pathways such as neurenergen, growth factors, cytokines, and transcription factors (Table 1). Previous studies have confirmed that neurotrophic factor-3, fibronectin, and PDGF-A promote OPC proliferation (Barres and Raff, 1994; Hill et al., 2013). PDGF-A and fibronectin promote OPC migration by recruitment of phosphorylated extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) and generation of parapodium (Tripathi et al., 2016). Cholinergic neurotrophic factor and leukemia inhibitory factor promote early maturation of OPCs. Knocking out these two cytokines causes delayed OPC development (Mayer et al., 1994; Barres et al., 1996; Ishibashi et al., 2009). Cytokine interleukin-17A promotes differentiation of OPCs into oligodendrocytes. Moreover, expression of key proteins (such as tau) also alters remyelination by affecting OPC differentiation (Ossola et al., 2016). Another study suggested that DEAD-box helicase 54 (Ddx54) may be a key factor for promoting OPC maturation (Tokunaga et al., 2016). Oligodendrocyte transcription factor 2 (Olig2) promotes OPC migration and myelination. Interestingly, single action of Olig2 leads to tumor-like growth of OPCs, and an interaction between Oligo1 and Oligo2 prevents the tumor-like growth pattern (Kim et al., 2011; Wegener et al., 2015). Hackett et al. (2016) revealed that deficit of signal transducer and activator of transcription 3 (STAT3) leads to reduced oligodendrogenesis, while knocking out suppressor of cytokine signaling 3 (SOCS3) results in enhanced OPC proliferation after SCI.
Table 1.
Factors known that regulate remyelination via different effects on OPCs
Nevertheless, the quality and integrity of regenerated myelin cannot meet demands owing to environmental change after injury (Alizadeh et al., 2015). In the microenvironment after SCI, degenerative myelin secretes many inhibitory molecules. Simultaneously, the extracellular matrix, glial cell proliferation, and downregulation of nutrients and growth factors affect remyelination (Meletis et al., 2008; Gauthier et al., 2013; Lukovic et al., 2015). The extracellular matrix can inhibit remyelination by blocking OPC migration (Siebert et al., 2011). Interleukin-beta limits OPC recruitment by activating the interleukin-1 receptor type 1 pathway (Kuroiwa et al., 2014). The glial scar produced by glial cells not only hinders OPC migration, but also results in a microenvironment that is not suitable for OPC proliferation. Degenerative myelin activates multiple microglial signaling pathways leading to release of inflammatory mediators (Sun et al., 2010). These molecules and cytokines inhibit axonal regeneration and destroy myelin integrity through the complement system (Chen et al., 2000).
In summary, there are several reasons for lack of remyelination: (1) the remyelination process lacks the necessary growth factors for promoting formation of intact mature myelin from newborn oligodendrocytes; or (2) there is death of newborn OPCs as there are not enough biochemical factors to promote production of related cells and myelin. Consequently, the microenvironment at the injured site after SCI has an inhibitory effect on remyelination. In view of this, OPCs should be at the core of studies on remyelination, relieving inhibition, and promoting proliferation and differentiation of OPCs.
Recently, increasing research has focused on promoting remyelination by improving OPC migration, proliferation, differentiation, and maturation after SCI. Many drugs, hormones, and even treatments have been used clinically and are shown to be effective (Table 1). A previous study reported that as a hormone, progesterone improves OPC survival rate at the injury site by mitigating the inflammatory response and improving reactive gliosis after SCI (Huang et al., 2015). The Chinese herbal medicine, dried tangerine peel, can improve remyelination by increasing bone morphogenetic protein (BMP) 2.5 expression and elevating Ddx54 expression in cerebral ventricles, the subventricular zone, and corpus callosum (Tokunaga et al., 2016). Amiloride is a potassium-conserving diuretic that has been shown to promote remyelination by reducing the endoplasmic reticulum stress response and reducing OPC apoptosis (Kuroiwa et al., 2014). In addition to these drugs, there is evidence that physical therapy also has a role in promoting remyelination. Huang et al. (2015) reported that electroacupuncture promotes OPC proliferation, reduces OPC death, and improves remyelination. There have also been breakthroughs in promoting remyelination by overexpressing certain molecules in OPCs. Yao et al. (2017) reported that PGDF-AA-overexpressing OPC transplantation in rats induces remyelination. Myelin regulatory factor (MRF)overexpression was also reported to stimulate OPC differentiation (Xie et al., 2016). Although the mechanism of remyelination is not fully understood, there are numerous ways to promote remyelination. Most of these methods are supported by compelling evidence, but there is still considerable distance between these factors and clinical applications, and a need for continued innovation.
Astrocytes and Remyelination
Astrocytes are widely present in the CNS. They are the most abundant glial cells in white matter and gray matter, and have a crucial role in neurophysiology. A recent study demonstrated that two kinds of astrocytes in brain tissue: fibrous astrocytes in the white matter of the corpus callosum, and protoplasmic astrocytes in the gray matter (Ding, 2014). The primary function of astrocytes was initially thought to support and supply neurons, but nowadays there is plenty of evidence showing that astrocytes are strongly associated with microglia, oligodendrocytes, and other astrocytes in the nervous system. Astrocytes regulate neurotransmitters, participate in synaptogenesis, mediate the immune response, express extracellular matrix molecules, promote cell migration, and promote differentiation and maturation of the CNS (Walz, 1989; Westergaard et al., 1995; Sofroniew and Vinters, 2010; Clarke and Barres, 2013). Astrocytes are associated with many pathological CNS processes, including inflammation, ischemia, infection, and degeneration. After activation, changes in cell morphology, gene expression, and cell physiology are observed in astrocytes (Sofroniew and Vinters, 2010). A previous study confirmed that astrocytes directly affect proliferation and survival of the oligodendrocyte line (Li et al., 2016), demonstrating that oligodendrocytes are strongly associated with remyelination. Astrocytes are involved in regulating the balance between Schwann cells and oligodendrocyte remyelination, with oligodendrocyte remyelination only observed in areas where astrocytes are present. A recent study showed that testosterone promoted oligodendrocyte remyelination via astrocyte recruitment (Bielecki et al., 2016). Indeed, increasing evidence shows that astrocytes directly or indirectly affect remyelination by acting on OPCs or oligodendrocytes (Figure 1).
Figure 1.
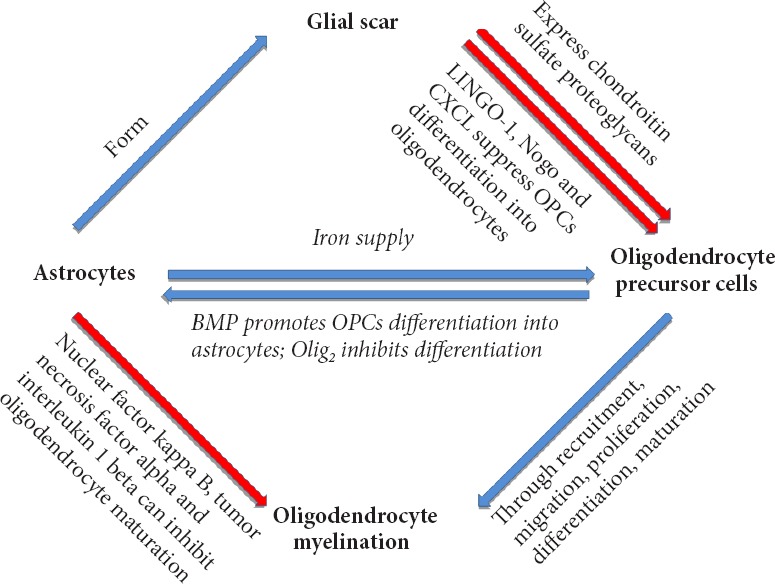
Astrocytes affect remyelination by affecting OPC differentiation and maturation or directly acting on oligodendrocytes.
BMP: Bone morphogenetic protein; OPC: oligodendrocyte precursor cell.
OPCs and astrocytes are homologous during development, and OPCs can directly differentiate into astrocytes in vitro (Raff et al., 1983). Furthermore, an in vivo study found that immature astrocytes are present within NG2+ cells after SCI (Lytle et al., 2009). While another study confirmed that after SCI, OPCs that differentiate into oligodendrocytes are limited. Further, some OPCs (4–13%) do not differentiate into oligodendrocytes, and instead differentiate into astrocytes (Sozmen et al., 2016). OPC differentiation into astrocytes will affect remyelination. BMP and Olig2 may be involved in differentiation of OPCs into astrocytes. Regarding BMP, current understanding is that BMP4 increases after SCI, with its potential source being reactive astrocytes after injury (Wang et al., 2011). BMP4 contributes to OPC differentiation into astrocytes, although BMP4 antagonists have only a limited inhibitory effect on differentiation into astrocytes (Hampton et al., 2007). Olig2 may inhibit OPC differentiation into astrocytes: Olig2 overexpression reduces differentiation of neural stem cells into astrocytes in vitro (Fukuda et al., 2004). During development, a large number of Olig2 knockout OPCs differentiate into astrocytes instead of myelin (Zhu et al., 2012). Reticulon 4 receptor (NgR1) is a Nogo receptor that can suppress OPC differentiation into oligodendrocytes. Its antagonist promotes OPC differentiation into mature oligodendrocytes (Hampton et al., 2007; Sozmen et al., 2016). Molecules that promote OPC differentiation into astrocytes also include hyaluronan, janus kinase (JAK)-Stat1, and jagged-1 (Back et al., 2005; Zhang et al., 2009). In addition, inhibition of leucine rich repeat and Ig domain containing 1 (LINGO-1) promotes OPC differentiation into mature oligodendrocytes, and LINGO-1 inhibitors have been used for treatment of multiple sclerosis (Mi et al., 2013). In conclusion, OPC differentiation into astrocytes and oligodendrocytes ensures remyelination is a fluctuating process. Specifically, excessive OPC differentiation into astrocytes reduces the number of mature oligodendrocytes. Astrocytes have a significant inhibitory effect on remyelination and axonal regeneration. Thus, recovery of neurological function worsens after SCI. Inhibition of astrocyte differentiation contributes to remyelination and ensures recovery of neurological function after SCI.
After SCI, astrocytes are immediately activated in the acute stage, and proliferate and secrete many cytokines, chemokines, and ligands in the subacute stage. These molecules have a certain inhibitory effect on remyelination. Activated astrocytes express the transcription factor, nuclear factor kappa B, to damage the oligodendrocyte line. Brambilla et al. (2014) verified that inflammatory cell infiltration is reduced to protect oligodendrocytes in GFAP-IκBα-dn mice after inactivation of astroglial nuclear factor kappa B. Furthermore, knocking out components of the nuclear factor kappa B signaling pathway in astrocytes (e.g., actin [ACT1] and inhibitor of kappa B [IkB] kinase 2) protects oligodendrocytes (Raasch et al., 2011). Cytokines that regulate astrocyte activation, such as tumor necrosis factor-alpha and interleukin-1 beta, are associated with oligodendrocyte survival (Deng et al., 2014). C-X-C motif chemokine ligand 10 (CXCL10) is expressed by astrocytes after the inflammatory response to suppress OPC differentiation (Moore et al., 2015). Astrocytes can excessively secrete fibroblast growth factor-2, which not only promotes OPC proliferation and growth, but also inhibits OPC maturation (Goddard et al., 1999). In certain environments, fibroblast growth factor-2 transforms mature oligodendrocytes into new forms (Bansal and Pfeiffer, 1997). A previous study demonstrated that ligand molecules secreted by astrocytes (such as Nogo-A) are strongly associated with the NOGO receptor complex, and that this complex can inhibit remyelination (Ji et al., 2006). Many astrocyte-associated ligands, such as p75, TROY, and BLyS, inhibit regeneration of the nervous system. Indeed, their interaction also provides evidence for the inhibitory effect of astrocytes on remyelination through the corresponding signaling pathways (Schwab and Strittmatter, 2014). Astrocytes are important carriers of iron in the CNS, and physiological activities of OPCs and oligodendrocytes require extracellular uptake of large amounts of iron (Todorich et al., 2009; Badaracco et al., 2010). Thus, destruction of the astrocyte-involved ferritin supply chain will affect remyelination.
Activated astrocytes lead to specific reactive gliosis. During this process, their morphology changes significantly and a large amount of intermediate filament proteins, mainly glial fibrillary acidic protein (GFAP) and nestin, are secreted to form the glial scar (Karimi-Abdolrezaee and Billakanti, 2012). There are two sources of activated astrocytes after SCI: (1) ependymal cell GFAP-astrocytes; and (2) in situ activated GFAP+ astrocytes. They play different roles in glial scar formation (Meletis et al., 2008; Barnabe-Heider et al., 2010). Activated astrocytes are harmful to remyelination and involved in scar tissue formation, inhibition of OPC migration, survival and differentiation after SCI, and even axonal regeneration (Wang et al., 2011). As a type of immunocyte in the CNS, astrocytes express many protein kinases, glycoproteins, and chondroitin sulfate proteoglycans after activation. These molecules induce inflammatory responses and the glial scar directly or indirectly causes severe damage to oligodendrocytes and neurons, chemically or physically (Silver and Miller, 2004). Glial scar formation limits the inflammatory reaction around the injury site, isolates damaged nerve tissue from normal tissue, and plays a supporting role in injured tissue. Simultaneously, the glial scar has a negative effect on remyelination and axonal regeneration. When axonal regeneration is inhibited, the link between axon and myelin is destroyed, and remyelination is not possible. Transplantation of OPCs and neural precursor cells into the injury site at the subacute stage contributes to axon myelination, but does not achieve a good outcome. This indicates that the internal environment after injury around the glial scar has an inhibitory effect on remyelination or myelination of axons (Keirstead et al., 2005; Karimi-Abdolrezaee et al., 2006). Activated astrocytes secrete a variety of chondroitin sulfate proteoglycans, mainly consisting of neuroncan and brevican, and versican in the nervous system (Yamada et al., 1994). They all have an inhibitory effect on remyelination and axonal regeneration (Dyck and Karimi-Abdolrezaee, 2015). Activated astrocytes affect OPC recruitment and maturation, and axonal ensheathment by secreting chondroitin sulfate proteoglycans, and finally inhibiting remyelination (Dyck and Karimi-Abdolrezaee, 2015). Chondroitin sulfate proteoglycans not only affect OPCs, but Karimi-Abdolezaee et al. (2010) found that chondroitin sulfate proteoglycans and the glial scar affect differentiation of neural precursor cells to oligodendrocytes. The glial scar is not only composed of astrocytes and microglia, and reactive activated OPCs are also involved in scar formation. OPCs also express chondroitin sulfate proteoglycans to inhibit axonal regeneration and repair myelin (Chen et al., 2002). Another inhibitory molecule secreted by astrocytes is hyaluronan, which is extensively found in the extracellular matrix and white matter of the CNS (Sherman et al., 2002). Hyaluronan can act on CD44 receptors of T cells and OPCs, and affect OPC maturation (Back et al., 2005; Lundgaard et al., 2014).
Reactive activated astrocytes after SCI participate in glial scar formation. Changes in their own cell products and the microenvironment surrounding glial scars have a strong inhibitory effect on remyelination (Wang et al., 2015). Some inflammatory factors mitigate scar formation in reactive gliosis by inhibiting astrocyte activation, which may be a way to improve remyelination after SCI. Wang et al. (2015) suggested that blocking the signaling pathway of platelet activating factor can reduce reactive gliosis and inhibit demyelination after SCI. Ishii et al. (2016) found that the RAS-related C3 botulinum substrate 1 (Rac1)–G1 to S phase transition 1 (GSPT1) signaling pathway is a new axis for regulating gliosis after SCI. These studies provide evidence for remyelination after SCI.
Microglia and Remyelination
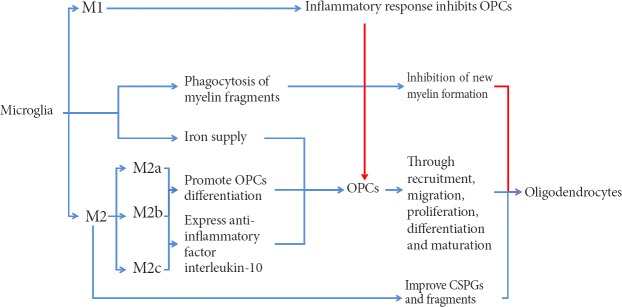
Microglia are macrophages present in the nervous system, and are involved in cellular immunity of the nervous system. Microglia are usually in a resting state, and in this state are in a “cruising” state to detect a pathological reaction at any time (Hanisch and Kettenmann, 2007). When a “crisis” arises, microglia can be immediately activated from the resting state, migrate to the injury site, and participate in formation of the outer layer of the glial scar to isolate damaged tissue from normal tissue (Davalos et al., 2005). Nevertheless, excessively activated microglia secrete large amounts of inflammatory factors, cytotoxic agents, and free radicals, thereby causing a severe inflammatory response, which undoubtedly inhibits remyelination. However, in recent years, more and more studies have focused on promoting the effect of microglia on remyelination. Microglia can be divided into different subtypes in the CNS, which play distinct roles in remyelination (Figure 2).
Figure 2.
Microglia and remyelination.
Different microglia subtypes have varied effects on remyelination. OPCs: Oligodendrocyte precursor cells; CSPGs: chondroitin sulfate proteoglycans.
Microglia have an important effect on remyelination. With demyelinating lesions following SCI, some myelin fragments may remain outside residual axons. If these residual myelin fragments cannot be removed, they will have an impact on new myelin. Microglia are responsible for removal of fragments (Kotter et al., 2006; Neumann et al., 2009). Both in vivo and in vivo, these residual fragments can influence differentiation, maturation, and myelination of OPCs (Nave, 2010). A previous study reported that this microglial function is dependent on downstream activation of the DAP12 signaling pathway by triggering receptor expressed on myeloid cells 2 (TREM2) (Poliani et al., 2015). A residual amount of these fragments is associated with phagocytic function of microglia/macrophages. Moreover, this function is largely determined by the age of the organism. If the blood of young animals is injected into the body of older animals, remyelination of older animals is improved (Miron and Franklin, 2014). Astrocytes recruit microglia to the site of injury by expressing the chemokine, CXCL10, which enhances phagocytosis of myelin fragments. If astrocytes are removed from the culture medium, removal of myelin fragments can be affected, resulting in inhibition of proliferation and myelination of OPCs (Skripuletz et al., 2013). Receptors associated with microglial phagocytosis of myelin fragments include CR3, SRA, and Fc gamma. A previous study found that CR3 can reduce phagocytosis by activating or downregulating microglial phagocytosis to act on phosphorylated cofilin via the spleen tyrosine kinase (Syk) signaling pathway (Hadas et al., 2012). Simultaneously, CR3 and SRA interact to mediate phagocytosis following axonal injury (Makranz et al., 2004). Expression of galectin-3/MAC-2 can alter phagocytosis of microglia by modulating CR3 and SRA (Rotshenker et al., 2008). The TLR4 agonist, E6020, promotes repair of damaged myelin by stimulating microglia phagocytosis and myelinating cell recruitment (Church et al., 2017). It also blocks the TLR4 signaling pathway leading to delayed phagocytosis and altered expression of cytokines such as insulin-like growth factor-1, fibroblast growth factor-2, and interleukin-1 beta, which ultimately reduces remyelination after SCI (Church et al., 2016).
Macrophages/microglia secrete a variety of cytokines, chemokines, and growth factors to affect remyelination after SCI. Microglia are divided into two subtypes, namely, M1 cells involved in the inflammatory response and M2 cells with anti-inflammatory and repair effects (Kigerl et al., 2009). M1 cells are strongly associated with the inflammatory response and suppress remyelination. M2 cells are classified into three subtypes: M2a, M2b, and M2c (Gensel and Zhang, 2015). Kigerl et al. (2009) found that M2 microglia ameliorate chondroitin sulfate proteoglycan-induced axonal degeneration and reduce residual myelin fragments. M2 microglia gradually occupy a dominant activated microglial position at 3–10 days after demyelination. This time window coincides with OPC recruitment and differentiation into mature oligodendrocytes at the site of injury (Miron et al., 2013). A further study verified that M2a and M2c microglia promote differentiation and maturation of oligodendrocytes by selectively removing M2 microglia (Miron and Franklin, 2014). These above studies confirm that M1 microglia inhibit remyelination after SCI. M2a and M2c (suspected) microglia may promote remyelination by promoting recruitment, proliferation, differentiation, and maturation of OPCs after SCI. Interleukin-10 is secreted by M2b microglia and an anti-inflammatory cytokine. After SCI, with activation of M2a cells, M2b cells reach a peak at 4–5 days after injury. Another study demonstrated that M2b cells protect against axonal degeneration. Although it is not clear if M2b cells have a direct effect on remyelination, there is enough evidence to show that M2b and M2c cells promote spinal cord tissue repair by modulating cell proliferation (including OPCs) at the proliferative stage after SCI (Gensel and Zhang, 2015). Bartus et al. (2014) have found that lentiviral introduction of the ChABC gene immediately after SCI promotes a neuroprotective form of M2 microglia and increases storage of neurons and axons after 12 weeks of SCI. They also reported that this effect of ChABC may be produced by increasing expression of the anti-inflammatory factor, interleukin-10, and reducing the inflammatory factor, interleukin-12 beta (Didangelos et al., 2014). To date, increasing pathways have been shown to shift M1/M2 polarization. The amount of M1/M2 polarization is associated with age, with more M1 polarization detected in infarcted brain from older stroke models and more M2 labels found in younger ones (Suenaga et al., 2015). Also, many mediators (such as interleukin-4 and -13) can enhance M2 polarization (Wang et al., 2014; Roszer, 2015). Wang et al. (2017) reported that heterochromatin protein 1c (HP-1c) activates the 5′AMP-activated protein kinase (AMPK)-Nrf2 pathway to alter M1/M2 polarization and reduce the inflammatory reaction in stroke models. Cocoa polyphenolic extract is reported to shift M1/M2 polarization, in which M1 polarization is reduced and alternatively, M2 polarization induced (Dugo et al., 2017). Although quite a few pathways are related to M1/M2 polarization, and many molecules have shown their anti-inflammatory potential by reducing/inducing M1/M2 polarization, alteration of M1/M2 polarization after SCI has yet to be fully understood.
Besides astrocytes, microglia are also associated with the iron supply chain in the nervous system. Increasing iron content in microglia increases the survival rate of co-cultured OPCs, verifying that microglia are a source of iron in OPCs (Zhang et al., 2006). Considering a similar role of astrocytes, microglia may improve iron protein content in both types of glial cells after SCI, improve iron supply in OPC–oligodendrocyte lines, and be helpful for remyelination after injury.
Summary
With an increasing number of SCI patients, the study of demyelination/remyelination after SCI has become increasingly significant. In addition to neurons, glial cells are resident cells in the CNS. Glial cells play supporting, nutritional, and immunological roles in the CNS. Simultaneously, glial cells are intimately associated with each other. After SCI, various signaling pathways are initiated, which can activate/injure glial cells and induce an inflammatory response, glial scar formation, neuronal injury, necrosis, and demyelination. In demyelinating lesions, OPCs in nerves replace lost oligodendrocytes and become new myelin via migration, proliferation, differentiation, and maturation. However, after glial cell activation, the surrounding environment is changed and OPC myelination is affected by many factors. Astrocytes are the most abundant glial cells in the CNS. They secrete chondroitin sulfate proteoglycans after activation. Astrocytes also induce glial scar formation, which has a large effect on remyelination. Microglia as major immune cells of the CNS initiate an inflammatory response after injury. Inflammatory cytokines expressed in microglia affect remyelination. M2 microglia promote OPC proliferation, differentiation, and maturation. Taken together, controlling reactive activation of glial cells after SCI to improve remyelination is an important approach to treat injured spinal cord and promote recovery of neurological function.
Footnotes
Conflicts of interest: None declared.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Na Lin, Kunming Medical University, Basic Medical Sciences, China.
Copyedited by James R, Frenchman B, Wang J, Li CH, Qiu Y, Song LP, Zhao M
Funding: This work was supported by the National Natural Science Foundation of China, No. 81601957.
References
- Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Myelin damage and repair in pathologic CNS: challenges and prospects. Front Mol Neurosci. 2015;8:35. doi: 10.3389/fnmol.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almad A, Sahinkaya FR, McTigue DM. Oligodendrocyte fate after spinal cord injury. Neurotherapeutics. 2011;8:262–273. doi: 10.1007/s13311-011-0033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azbill RD, Mu X, Bruce-Keller AJ, Mattson MP, Springer JE. Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res. 1997;765:283–290. doi: 10.1016/s0006-8993(97)00573-8. [DOI] [PubMed] [Google Scholar]
- Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, Trapp BD, Bebo BF, Jr, Rao MS, Sherman LS. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- Badaracco ME, Siri MV, Pasquini JM. Oligodendrogenesis: the role of iron. BioFactors (Oxford, England) 2010;36:98–102. doi: 10.1002/biof.90. [DOI] [PubMed] [Google Scholar]
- Balentine JD. Pathology of experimental spinal cord trauma. I. The necrotic lesion as a function of vascular injury. Lab Invest. 1978;39:236–253. [PubMed] [Google Scholar]
- Bansal R, Pfeiffer SE. FGF-2 converts mature oligodendrocytes to a novel phenotype. J Neurosci Res. 1997;50:215–228. doi: 10.1002/(SICI)1097-4547(19971015)50:2<215::AID-JNR10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, Frisen J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Control of oligodendrocyte number in the developing rat optic nerve. Neuron. 1994;12:935–942. doi: 10.1016/0896-6273(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Barres BA, Burne JF, Holtmann B, Thoenen H, Sendtner M, Raff MC. Ciliary neurotrophic factor enhances the rate of oligodendrocyte generation. Mol Cell Neurosci. 1996;8:146–156. doi: 10.1006/mcne.1996.0053. [DOI] [PubMed] [Google Scholar]
- Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yanez-Munoz RJ, Rogers JH, Schneider BL, Muir EM, Bradbury EJ. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci. 2014;34:4822–4836. doi: 10.1523/JNEUROSCI.4369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielecki B, Mattern C, Ghoumari AM, Javaid S, Smietanka K, Abi Ghanem C, Mhaouty-Kodja S, Ghandour MS, Baulieu EE, Franklin RJM, Schumacher M, Traiffort E. Unexpected central role of the androgen receptor in the spontaneous regeneration of myelin. Proc Natl Acad Sci U S A. 2016;113:14829–14834. doi: 10.1073/pnas.1614826113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Morton PD, Ashbaugh JJ, Karmally S, Lambertsen KL, Bethea JR. Astrocytes play a key role in EAE pathophysiology by orchestrating in the CNS the inflammatory response of resident and peripheral immune cells and by suppressing remyelination. Glia. 2014;62:452–467. doi: 10.1002/glia.22616. [DOI] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Negra M, Levine A, Ughrin Y, Levine JM. Oligodendrocyte precursor cells: Reactive cells that inhibit axon growth and regeneration. J Neurocytol. 2002;31:481–495. doi: 10.1023/a:1025791614468. [DOI] [PubMed] [Google Scholar]
- Chong SY, Rosenberg SS, Fancy SP, Zhao C, Shen YA, Hahn AT, McGee AW, Xu X, Zheng B, Zhang LI, Rowitch DH, Franklin RJ, Lu QR, Chan JR. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci U S A. 2012;109:1299–1304. doi: 10.1073/pnas.1113540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JS, Kigerl KA, Lerch JK, Popovich PG, McTigue DM. TLR4 deficiency impairs oligodendrocyte formation in the injured spinal cord. J Neurosci. 2016;36:6352–6364. doi: 10.1523/JNEUROSCI.0353-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JS, Milich LM, Lerch JK, Popovich PG, McTigue DM. E6020, a synthetic TLR4 agonist, accelerates myelin debris clearance, Schwann cell infiltration, and remyelination in the rat spinal cord. Glia. 2017;65:883–899. doi: 10.1002/glia.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Deng Y, Xie D, Fang M, Zhu G, Chen C, Zeng H, Lu J, Charanjit K. Astrocyte-derived proinflammatory cytokines induce hypomyelination in the periventricular white matter in the hypoxic neonatal brain. PLoS One. 2014;9:e87420. doi: 10.1371/journal.pone.0087420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didangelos A, Iberl M, Vinsland E, Bartus K, Bradbury EJ. Regulation of IL-10 by chondroitinase ABC promotes a distinct immune response following spinal cord injury. J Neurosci. 2014;34:16424–16432. doi: 10.1523/JNEUROSCI.2927-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincman TA, Beare JE, Ohri SS, Whittemore SR. Isolation of cortical mouse oligodendrocyte precursor cells. J Neurosci Methods. 2012;209:219–226. doi: 10.1016/j.jneumeth.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues HS, Portugal CC, Socodato R, Relvas JB. Oligodendrocyte, astrocyte, and microglia crosstalk in myelin development, damage, and repair. Front Neurol. 2016;4:71. doi: 10.3389/fcell.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugo L, Belluomo MG, Fanali C, Russo M, Cacciola F, Maccarrone M, Sardanelli AM. Effect of Cocoa polyphenolic extract on macrophage polarization from proinflammatory M1 to anti-inflammatory M2 state. Oxid Med Cell Longev. 2017;2017:6293740. doi: 10.1155/2017/6293740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck SM, Karimi-Abdolrezaee S. Chondroitin sulfate proteoglycans: Key modulators in the developing and pathologic central nervous system. Exp Neurol. 2015;269:169–187. doi: 10.1016/j.expneurol.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Kondo T, Takebayashi H, Taga T. Negative regulatory effect of an oligodendrocytic bHLH factor OLIG2 on the astrocytic differentiation pathway. Cell Death Differ. 2004;11:196–202. doi: 10.1038/sj.cdd.4401332. [DOI] [PubMed] [Google Scholar]
- Gauthier MK, Kosciuczyk K, Tapley L, Karimi-Abdolrezaee S. Dysregulation of the neuregulin-1-ErbB network modulates endogenous oligodendrocyte differentiation and preservation after spinal cord injury. Eur J Neurosci. 2013;38:2693–2715. doi: 10.1111/ejn.12268. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11. doi: 10.1016/j.brainres.2014.12.045. [DOI] [PubMed] [Google Scholar]
- Goddard DR, Berry M, Butt AM. In vivo actions of fibroblast growth factor-2 and insulin-like growth factor-I on oligodendrocyte development and myelination in the central nervous system. J Neurosci Res. 1999;57:74–85. doi: 10.1002/(SICI)1097-4547(19990701)57:1<74::AID-JNR8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Hackett AR, Lee DH, Dawood A, Rodriguez M, Funk L, Tsoulfas P, Lee JK. STAT3 and SOCS3 regulate NG2 cell proliferation and differentiation after contusive spinal cord injury. Neurobiol Dis. 2016;89:10–22. doi: 10.1016/j.nbd.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadas S, Spira M, Hanisch UK, Reichert F, Rotshenker S. Complement receptor-3 negatively regulates the phagocytosis of degenerated myelin through tyrosine kinase Syk and cofilin. J Neuroinflammation. 2012;9:166. doi: 10.1186/1742-2094-9-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton DW, Asher RA, Kondo T, Steeves JD, Ramer MS, Fawcett JW. A potential role for bone morphogenetic protein signalling in glial cell fate determination following adult central nervous system injury in vivo. Eur J Neurosci. 2007;26:3024–3035. doi: 10.1111/j.1460-9568.2007.05940.x. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hesp ZC, Goldstein EZ, Miranda CJ, Kaspar BK, McTigue DM. Chronic oligodendrogenesis and remyelination after spinal cord injury in mice and rats. J Neurosci. 2015;35:1274–1290. doi: 10.1523/JNEUROSCI.2568-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Patel KD, Medved J, Reiss AM, Nishiyama A. NG2 cells in white matter but not gray matter proliferate in response to PDGF. J Neurosci. 2013;33:14558–14566. doi: 10.1523/JNEUROSCI.2001-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Tang C, Sun S, Cao W, Qi W, Xu J, Huang J, Lu W, Liu Q, Gong B, Zhang Y, Jiang J. Protective effect of electroacupuncture on neural myelin sheaths is mediated via promotion of oligodendrocyte proliferation and inhibition of oligodendrocyte death after compressed spinal cord injury. Mol Neurobiol. 2015;52:1870–1881. doi: 10.1007/s12035-014-9022-0. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Lee PR, Baba H, Fields RD. Leukemia inhibitory factor regulates the timing of oligodendrocyte development and myelination in the postnatal optic nerve. J Neurosci Res. 2009;87:3343–3355. doi: 10.1002/jnr.22173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Ueyama T, Shigyo M, Kohta M, Kondoh T, Kuboyama T, Uebi T, Hamada T, Gutmann DH, Aiba A, Kohmura E, Tohda C, Saito N. A novel Rac1-GSPT1 signaling pathway controls astrogliosis following central nervous system injury. J Biol Chem. 2016;292:1240–1250. doi: 10.1074/jbc.M116.748871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Li M, Wu WT, Yick LW, Lee X, Shao Z, Wang J, So KF, McCoy JM, Pepinsky RB, Mi S, Relton JK. LINGO-1 antagonist promotes functional recovery and axonal sprouting after spinal cord injury. Mol Cell Neurosci. 2006;33:311–320. doi: 10.1016/j.mcn.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Billakanti R. Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol Neurobiol. 2012;46:251–264. doi: 10.1007/s12035-012-8287-4. [DOI] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657–1676. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Hwang DH, Choi JY, Park CH, Suh-Kim H, Kim SU, Kim BG. Differential and cooperative actions of Olig1 and Olig2 transcription factors on immature proliferating cells after contusive spinal cord injury. Glia. 2011;59:1094–1106. doi: 10.1002/glia.21182. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kotter MR, Li WW, Zhao C, Franklin RJ. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26:328–332. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa M, Watanabe M, Katoh H, Suyama K, Matsuyama D, Imai T, Mochida J. Effect of amiloride on endoplasmic reticulum stress response in the injured spinal cord of rats. Eur J Neurosci. 2014;40:3120–3127. doi: 10.1111/ejn.12647. [DOI] [PubMed] [Google Scholar]
- Kwo S, Young W, Decrescito V. Spinal cord sodium, potassium, calcium, and water concentration changes in rats after graded contusion injury. J Neurotrauma. 1989;6:13–24. doi: 10.1089/neu.1989.6.13. [DOI] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang L, Chu Y, Namaka M, Deng B, Kong J, Bi X. Astrocytes in oligodendrocyte lineage development and white matter pathology. Front Cell Neurosci. 2016;10:119. doi: 10.3389/fncel.2016.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Leung GK. Oligodendrocyte precursor cells in spinal cord injury: a review and update. Biomed Res Int. 2015;2015:235195. doi: 10.1155/2015/235195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S. Demyelinating diseases. J Clin Pathol. 2006;59:1151–1159. doi: 10.1136/jcp.2005.031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukovic D, Stojkovic M, Moreno-Manzano V, Jendelova P, Sykova E, Bhattacharya SS, Erceg S. Concise review: reactive astrocytes and stem cells in spinal cord injury: good guys or bad guys. Stem Cells. 2015;33:1036–1041. doi: 10.1002/stem.1959. [DOI] [PubMed] [Google Scholar]
- Lundgaard I, Osorio MJ, Kress BT, Sanggaard S, Nedergaard M. White matter astrocytes in health and disease. Neurosci. 2014;276:161–173. doi: 10.1016/j.neuroscience.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle JM, Chittajallu R, Wrathall JR, Gallo V. NG2 cell response in the CNP-EGFP mouse after contusive spinal cord injury. Glia. 2009;57:270–285. doi: 10.1002/glia.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makranz C, Cohen G, Baron A, Levidor L, Kodama T, Reichert F, Rotshenker S. Phosphatidylinositol 3-kinase, phosphoinositide-specific phospholipase-Cgamma and protein kinase-C signal myelin phagocytosis mediated by complement receptor-3 alone and combined with scavenger receptor-AI/II in macrophages. Neurobiol Dis. 2004;15:279–286. doi: 10.1016/j.nbd.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Mayer M, Bhakoo K, Noble M. Ciliary neurotrophic factor and leukemia inhibitory factor promote the generation, maturation and survival of oligodendrocytes in vitro. Development. 1994;120:143–153. doi: 10.1242/dev.120.1.143. [DOI] [PubMed] [Google Scholar]
- Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O, Frisen J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Pepinsky RB, Cadavid D. Blocking LINGO-1 as a therapy to promote CNS repair: from concept to the clinic. CNS Drugs. 2013;27:493–503. doi: 10.1007/s40263-013-0068-8. [DOI] [PubMed] [Google Scholar]
- Miron VE, Franklin RJ. Macrophages and CNS remyelination. J Neurochem. 2014;130:165–171. doi: 10.1111/jnc.12705. [DOI] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJ, ffrench-Constant C. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CS, Cui QL, Warsi NM, Durafourt BA, Zorko N, Owen DR, Antel JP, Bar-Or A. Direct and indirect effects of immune and central nervous system-resident cells on human oligodendrocyte progenitor cell differentiation. J Immunol. 2015;194:761–772. doi: 10.4049/jimmunol.1401156. [DOI] [PubMed] [Google Scholar]
- Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11:275–283. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- Nave KA, Werner HB. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJM. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning GZ, Wu Q, Li YL, Feng SQ. Epidemiology of traumatic spinal cord injury in Asia: a systematic review. J Spinal Cord Med. 2012;35:229–239. doi: 10.1179/2045772312Y.0000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, 2nd, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- O’Rourke M, Gasperini R, Young KM. Adult myelination: wrapping up neuronal plasticity. Neural Regen Res. 2014;9:1261–1264. doi: 10.4103/1673-5374.137571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossola B, Zhao C, Compston A, Pluchino S, Franklin RJ, Spillantini MG. Neuronal expression of pathological tau accelerates oligodendrocyte progenitor cell differentiation. Glia. 2016;64:457–471. doi: 10.1002/glia.22940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, Tetzlaff W. Remyelination after spinal cord injury: is it a target for repair. Prog Neurobiol. 2014;117:54–72. doi: 10.1016/j.pneurobio.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Poliani PL, Wang Y, Fontana E, Robinette ML, Yamanishi Y, Gilfillan S, Colonna M. TREM2 sustains microglial expansion during aging and response to demyelination. J Clin Invest. 2015;125:2161–2170. doi: 10.1172/JCI77983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raasch J, Zeller N, van Loo G, Merkler D, Mildner A, Erny D, Knobeloch K-P, Bethea JR, Waisman A, Knust M, Del Turco D, Deller T, Blank T, Priller J, Brück W, Pasparakis M, Prinz M. IκB kinase 2 determines oligodendrocyte loss by non-cell-autonomous activation of NF-κB in the central nervous system. Brain. 2011;134:1184–1198. doi: 10.1093/brain/awq359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Ray SK, Samntaray S, Banik NL. Future directions for using estrogen receptor agonists in the treatment of acute and chronic spinal cord injury. Neural Regen Res. 2016;11:1418–1419. doi: 10.4103/1673-5374.191212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshenker S, Reichert F, Gitik M, Haklai R, Elad-Sfadia G, Kloog Y. Galectin-3/MAC-2, Ras and PI3K activate complement receptor-3 and scavenger receptor-AI/II mediated myelin phagocytosis in microglia. Glia. 2008;56:1607–1613. doi: 10.1002/glia.20713. [DOI] [PubMed] [Google Scholar]
- Salgado-Ceballos H, Guizar-Sahagun G, Feria-Velasco A, Grijalva I, Espitia L, Ibarra A, Madrazo I. Spontaneous long-term remyelination after traumatic spinal cord injury in rats. Brain Res. 1998;782:126–135. doi: 10.1016/s0006-8993(97)01252-3. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Strittmatter SM. Nogo limits neural plasticity and recovery from injury. Curr Opin Neurobiol. 2014;27:53–60. doi: 10.1016/j.conb.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LS, Struve JN, Rangwala R, Wallingford NM, Tuohy TM, Kuntz Ct Hyaluronate-based extracellular matrix: keeping glia in their place. Glia. 2002;38:93–102. doi: 10.1002/glia.10053. [DOI] [PubMed] [Google Scholar]
- Siebert JR, Stelzner DJ, Osterhout DJ. Chondroitinase treatment following spinal contusion injury increases migration of oligodendrocyte progenitor cells. Exp Neurol. 2011;231:19–29. doi: 10.1016/j.expneurol.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Skripuletz T, Hackstette D, Bauer K, Gudi V, Pul R, Voss E, Berger K, Kipp M, Baumgartner W, Stangel M. Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain. 2013;136:147–167. doi: 10.1093/brain/aws262. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozmen EG, Rosenzweig S, Llorente IL, DiTullio DJ, Machnicki M, Vinters HV, Havton LA, Giger RJ, Hinman JD, Carmichael ST. Nogo receptor blockade overcomes remyelination failure after white matter stroke and stimulates functional recovery in aged mice. Proc Natl Acad Sci U S A. 2016;113:E8453–8462. doi: 10.1073/pnas.1615322113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaga J, Hu X, Pu H, Shi Y, Hassan SH, Xu M, Leak RK, Stetler RA, Gao Y, Chen J. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol. 2015;272:109–119. doi: 10.1016/j.expneurol.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Wang X, Chen T, Li T, Cao K, Lu A, Chen Y, Sun D, Luo J, Fan J, Young W, Ren Y. Myelin activates FAK/Akt/NF-kappaB pathways and provokes CR3-dependent inflammatory response in murine system. PLoS One. 2010;5:e9380. doi: 10.1371/journal.pone.0009380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57:467–478. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- Tognatta R, Sun W, Goebbels S, Nave KA, Nishiyama A, Schoch S, Dimou L, Dietrich D. Transient Cnp expression by early progenitors causes Cre-Lox-based reporter lines to map profoundly different fates. Glia. 2017;65:342–359. doi: 10.1002/glia.23095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga H, Seiwa C, Yoshioka N, Mizoguchi K, Yamamoto M, Asou H, Aiso S. An extract of chinpi, the dried peel of the citrus fruit unshiu, enhances axonal remyelination via promoting the proliferation of oligodendrocyte progenitor cells. Evid Based Complement Alternat Med. 2016;2016:8692698. doi: 10.1155/2016/8692698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Parikh ZS, Vora P, Frost EE, Pillai PP. pERK1/2 peripheral recruitment and filopodia protrusion augment oligodendrocyte progenitor cell migration: combined effects of PDGF-A and fibronectin. Cell Mol Neurobiol. 2016;37:183–194. doi: 10.1007/s10571-016-0359-y. [DOI] [PubMed] [Google Scholar]
- Tripathi R, McTigue DM. Prominent oligodendrocyte genesis along the border of spinal contusion lesions. Glia. 2007;55:698–711. doi: 10.1002/glia.20491. [DOI] [PubMed] [Google Scholar]
- Walz W. Role of glial cells in the regulation of the brain ion microenvironment. Prog Neurobiol. 1989;33:309–333. doi: 10.1016/0301-0082(89)90005-1. [DOI] [PubMed] [Google Scholar]
- Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cheng X, He Q, Zheng Y, Kim DH, Whittemore SR, Cao QL. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci. 2011;31:6053–6058. doi: 10.1523/JNEUROSCI.5524-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gao Z, Zhang Y, Feng SQ, Liu Y, Shields LB, Zhao YZ, Zhu Q, Gozal D, Shields CB, Cai J. Attenuated reactive gliosis and enhanced functional recovery following spinal cord injury in null mutant mice of platelet-activating factor receptor. Mol Neurobiol. 2015;53:3448–3461. doi: 10.1007/s12035-015-9263-6. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang Y, Xu Y, Ruan W, Wang H, Zhang Y, Saavedra JM, Zhang L, Huang Z, Pang T. A dual AMPK/Nrf2 activator reduces brain inflammation after stroke by enhancing microglia M2 polarization. Antioxid Redox Signal. 2017 doi: 10.1089/ars.2017.7003. doi: 10.1089/ars.2017.7003. [DOI] [PubMed] [Google Scholar]
- Wegener A, Deboux C, Bachelin C, Frah M, Kerninon C, Seilhean D, Weider M, Wegner M, Nait-Oumesmar B. Gain of Olig2 function in oligodendrocyte progenitors promotes remyelination. Brain. 2015;138:120–135. doi: 10.1093/brain/awu375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard N, Sonnewald U, Schousboe A. Metabolic trafficking between neurons and astrocytes: the glutamate/glutamine cycle revisited. Dev Neurosci. 1995;17:203–211. doi: 10.1159/000111288. [DOI] [PubMed] [Google Scholar]
- Wrathall JR, Teng YD, Choiniere D. Amelioration of functional deficits from spinal cord trauma with systemically administered NBQX, an antagonist of non-N-methyl-D-aspartate receptors. Exp Neurol. 1996;137:119–126. doi: 10.1006/exnr.1996.0012. [DOI] [PubMed] [Google Scholar]
- Xie XM, Shi LL, Shen L, Wang R, Qi Q, Wang QY, Zhang LJ, Lu HZ, Hu JG. Co-transplantation of MRF-overexpressing oligodendrocyte precursor cells and Schwann cells promotes recovery in rat after spinal cord injury. Neurobiol Dis. 2016;94:196–204. doi: 10.1016/j.nbd.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Yamada H, Watanabe K, Shimonaka M, Yamaguchi Y. Molecular cloning of brevican, a novel brain proteoglycan of the aggrecan/versican family. J Biol Chem. 1994;269:10119–10126. [PubMed] [Google Scholar]
- Yao ZF, Wang Y, Lin YH, Wu Y, Zhu AY, Wang R, Shen L, Xi J, Qi Q, Jiang ZQ, Lu HZ, Hu JG. Transplantation of PDGF-AA-overexpressing oligodendrocyte precursor cells promotes recovery in rat following spinal cord injury. Front Cell Neurosci. 2017;11:79. doi: 10.3389/fncel.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YQ, Hu NC, Duan JA, Li DP, Liu C. Neuroprotective effects of sufentanil preconditioning on spinal cord injury in mouse models. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:5966–5972. [Google Scholar]
- Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, Rowitch DH, Kessaris N, Suter U, Richardson WD, Franklin RJ. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Surguladze N, Slagle-Webb B, Cozzi A, Connor JR. Cellular iron status influences the functional relationship between microglia and oligodendrocytes. Glia. 2006;54:795–804. doi: 10.1002/glia.20416. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Argaw AT, Gurfein BT, Zameer A, Snyder BJ, Ge C, Lu QR, Rowitch DH, Raine CS, Brosnan CF, John GR. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc Natl Acad Sci U S A. 2009;106:19162–19167. doi: 10.1073/pnas.0902834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zuo H, Maher BJ, Serwanski DR, LoTurco JJ, Lu QR, Nishiyama A. Olig2-dependent developmental fate switch of NG2 cells. Development. 2012;139:2299–2307. doi: 10.1242/dev.078873. [DOI] [PMC free article] [PubMed] [Google Scholar]