Abstract
We present an innovative application of the Flexi-Seal® Faecal Management System (FMS) for the diversion of upper and lower gastrointestinal secretions from enterocutaneous fistulae associated with complex wounds. Fistula is a common complication after the formation of a laparostomy, secondary to cases of severe intra-abdominal sepsis, acute mesenteric ischaemia, necrotising infection of the abdominal wall, or intra-abdominal hypertension. A significant mortality rate is associated with such fistula. With the successful continent diversion of gastrointestinal secretions by the Flexi-Seal® FMS, abdominal wounds can be successfully skin-grafted, and wound healing expedited.
Keywords: Flexi-seal, Wound, Laparostomy, Enterocutaneous fistula, Split skin graft
A laparostomy is an established means of treatment in cases of severe intra-abdominal sepsis, acute mesenteric ischaemia, necrotising infection of the abdominal wall, or intra-abdominal hypertension. The resulting large cavity heals by granulation from the omentum and abdominal viscera, often assisted by split skin grafting or vacuum-assisted closure (VAC).1 A number of patients, however, develop complications such as enteric fistulation within the laparostomy wound.2 Fistulation can occur between the skin and stomach, small bowel and/or colon, as well as the other viscera, and can be multiple. It has been reported to occur in up to 25% of laparostomy wounds.3 In an established enterocutaneous fistula, control of fistula effluent is essential for successful healing.2 The mortality rate associated with enterocutaneous fistulae is reported to be 4.8–20%.4
Current treatment strategies include the use of octreotide and proton-pump inhibitors to reduce acidic intestinal secretions and stoma bags for diversion, and measurement, of the fistula output. Wounds involving high-output enterocutaneous fistulae can present a challenging surgical problem, and traditional stoma bag application can be hampered in the absence of an epithelialised wound bed. If the laparostomy wound is to be skin grafted, a reliable method of diversion of faeces or upper gastrointestinal secretions is required for protection of skin grafts in close proximity. After successful skin graft take, traditional stoma bags can then be used to collect secretions, prior to potential definitive surgical treatment of the fistula, by resection.
The Flexi-Seal® Faecal Management System (FMS; Convatec, Rhymney, UK) consists of a soft silicone catheter approximately 1-m long, syringe and collection bag. At one end, the silicone catheter (23-mm diameter, collapsing to 8 mm for insertion) has a retention balloon, which is traditionally inserted into the rectum. This balloon is inflated with 45 ml of water, to a diameter of about 53 mm. A third port allows flushing of the tube if necessary. The manufacturer recommends changing the FMS at 29 days. The FMS allows diversion of liquid, or semi-liquid, stool away from surrounding skin, preventing skin breakdown and infection. The FMS is used as an alternative to the use of diverting colostomies in the intensive care unit (ICU).5 We present two cases where the Flexi-Seal® FMS has been used to allow diversion of gastric and colonic secretions in patients with enterocutaneous fistulae, in conjunction with split skin autografting to laparostomy wounds.
Case history 1
A 68-year-old man, with a past medical history of angina and carcinoma of the lung, underwent an emergency abdominal aortic aneurysm repair, with subsequent development of an abdominal compartment syndrome. The abdomen was left open with the formation of a laparostomy. Further surgery included resection of necrotic small bowel (distal ileum) with the formation of ileostomies (×2). A gastrocutaneous fistula at the superior wound margin was subsequently diagnosed by fistulogram. Total parenteral nutrition (TPN) was commenced and a plastic surgery opinion sought regarding skin grafting to the granulating abdominal wound (Fig. 1A). Pre-operative ocreotide and a proton-pump inhibitor were commenced. Under general anaesthesia, a Flexi-Seal® FMS was sited in the lumen of the fistula (25-mm diameter), a meshed split skin graft was sited on the wound, and dressed with paraffin gauze and polyurethane foam (Fig. 1B). In the postoperative period, gastric contents were successfully diverted from the wound site without the need for a traditional stoma bag, allowing successful grafting to the perifistula site (Fig. 1C). At 13 days’ post-grafting, a stoma bag was then placed onto the grafted tissue (Fig. 1D). A fluid-tight seal was formed and the wound was allowed to mature, without the need for dressings.
Figure 1.
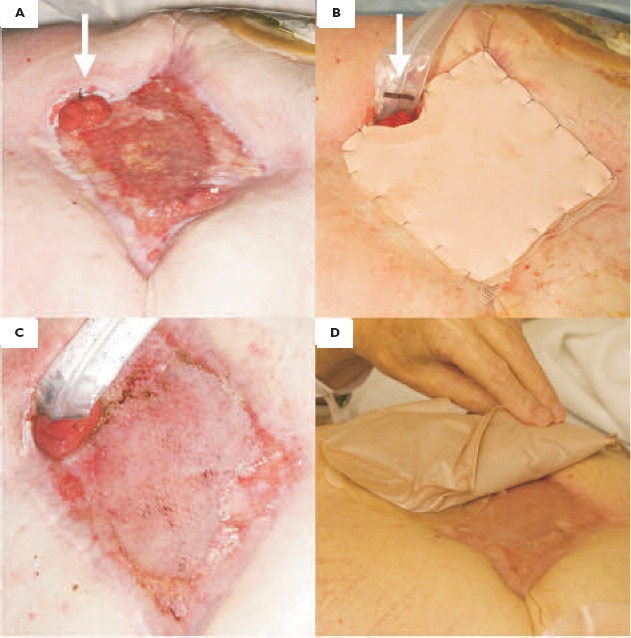
(A) A granulating abdominal wound, secondary to laparostomy, with a gastrocutaneous fistula (arrowed). (B) The application of a Flexi-Seal® FMS to the fistula (arrowed) and skin graft dressed with paraffin gauze and polyurethane foam. (C) The skin graft at the first wound check (day 5), with continent diversion of secretions with the FMS. (D) The graft at day 13, with successful adherence of a stoma bag to the grafted tissue.
Case history 2
A 77-year-old man, with a past medical history of myocardial infarction and poor exercise tolerance, underwent an emergency aortic aneurysm repair. Postoperative recovery was complicated by infection of the graft site, necessitating the formation of a laparostomy. Subsequently, a colocutaneous fistula to the left iliac fossa (LIF) and a caecocutaneous fistula to the right iliac fossa (RIF) were diagnosed. A plastic surgery opinion was sought regarding skin grafting to the granulating abdominal wound. Under general anaesthesia, a Flexi-Seal® FMS was sited in the lumen of the RIF fistula and a meshed split skin graft was sited on the abdominal and perifistula wound, and dressed as above. A traditional stoma bag was applied to the LIF fistula, where there was adequate tissues to allow bag adhesion and formation of a fluid-tight seal. In the postoperative period, large-bowel contents were continently diverted from the RIF wound site by the FMS, and after graft-take and healing of the abdominal wound, the FMS was replaced with a stoma bag. The stoma bag adhered well to the matured graft site.
Discussion
This is an innovative application for an FMS. In complex abdominal wounds involving fistula(e), diversion of secretions is essential for successful wound healing, in the medically co-morbid patient, contributing to patient optimisation, prior to definitive treatment. Although originally designed for faecal diversion in the ICU, the FMS has previously been applied successfully in perineal burn wound healing,6 and in the cases presented here, has allowed expedited wound healing in both cases of upper and lower gastrointestinal fistulae. In both cases, the FMS enabled effective wound management and nursing, as well as being well-tolerated by the patient. It must be remembered, though, in both of the aforementioned cases, skin-grafting was carried out after significant granulation tissue formation had occurred. This technique may not be appropriate in the acute setting.
The intubation of fistulae to allow skin grafting in conjunction with vacuum-assisted closure (VAC) has been described using a Malecot catheter.7 The Flexi-Seal® FMS is of a significantly larger diameter (23 mm vs 13.3 mm), and has the advantage of permitting the diversion of both liquid and semi-liquid bowel contents, thus allowing application to small and large bowel fistulae, as shown in these case reports. It also has the benefits of expediting wound closure in the sick patient, who may not be fit for formal repair by surgical faecal diversion, fistula excision and component separation techniques. VAC therapy could be used in conjunction with the Flexi-Seal® FMS, but one must be aware of the risks of further fistula formation.1
The Flexi-Seal® FMS is suitable for larger fistula between approximately 20–40 mm due to the dimensions of the silicone tube and retention balloon. If leakage does occur from around the FMS, it can simply be plugged with a gauze dressing pad. After successful healing of the abdominal wounds, subsequent fistula resection can be considered. The use of a FMS in these cases negated the need for a diverting stoma and thus reduced the need for further surgery in the critically ill patient, and has reduced the morbidity associated with such complex wounds.
References
- 1.Stevens P. Vacuum-assisted closure of laparostomy wounds: a critical review of the literature. Int Wound J 2009; : 259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schecter WP, Ivatury RR, Rotondo MF, Hirshberg A. Open abdomen after trauma and abdominal sepsis: a strategy for management. J Am Coll Surg 2006; : 390–6. [DOI] [PubMed] [Google Scholar]
- 3.Connolly PT, Teubner A, Lees NP, Anderson ID, Scott NA, Carlson GL. Outcome of reconstructive surgery for intestinal fistula in the open abdomen. Ann Surg 2008; : 440–4. [DOI] [PubMed] [Google Scholar]
- 4.Fisher JE. The importance of reconstruction of the abdominal wall after gastrointestinal fistula closure. Am J Surg 2009; : 131–2. [DOI] [PubMed] [Google Scholar]
- 5.Padmanabhan A, Stern M, Wishin J, Mangino M, Richey K, DeSane M. Clinical evaluation of a flexible fecal incontinence management system. Am J Crit Care 2007; : 384–93. [PubMed] [Google Scholar]
- 6.Bordes J, Goutorbe P, Asenio Y, Meaudre E, Dantzer E. A non-surgical device for faecal diversion in the management of perineal burns. Burns 2008; : 840–4. [DOI] [PubMed] [Google Scholar]
- 7.Al-Khoury G, Kaufman D, Hirshberg A. Improved control of exposed fistula in the open abdomen. J Am Coll Surg 2008; : 397–8. [DOI] [PubMed] [Google Scholar]


