Abstract
The nuclear receptor coactivators participate in the transcriptional activation of specific genes by nuclear receptors. In this study, we report the isolation of a nuclear receptor coactivator-interacting protein from a human liver cDNA library by using the coactivator peroxisome proliferator-activated receptor-interacting protein (PRIP) (ASC2/AIB3/RAP250/NRC/TRBP) as bait in a yeast two-hybrid screen. Human PRIP-interacting protein cDNA has an ORF of 2,556 nucleotides, encodes a protein with 852 amino acids, and contains a 9-aa VVDAFCGVG methyltransferase motif I and an invariant GXXGXXI segment found in K-homology motifs of many RNA-binding proteins. The gene encoding this protein, designated PRIP-interacting protein with methyltransferase domain (PIMT), is localized on chromosome 8q11 and spans more than 40 kb. PIMT mRNA is ubiquitously expressed, with a high level of expression in heart, skeletal muscle, kidney, liver, and placenta. Using the immunofluorescence localization method, we found that PIMT and PRIP proteins appear colocalized in the nucleus. PIMT strongly interacts with PRIP under in vitro and in vivo conditions, and the PIMT-binding site on PRIP is in the region encompassing amino acids 773–927. PIMT binds S-adenosyl-l-methionine, the methyl donor for methyltransfer reaction, and it also binds RNA, suggesting that it is a putative RNA methyltransferase. PIMT enhances the transcriptional activity of peroxisome proliferator-activated receptor γ and retinoid-X-receptor α, which is further stimulated by coexpression of PRIP, implying that PIMT is a component of nuclear receptor signal transduction apparatus acting through PRIP. Definitive identification of the specific substrate of PIMT and the role of this RNA-binding protein in transcriptional regulation remain to be determined.
The nuclear receptor superfamily consists of members of ligand-regulated transcriptional factors comprising receptors for steroid and thyroid hormones, vitamin D3, retinoic acid, and peroxisome proliferators, among others (1, 2). All nuclear receptors share a common structure with a highly conserved DNA-binding domain consisting of two zinc fingers, a C-terminal hormone-binding domain, and two transcriptional activation function (AF) domains, termed AF-1 in the N-terminal domain and AF-2 in the hormone-binding domain (3). Transactivation of the AF-2 domain is ligand-dependent and can be blocked by the binding of antagonists. The activities of AF-1 and -2 vary depending on responsive cell types as well as a short DNA sequence, termed response element, located in the promoter regions of target genes (2, 3).
The molecular mechanism(s) by which nuclear receptors achieve transcriptional activation in a tissue-specific fashion is not fully understood. The current models call for the participation of additional factors for modifying the chromatin structure and mediating the interaction between the activated nuclear receptor and the basal transcriptional machinery in a ligand-dependent fashion. During the past few years, many nuclear receptor-binding proteins have been identified (4, 5). Most of them contain the conserved LXXLL (where L is leucine and X is any amino acid) signature motif, which mediates recognition of nuclear receptors (4). The nuclear receptor coactivators identified to date include the well-characterized steroid receptor coactivator-1 (SRC-1) family with three members [SRC-1 (6), TIF-2 (SRC-2, GRIP1) (7), p/CIP (ACTR, AIB1, RAC3, and SRC-3) (8–10)], CREB-binding protein (CBP)/p300 (11), PPAR-binding protein (PBP) (12), and PPARγ coactivator-1 (PGC-1) (13). SRC-1 and CBP/p300 possess intrinsic histone acetyltransferase activity and also recruit other proteins with histone acetyltransferase activity, indicating their role in chromatin modification (2, 4, 8, 14). In addition to histone acetylation, there is emerging evidence that suggests a role for histone methylation in the regulation of nuclear receptor transcriptional activity. A protein, designated as coactivator-associated arginine methyltransferase 1 (CARM1), which associates with SRC-2, can methylate histones, and the methyltransferase activity of CARM1 appears necessary for this protein to potentiate nuclear receptor activity (15). Furthermore, protein arginine methyltransferase 1, which shares a region of homology with CARM1, appears to enhance transcriptional activity by acting synergistically with CARM1 (16). Recent evidence suggests that coactivator proteins form distinct coactivator complexes, such as the one anchored by CBP/p300 that integrates the SRC-1 family of proteins and p/CAF to carry out histone acetyltransferase reactions, and the other thyroid hormone receptor-associated protein (TRAP)/vitamin D receptor-interacting protein (DRIP)/activator-recruited cofactor (ARC) complex anchored by PBP (TRAP220/DRIP205) (12, 17–19), which links to the basal transcription machinery (4, 20). PBP, with no histone acetyltransferase activity, serves as an anchor protein to recruit the multiprotein complex that appears to act more directly on the transcriptional apparatus, presumably after the unwinding of chromatin facilitated by the CBP/p300 anchored multiprotein complex (4). In agreement with the central role of CBP/p300 and PBP in the coactivator complex configuration and in nuclear receptor-mediated transcriptional activity, knockout experiments revealed that both CBP, p300, and PBP null mutations lead to embryonic lethality (21–26), indicating that disruption of these pivotal anchoring coactivators affects the function of many nuclear receptors and possibly other transcription factors.
In this work, we report the cloning of a protein that binds the recently identified nuclear receptor coactivator peroxisome proliferator-activated receptor-(PPAR) interacting protein (PRIP) (ASC2/AIB3/RAP250/NRC/TRBP) (27–31). We cloned PRIP by using PPARγ as bait in the yeast two-hybrid system (27); others have cloned this coactivator by using thyroid hormone receptor and retinoid-X-receptor for 9-cis-retinoic acid (RXR) (28–31). To elucidate the role of PRIP, we set out to isolate the PRIP-interacting protein(s) with a two-hybrid system. One of the proteins identified is designated PRIP-interacting protein with methyltransferase domain (PIMT); this protein is a ubiquitously expressed nuclear protein containing a methyltransferase domain. PIMT is able to enhance the transcriptional activity of PPARγ and RXRα, which is further potentiated by overexpression of PRIP. We also show that PIMT binds S-adenosyl-l-methionine (AdoMet) and RNA, implying that PIMT may function as a putative RNA methyltransferase.
Materials and Methods
Plasmids.
GAL4-PRIP (amino acids 773-2068), GAL4-PPARγ, PCMV-PRIP, PCMV-PPARγ, peroxisome proliferator response element (PPRE)-luciferase (LUC), PCMX-RXRα, and RXRE-LUC have been described elsewhere (12, 27, 32). PCMV-PIMT was constructed by inserting the full-length coding region of PIMT cDNA into HindIII/XhoI site of PCDNA 3.1(+) (Invitrogen). PCMV-PIMT-FLAG was made by inserting full-length PIMT cDNA into HindIII/BamHI site of p3XFLAG-CMV-14 (Sigma).
Isolation of Human and Mouse PIMT cDNA.
A partial cDNA encoding a protein that interacts with PRIP was first isolated by yeast two-hybrid screening of a human liver cDNA library, as described previously (12, 27). We then obtained the full-length cDNA by checking human expressed sequence tag (EST) in GenBank. The full-length cDNA we cloned has been designated PIMT to reflect its ability to bind PRIP and its putative functional domain. We then searched the mouse EST database in GenBank, by using the human PIMT cDNA sequence. Several ESTs, including ESTs covering the 5′ and 3′ of PIMT cDNA, were found. The primers 5′-GAGCCCGGATAACGAAATGT-3′ and 5′-CTTCCTCCTGTCTCTCACTT-3′ were synthesized on the basis of the EST sequences. The full-length mouse PIMT cDNA was amplified by using spleen mouse cDNA and sequenced.
Northern Blot Analysis and in Situ Hybridization.
Human multiple tissue Northern blot (CLONTECH) containing 2 μg of poly(A) RNA in each lane was probed with 32P-labeled PIMT full-length cDNA, according to the conditions outlined by the manufacturer. For in situ hybridization, tissues were fixed in 4% paraformaldehyde at 4°C for 14–18 h, dehydrated, embedded in paraffin, and 7-μm-thick sections were cut under ribonuclease-free conditions. RNA riboprobes (antisense and sense riboprobes) for PIMT were synthesized in the presence of digoxigenin-labeled UTP (Roche Diagnostics). Prehybridization, hybridization, washing, and immunological detection were performed as described (33).
Glutathione S-Transferase (GST) Pull-Down Assays.
The GST alone or GST-fusion protein, GST-PRIP (amino acids 772–1317), was expressed in Escherichia coli BL21 and bound to glutathione-Sepharose-4B beads according to the manufacturer's instructions (Amersham Pharmacia). In vitro translation was performed by using rabbit reticulocyte lysate (Promega) and labeled with [35S]methionine. In a GST pull-down assay, 15 μl of GST fusion protein on glutathione-Sepharose beads was incubated with 5 μl of [35S]methionine-labeled in vitro-translated proteins for 2 h in 600 μl of 20 mM Tris⋅HCl, pH 7.5/100 mM KCl/0.7 mM EDTA/0.05% Nonidet P-40/1 mM phenylmethylsulfonyl fluoride (NETN). Bound proteins were washed four times with 800 μl of NETN, eluted by boiling for 2 min in 24 μl of SDS loading buffer, separated by SDS/PAGE, and autoradiographed.
Immunoprecipitation.
COS-1 cells are transfected with PCMV-PIMT-FLAG by the calcium precipitation method. Twenty-four hours after transfection, the cell was harvested. The lysate was immunoprecipitated with anti-PRIP or preimmune serum. The precipitates were resolved on SDS/PAGE and subjected to Western blot analysis by using anti-FLAG (Sigma).
Cell Culture and Transfection.
CV-1 cells (1 × 105) were plated in six-well plates and cultured in DMEM containing 10% FCS for 24 h before transfection. Cells were transfected for 5 h with 1.5 μg of luciferase reporter plasmid DNA, 1.5 μg of appropriate expression plasmid DNA, and 0.4 μg of β-galactosidase expression vector pCMVβ (CLONTECH) DNA by using the Lipofectamine 2000-mediated transfection method (GIBCO/BRL). Cell extracts were prepared 24 h after transfection and assayed for luciferase and β-galactosidase activities (Tropix, Bedford, MA).
Immunofluorescence.
COS-1 cells were transfected with PCMV-PIMT-FLAG by using Lipofectamine 2000 (GIBCO/BRL), and 24 h after transfection, the cells were fixed in 1% formaldehyde and washed once with PBS, pH 7.4, after which autofluorescence was quenched with 50 mM ammonium chloride in PBS. Cells were washed with PBS, permeabilized with 0.5% saponin, and then blocked with 0.2% fish skin gelatin. The cells were incubated with the primary antibody anti-FLAG (Sigma) and anti-PRIP, followed by incubation with secondary antibodies. Fluorescence microscopy and digital image collection were performed by using an Olympus (New Hyde Park, NY) microscope and a photometrix cooled charge-coupled device camera driven by deltavision software from Applied Precision (Seattle).
AdoMet-Binding Assay.
Purified GST or GST-PIMT (10 μg) was incubated with 20 μCi of S-adenosyl-l-[methyl-3H]methionine (Amersham Pharmacia) in a buffer containing 20 mM Tris⋅HCl, pH 7.5, 150 mM NaCl, and 2 mM MgCl2, at 37°C for 10 min. The protein was trapped on a HAWP 02500 filter (Millipore). Unbound S-adenosyl-l-[methyl-3H]methionine was removed by washing with the buffer (20 mM Tris⋅HCl, pH 7.5/150 mM NaCl/2 mM MgCl2). The filters were dried, and the amount of bound S-adenosyl-l-[methyl-3H]methionine was quantified by liquid scintillation counting.
RNA-Binding Gel Retardation Assay.
The degenerate 32P-labeled RNA (GGAGACCGGCCAGGAUCCAAGCUNNNNNNNNCAUAAGGAUCCAAGCUAA) was generated by in vitro transcription (Ambion, Austin, TX). Reactions (20 μl) containing 0.1 μg of protein indicated, 1 μg of anti-FLAG, 10,000 cpm of RNA, and 1× buffer (20 mM Tris⋅HCl, pH 7.5/150 mM NaCl/2 mM MgCl2) was assembled and incubated for 15 min on ice. The reactions were loaded on a native polyacrylamide gel (Invitrogen) running at 300 V at 4°C in 0.5× TBE buffer (90 mM Tris/64.6 mM boric acid/2.5 mM EDTA, pH 8.3). The gels were dried and autoradiographed.
Results
Isolation of PIMT.
Using PRIP (amino acids 773-2068) as bait in a yeast two-hybrid system, we isolated from human liver cDNA library a partial cDNA encoding a PRIP-interacting protein, which was designated as PIMT. The rest of the cDNA was obtained from a human EST clone that was identified by searching for the homologous sequence in GenBank's human EST. The full-length human PIMT has an ORF of 2,556 nucleotides that encodes a protein of 852 amino acids (Fig. 1) with an estimated molecular mass of 96.5 kDa. The PIMT cDNA fragment directly recovered by yeast two-hybrid screening encodes amino acids from 1 to 384. The human PIMT cDNA contains a short 5′ (165-bp) and a long 3′ (743-bp) untranslated region (Fig. 1). The start of the coding sequence was defined by the first ATG downstream of an in-frame stop codon at position −150. The gene encoding human PIMT is localized on chromosome 8q11, spans more than 40 kb, and consists of more than 13 exons (data not presented). The full-length mouse PIMT cDNA we cloned is 2,648 bp in length, with an ORF of 2,559, which encodes a peptide of 853 amino acids that is 73% identical and 84% similar to the human PIMT (data not shown).
Figure 1.
The complete nucleotide and deducted amino acid sequence of human PIMT cDNA. The nucleotide numbering (Upper Right) begins as +1 from the initial ATG codon, and the amino acids for the single longest ORF are numbered starting with the first methionine (Lower Right). The initiation codon ATG is shown in boldface, and the termination codon TAA and the in-frame stop codon TAG at nucleotide position −150 are underlined. The consensus GXXGXXI found in the K-homology RNA-binding domain is underlined. Putative methyltransferase motifs I, II, and III are shown in boldface (motif I), underlined (motif II), and double underlined (motif III). Motif I and Post I (dotted) are known to interact directly with S-adenosyl-l-methionine.
When the nucleotide-derived amino acid sequences of human and mouse PIMTs were used to search the databases, no protein that showed significant overall similarity was detected, but the carboxyl region of human PIMT from amino acids 640–852 shows 56% identity and 68% similarity of amino acids with a protein of unknown function in Caenorhabditis elegans and 35% identity and 52% similarity of amino acids with the YPL157WP of the yeast (Fig. 2). They most likely represent homologues of human PIMT. Protein with such a high homology is not found in Drosophila. In this region, the human PIMT contains VVDAFCGVG (amino acids 693–701), which is similar to the highly conserved motif I with a consensus hh(D/S)(L/P)FXGXG, where “h” represents a hydrophobic residue, and “X” represents any amino acid, found in a number of m5C and m5A methyltransferases and other enzymes that use AdoMet as substrate (34–38). The C-terminal part of human PIMT also reveals regions that are similar to methyltransferase motif II, motif III, and Post 1 (Fig. 2).
Figure 2.
Homology of putative methyltransferase motifs (I, II, and III), and Post I, between human PIMT (amino acids 640–853), mouse PIMT, and C. elegans (GenBank accession no. T24696), Arabidopsis thaliana (GenBank accession no. AC009917), and yeast gene YPL157WP (GenBank accession no. Z73513) proteins. Dashes represent the same amino acid as in human PIMT, and asterisks represent spaces inserted for optimum alignment.
Tissue Distribution of the PIMT Transcript.
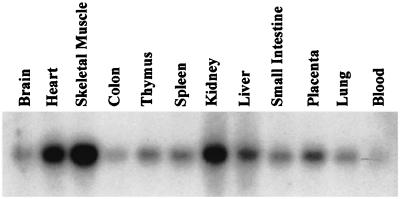
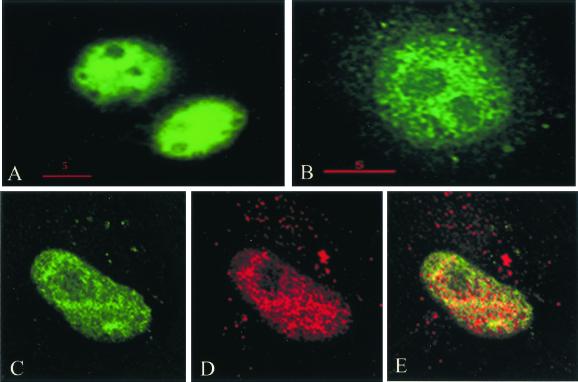
Human PIMT is expressed as a 3.2-kb transcript in all tissues examined, with high levels of expression of PIMT mRNA found in heart, skeletal muscle, and kidney (Fig. 3). By in situ hybridization, using mouse PIMT as a probe, we found the presence of PIMT transcripts in several tissues of adult mouse, including liver parenchymal cells, proximal tubular epithelium of kidney, intestinal epithelium, exocrine and endocrine pancreatic tissues, thyroid follicles, and prostate epithelium, among others (not illustrated). To localize PIMT protein, a plasmid containing three FLAG-epitopes linked to the C-terminal portion of the PIMT protein was transfected into COS-1 cells derived from African green monkey kidney (American Type Culture Collection CRL1651). Immunofluoresence with anti-FLAG revealed the expressed PIMT protein is localized in the nucleus (Fig. 4). Localization of PRIP by using anti-PRIP revealed that PRIP is also localized in the nucleus and, when the images of PIMT and PRIP localization were merged, an overlapping localization of PRIP and PIMT has been noted, suggesting colocalization of these molecules in the nucleus (Fig. 4).
Figure 3.
PIMT mRNA expression in various human tissues. A human multiple tissue Northern blot (CLONTECH) containing 2 μg of poly(A) RNA for each tissue was probed with 32P-labeled full-length human PIMT cDNA and then exposed to film at −80°C with an intensifier screen for 24 h. The transcript size of PIMT is 3.2 kb in all tissues examined.
Figure 4.
PIMT and PRIP colocalize in the nucleus. PRIP was visualized by using conventional fluorescence microscopy (A) and by deltavision deconvolution microscopy (B). By deltavision microscopy, a speckled pattern of distribution of this coactivator is noted. PIMT was expressed transiently in COS-1 cells by using three FLAG epitopes linked to the C terminus of PIMT, and the FLAG epitope of PIMT was visualized by deconvolution microscopy by using anti-FLAG antibodies (C). Merging of the PIMT (D) and PRIP (D) localization images reveals overlapping localization (yellow) of these two proteins (E).
Interaction of PIMT with PRIP in Vitro and in Vivo.
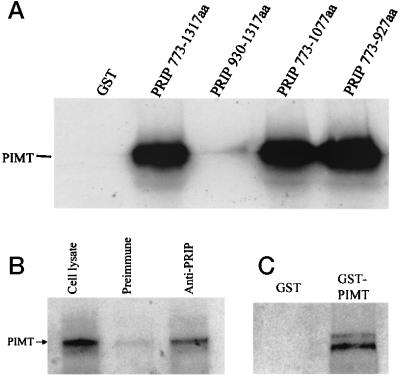
The direct interaction between PRIP and PIMT was further tested by using in vitro a GST-binding assay with a bacterially generated GST-PRIP fusion protein (amino acids 773-1317) and radioactively labeled in vitro-translated PIMT. As shown in Fig. 5, PIMT specifically interacted with the immobilized GST-PRIP but not with GST. Using further truncated PRIP, we narrowed the PIMT-binding site on PRIP to a region encompassing amino acids 773–927 (Fig. 5A). To determine whether PIMT and PRIP interact within the context of intact cells, a vector encoding human PIMT with C-terminal FLAG epitopes was transfected into COS-1 cells. The potential complex between PRIP and PIMT was immunoprecipitated by using anti-PRIP, and the product was analyzed by immunoblotting by using anti-FLAG to demonstrate PIMT in the precipitate (Fig. 5B). The results showed that anti-PRIP precipitated PIMT but not control serum, demonstrating the existence of a PRIP and PIMT complex in vivo (Fig. 5B). Because PRIP is able to form homodimers, we examined whether PIMT can also form homodimers. A GST pull-down assay was performed with GST-PIMT fusion protein (amino acids 326–852) and in vitro-translated [35S]methionine-labeled human PIMT. The results demonstrated that in vitro-translated PIMT interacts with GST-PIMT, suggesting that PIMT may form homooligomers (Fig. 5C).
Figure 5.
In vitro interaction of PRIP with PIMT. (A) [35S]Methionine-labeled full-length PIMT generated by in vitro translation was incubated with glutathione-Sepharose beads bound with purified E. coli-expressed GST-PRIP (amino acids 773-1317, 930-1317, 773-1077, and 773–927) or GST. The bound proteins were eluted and analyzed by using 10% SDS/PAGE and autoradiographed. (B) Coimmunoprecipitation of PIMT and PRIP in intact cells. pcDNA3.1-FLAG-PIMT were transfected into COS-1 cells and, 24 h after transfection, cells were harvested. Cell lysate was immunoprecipitated with anti-PRIP or control serum, and immunoprecipitates were subjected to immunoblotting with anti-FLAG. Epitope-tagged protein PIMT can be coprecipitated by anti-PRIP (lane 3) but not by preimmune serum (lane 2). Lane 1, 1/20 input cell lysate. (C) PIMT forms homodimers. GST-PIMT (326-852 aa) or GST alone was incubated with PIMT labeled with [35S]methionine. The bound protein was electrophoresed and visualized by fluorography. GST-PIMT, but not GST alone, binds PIMT.
PIMT Binds AdoMet.
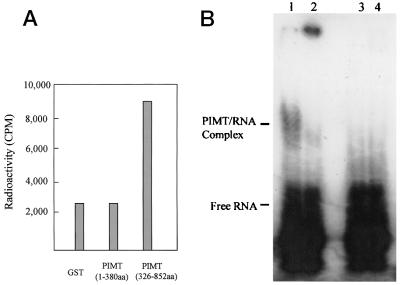
Human PIMT contains discernible methyltransferase motifs I, II, and III, and also a conserved motif called Post 1 (Figs. 1 and 2). Because motif I (FXGXG) is known to interact with an adenosyl moiety of the cofactor (34, 35), we assessed the ability of human PIMT to bind AdoMet by a filter-binding assay. The partial PIMT (amino acid residues 326–852), which contains the putative methyltransferase domain, binds AdoMet, whereas the N-terminal PIMT fragment (amino acids 1–384), which does not contain these motifs, failed to bind AdoMet (Fig. 6A).
Figure 6.
PIMT binds AdoMet and RNA. (A) PIMT binds AdoMet. Purified protein was incubated with S-adenosyl-l-[methyl-3H]methionine, and the amount of protein bound to AdoMet was trapped on a filter and quantified by liquid scintillation. GST-PIMT (amino acids 326–852), which contained the methyltransferase domain, retained a significant amount of labeled AdoMet, whereas the filter with GST-PIMT (amino acids 1–384) showed background radioactivity as that with GST alone. (B) PIMT is an RNA-binding protein. 32P-labeled RNA produced by in vitro transcription is incubated with purified FLAG-PIMT (amino acids 1–384). A PIMT–RNA complex is seen in addition to the free RNA (lane 1). The addition of anti-FLAG markedly diminished the complex; instead, antibody and PIMT–RNA formed a big complex, which hardly migrated into the gel (lane 2). Lanes 3 and 4 represent controls by using purified FLAG-PRIP (amino acids 786-1132) and purified FLAG-PRIP (amino acids 786-1132) plus anti-FLAG, respectively.
PIMT Is an RNA-Binding Protein.
In an effort to search for the potential substrate of PIMT, we examined whether PIMT can bind to RNA by using a gel-shift assay. When purified FLAG-PIMT (amino acids 1–384) was incubated with 32P-labeled RNA produced by in vitro transcription, a PIMT–RNA complex was produced (Fig. 6B, lane 1). The addition of anti-FLAG markedly diminished the formation of the PIMT–RNA complex; instead, antibody and PIMT–RNA formed a high molecular-weight complex, which failed to migrate into the gel (Fig. 6B, lane 2). As a control, when purified FLAG-PRIP (amino acids 786-1132) was used in an RNA-binding assay with or without anti-FLAG, no detectable RNA binding was observed (Fig. 6B, lanes 3 and 4). Close examination of PIMT amino acids 1–384 revealed that they contained a consensus sequence GXXGXXI (Fig. 1), which is present in virtually all K-homology domains of RNA-binding proteins (39, 40). A gel retardation experiment revealed that FLAG-PIMT (amino acids 1–384) was unable to bind single- and double-stranded DNA (data not shown).
PIMT Potentiates the Transcriptional Activity of PPARγ.
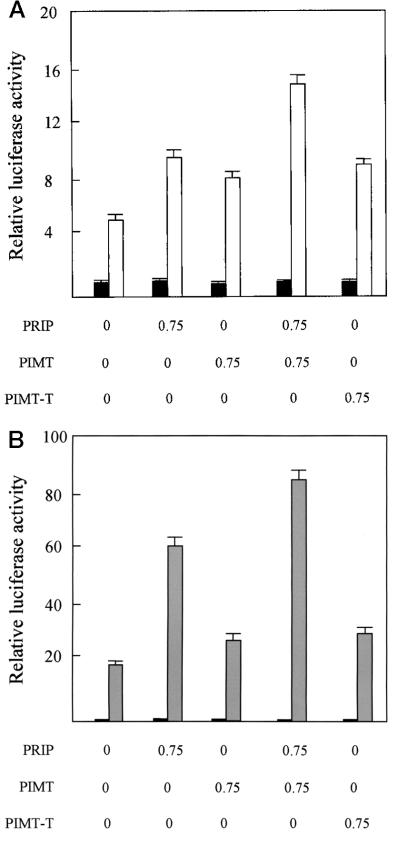
Το determine the functional relevance of the interaction of PIMT with PRIP, we transiently overexpressed PIMT and PRIP in CV-1 cells along with PPARγ and monitored the transcriptional activity of PPARγ with expression of the PPRE-linked reporter luciferase gene. Both PIMT and PRIP, when transfected individually, consistently increased the transcription of the PPARγ-mediated luciferase gene by about 1.6-fold in the presence of PPARγ ligand BRL49653 (Fig. 7A). Cotransfection of PIMT and PRIP resulted in further enhancement of ligand-dependent reporter gene expression, indicating that PIMT synergizes PRIP action. The truncated PIMT (amino acids 1–384), without methyltransferase domains, can also enhance PPARγ transcriptional activity by about 1.8-fold, suggesting the methyltransferase domain is not necessary for PIMT's ability to enhance the transcriptional activity of PPARγ under the conditions of transient transfection. Similar results were observed with RXRα (Fig. 7B).
Figure 7.
PIMT increases PPARγ- and RXRα-mediated transactivation of reporter expression in CV-1 cells. (A) CV-1 cells were cotransfected with 1.5 μg of reporter construct PPRE-TK-LUC, 25 ng of PCMV-mPPARγ, and 0.4 μg of PCMVβ, along with the indicated plasmid in the absence (solid bar) or presence (open bar) of 10−5 M BRL49653. Transfection, without the indicated plasmid, was compensated by adding the same amount of PCDNA3.1. The activity obtained on transfection of PPRE-TK-LUC, without exogenous PIMT in the absence of ligand was taken as 1. Results are the mean of three independent transfections. (B) Transfection analysis for RXRα was performed in the same way as PPARγ, except for using RXRE-TK-LUC and PCMX-RXRα in the absence (solid bar) and presence (hatched bar) of ligand 9-cis-retinoic acid.
Discussion
In a previous study, using the yeast two-hybrid system, we isolated and characterized mouse PRIP as a PPAR coactivator (27). The cloning of this nuclear receptor coactivator from human and rat has also been reported (28–31). PRIP has been shown to interact with a variety of nuclear receptors and also CBP and is widely expressed with the highest expression in heart, ovary, testis, prostate, and skeletal muscle (27–31). These observations suggested this coactivator may prove as indispensable as CBP, p300, and PBP in mediating the transcriptional activity of nuclear receptors and other transcription factors. In this study, we used a two-hybrid system with PRIP as bait and isolated PIMT, a PRIP-interacting protein, which has a methyltransferase domain. The mRNA of PIMT is ubiquitously expressed, and its protein appears colocalized in the nucleus with PRIP. It appears that both PRIP and PIMT are expressed abundantly in the same tissues and cell types, suggesting these two proteins play a synergistic function in vivo. The PIMT-binding region in PRIP (amino acids 773–927) has also been determined by using truncated portions of PRIP in GST pull-down assays; this region avidly binds PIMT. Overexpression of PIMT enhances the transcriptional activity of PPARγ and RXR, and this enhancement is further stimulated by overexpression of PRIP, suggesting that PIMT is a component of nuclear receptor signal transduction that acts through PRIP.
PIMT reveals the presence of an AdoMet-binding site VVDAFCGVG, which is similar to the highly conserved methylltransferase motif I with a consensus hh(D/S)(L/P)FXGXG, (where “h” represents a hydrophobic residue, and “X ” represents any amino acid) (34, 35). This motif is found in several methyltransferases and other enzymes that use AdoMet as substrate (34–36). Of considerable interest is that the motif FCGVG present in PIMT corresponds to the sequence (FXGXG) found in many RNA and DNA methyltransferases (34, 35, 41). The Phe in this motif is known to interact with the adenosyl moiety of the cofactor AdoMet (35). In other methyltransferases, particularly in protein arginine methyltransferases, Phe is often substituted by Gly (34, 35). The binding of AdoMet, the donor of methyl groups, to purified PIMT under in vitro conditions essentially confirms that PIMT is a putative methyltransferase, but the target of PIMT remains unknown. Although PIMT failed to methylate in vitro either histone, unlike CARM1 (15), or DNA (Y.Z., unpublished work), we found that it binds RNA. The segment of PIMT (amino acids 1–384) that binds RNA contains an invariant GXXGXXI motif present in the K-homology domains of RNA-binding proteins (39, 40). It is estimated that the human genome contains ≈1,500 RNA-binding proteins, but their distribution across global, group-specific, and type-specific classes is unknown (42). PIMT may function as a putative RNA methyltransferase, raising the possibility that some coactivators and/or their cofactors may influence transcription by binding and methylating RNA. PIMT shows high homology with an uncharacterized protein YPL157W (GenBank accession no. Z73513) from yeast and a functionally unknown protein (GenBank accession no. T24696) from C. elegans. They most probably represent the homologues of PIMT and should have similar substrates. The yeast strain with null mutation of YPL157W gene has been generated by the Saccharomyces Genome Deletion Project and has turned out to be viable. We have analyzed the methyl proteins in the YPL157W mutated and wild strains by in vivo labeling with S-adenosyl-l-[methyl-3H]methionine. The methyl protein pattern in the mutated strain appeared similar to that of wild type, but we cannot rule out the possibility that the YPL157W protein methylates a less abundant protein not identified by SDS/PAGE gel (Y.Z., unpublished work).
Methylation plays an important role in the regulation of gene expression (43, 44). Methylation of the promoter participates in the inactivation of gene transcription, which contributes to the silencing of tumor suppressor genes in cancers, whereas methylation of sites downstream of transcriptional initiation is associated with increased transcription (41, 44). In addition to DNA methylation, the importance of histone methylation in the transcriptional regulation is increasingly appreciated (15, 16). Although PIMT does not methylate the histone, it could be involved in transcriptional regulation by methylating other transcriptional components. Truncated PIMT, without the methyltransferase domain, still showed its ability to enhance the transcriptional activity of PPARγ and RXRα, suggesting this enzyme activity may not be crucial to transcription under transient transfection conditions. Given the oversimplified transient transfection system, the role of PIMT's methyltransferase activity in transcriptional regulation remains to be appreciated in the context of in vivo physiological condition.
Acknowledgments
We thank Travis Harrison for his expert technical assistance with DeltaVision Microscopy. This work was supported by National Institutes of Health Grants GM23750 (J.K.R.), CA84472 (M.S.R.), CA88898 (Y.Z.), and K08 ES00356 (Y.Z.), and by the Joseph L. Mayberry, Sr., Endowment Fund, Northwestern University.
Abbreviations
- PPAR
peroxisome proliferator-activated receptor
- PPRE
peroxisome proliferator response element
- SRC-1
steroid receptor coactivator-1
- CARM1
coactivator-associated arginine methyltransferase 1
- PRIP
PPAR-interacting protein
- PIMT
PRIP-interacting protein with methyltransferase domain
- EST
expressed sequence tag
- RXR
retinoid-X-receptor for 9-cis-retinoic acid
- GST
glutathione S-transferase
- AdoMet
S-adenosyl-l-methionine
- PBP
PPAR-binding protein
Footnotes
References
- 1.Evans R M. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKenna N J, Lanz R B, O'Malley B W. Endocrine Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 3.Nagpal S, Friant S, Nakshatri H, Chambon P. EMBO J. 1993;12:2349–2360. doi: 10.1002/j.1460-2075.1993.tb05889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glass C K, Rosenfeld M G. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- 5.Shang Y, Hu X, DiRenzo J, Lazar M A, Brown M. Cell. 2000;103:843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 6.Onate SA, Tsai S Y, Tsai M J, O'Malley B W. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 7.Voegel J J, Heine M J S, Zechel C, Chambon P, Gronemeyer H. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Lin R J, Schiltz R L, Chakravarti D, Nash A, Nagy L, Privalsky M L, Nakatani Y, Evans R M. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 9.Anzick S L, Kononen J, Walker R L, Azorsa D O, Tanner M M, Guan X Y, Sauter G, Kallioniemi O P, Trent J M, Meltzer P S. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 10.Torchia J, Rose D W, Inostroza J, Kamei Y, Westin S, Glass C K, Rosenfeld M G. Nature (London) 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 11.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Qi C, Jain S, Rao M S, Reddy J K. J Biol Chem. 1997;272:25500–25506. doi: 10.1074/jbc.272.41.25500. [DOI] [PubMed] [Google Scholar]
- 13.Puigserver P, Wu Z, Park C W, Graves R, Wright M, Spiegelman B M. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 14.Chakravarti D, LaMorte V J, Nelson M C, Nakajima T, Schulman I G, Juguilon H, Montminy M, Evans R M. Nature (London) 1996;383:99–102. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- 15.Chen D, Ma H, Hong H, Koh S S, Huang S-M, Schurter B T, Aswad D W, Stallcup M R. Science. 1999;284:2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- 16.Koh S S, Chen D, Lee Y-H, Stallcup M R. J Biol Chem. 2001;276:1089–1098. doi: 10.1074/jbc.M004228200. [DOI] [PubMed] [Google Scholar]
- 17.Yuan C X, Ito M, Fondell J D, Fu Z Y, Roeder R G. Proc Natl Acad Sci USA. 1998;95:7939–7944. doi: 10.1073/pnas.95.14.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rachez C, Lemon B D, Suldan Z, Bromleigh V, Gamble M, Naar A M, Erdjument-Bromage H, Tempst P, Freedman L P. Nature (London) 1999;398:824–828. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 19.Naar A M, Beaurang P A, Zhou S, Abraham S, Solomon W, Tjian R. Nature (London) 1999;398:828–832. doi: 10.1038/19789. [DOI] [PubMed] [Google Scholar]
- 20.Qi C, Zhu Y, Reddy J K. Cell Biochem Biophys. 1999;31:1–18. [Google Scholar]
- 21.Yao T-P, Oh S P, Fuchs M, Zhou N-D, Ch'ng L-E, Newsome D, Bronson R T, Li E, Livingston D L, Eckner R. Cell. 1998;93:361–372. doi: 10.1016/s0092-8674(00)81165-4. [DOI] [PubMed] [Google Scholar]
- 22.Oike Y, Takakura N, Hata A, Kaname T, Akizuki M, Yamaguchi Y, Yasue H, Araki K, Yamamura K, Suda T. Blood. 1999;93:2771–2779. [PubMed] [Google Scholar]
- 23.Kung A L, Rebel V I, Bronson R T, Ch'ng L-E, Siefff C A, Livingston D M, Yao T-P. Genes Dev. 2000;14:272–277. [PMC free article] [PubMed] [Google Scholar]
- 24.Goodman R H, Smolik S. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- 25.Zhu Y, Qi C, Jia Y, Nye J S, Rao M S, Reddy J K. J Biol Chem. 2000;275:14779–14782. doi: 10.1074/jbc.C000121200. [DOI] [PubMed] [Google Scholar]
- 26.Ito M, Yuan C-X, Okano H J, Darnell R B, Roeder R G. Mol Cell. 2000;5:683–693. doi: 10.1016/s1097-2765(00)80247-6. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Y, Kan L, Qi C, Kanwar Y S, Yeldandi A V, Rao M S, Reddy J K. J Biol Chem. 2000;275:13510–13516. doi: 10.1074/jbc.275.18.13510. [DOI] [PubMed] [Google Scholar]
- 28.Lee S-K, Anzick S L, Choi J-E, Bubendorf L, Guan X-Y, Jung Y-K, Kallioniemi O P, Kononen J, Trent J M, Azorsa D, et al. J Biol Chem. 1999;274:34283–34293. doi: 10.1074/jbc.274.48.34283. [DOI] [PubMed] [Google Scholar]
- 29.Mahajan M, Samuels H H. Mol Cell Biol. 2000;20:5048–5063. doi: 10.1128/mcb.20.14.5048-5063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caira F, Antonson P, Pelto-Huikko M, Treuter E, Gustafsson J-A. J Biol Chem. 2000;275:5308–5317. doi: 10.1074/jbc.275.8.5308. [DOI] [PubMed] [Google Scholar]
- 31.Ko L, Cardona G R, Chin W W. Proc Natl Acad Sci USA. 2000;97:6212–6217. doi: 10.1073/pnas.97.11.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu Y, Qi C, Calandra C, Rao M S, Reddy J K. Gene Expression. 1996;6:185–195. [PMC free article] [PubMed] [Google Scholar]
- 33.Komminoth P, Merk F B, Leav I, Wolfe H J, Roth J. Histochemistry. 1992;98:217–228. doi: 10.1007/BF00271035. [DOI] [PubMed] [Google Scholar]
- 34.Lauster R. J Mol Biol. 1989;206:313–321. doi: 10.1016/0022-2836(89)90481-6. [DOI] [PubMed] [Google Scholar]
- 35.Kagan R M, Clarke S. Arch Biochem Biophys. 1994;310:417–427. doi: 10.1006/abbi.1994.1187. [DOI] [PubMed] [Google Scholar]
- 36.Kumar S, Cheng X, Klimasaukas S, Mi S, Posfai J, Roberts R J, Wilson G. Nucleic Acids Res. 1994;22:1–10. doi: 10.1093/nar/22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang J, Gary J D, Clarke S, Herschman H R. J Biol Chem. 1998;273:16935–16945. doi: 10.1074/jbc.273.27.16935. [DOI] [PubMed] [Google Scholar]
- 38.Jeltsch A, Christ F, Fatemi M, Roth M. J Biol Chem. 1999;274:19538–19544. doi: 10.1074/jbc.274.28.19538. [DOI] [PubMed] [Google Scholar]
- 39.Burd C G, Dreyfuss G. Science. 1994;265:615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- 40.Lewis H A, Musunuru K, Jensen K B, Edo C, Chen H, Darnell R B, Burley S K. Cell. 2000;100:323–332. doi: 10.1016/s0092-8674(00)80668-6. [DOI] [PubMed] [Google Scholar]
- 41.Wu G, William H D, Zamanian M, Gibson F, Poole R K. J Gen Virol. 1992;138:2101–2112. doi: 10.1099/00221287-138-10-2101. [DOI] [PubMed] [Google Scholar]
- 42.Keene J D. Proc Natl Acad Sci USA. 2001;98:7018–7024. doi: 10.1073/pnas.111145598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colota V, Rossignol J-L. BioEssays. 1999;21:402–411. doi: 10.1002/(SICI)1521-1878(199905)21:5<402::AID-BIES7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 44.Smith S S. Int J Mol Med. 1998;1:147–156. [PubMed] [Google Scholar]