Abstract
INTRODUCTION
Direct home discharge (DHD) following hip fracture surgery represents a challenging proposition. The aim of this study was to identify factors influencing the discharge destination (home vs alternative location) for patients admitted from their own home with a fractured neck of femur.
METHODS
A retrospective cohort study of prospectively collected major trauma centre data was performed, identifying 10,044 consecutive hip fracture admissions between 2000 and 2012.
RESULTS
Two-thirds of the patients (n=6,742, 67%) were admitted from their own home. Half of these (n=3,509, 52%) returned directly to their own home while two-fifths (n=2,640, 39%) were discharged to an alternative location; 593 (9%) died. The following were identified as independent variables associated with a higher likelihood of DHD: younger patients, female sex, an abbreviated mental test score of 10, absence of certain co-morbidities, cohabiting, walking independently outdoors, no use of walking aids, no assistance required with basic activities of daily living and intracapsular fracture.
CONCLUSIONS
Identifying those at risk of being discharged to an alternative location following admission from home on the basis of identified preoperative indices could assist in streamlining the postoperative care phase. Pre-emptive action may help increase the numbers of patients discharged directly home and reduce the number requiring additional rehabilitation prior to discharge home with its associated socioeconomic effect.
Keywords: Hip fracture, Trauma, Epidemiology, Direct home discharge, Discharge to alternative location
Over the last 20 years there has been a year-on-year increase in the number of hip fracture patients admitted to hospitals in England.1,2 Our unit has previously reported on the factors that influence discharge destination following hip fracture.3 These include the level of preinjury dependence, age, sex and whether the injury was sustained in hospital. Following hip fractures, many patients are unable to reach premorbid levels of ambulatory function3 and return to their premorbid living arrangements.4 This occurs despite increasing community support and rehabilitation facilities initiated as part of strategies linked to the National Service Framework for Older People5 and National Institute for Health and Care Excellence (NICE) guidance on hip fractures.6
Despite the availability of improved postoperative rehabilitation facilities, there remains a ‘downward drift’ in discharge destination, with approximately 20% of patients moving from their own home to institution-based care provision following hip fracture.7 This increased care requirement will amplify the socioeconomic burden associated with this patient group. The aim of hip fracture management should be to rehabilitate patients as quickly and as safely as possible back to their premorbid function.3 Identifying patients who are suitable and capable of direct home discharge (DHD) will enable us to maximise the use of community care and the enhanced community rehabilitation infrastructure that has evolved over recent years.
Currently, is it not clear which preoperative factors influence discharge destination (specifically DHD).3,8 The aim of the present study was therefore to determine which parameters are associated with DHD vs discharge to an alternative location [DAL] for patients admitted from their own home with a fractured neck of femur.
Methods
The present study was conducted as a retrospective cohort study using the information held in a major trauma centre hip fracture database on all hip fracture patients admitted between 1 January 2000 and 31 December 2012. This cohort and the methods of data extraction have been described previously.1 These dates were chosen as they represented 13 consecutive years for which complete data were available for the entire year.
Data for all hip fracture patients are recorded using a modified version of the Standardised Audit of Hip Fractures in Europe (SAHFE) audit form. SAHFE data completion is a mandatory requisite for all hip fracture cases admitted to our hospital. In 2012–2013 our trust scored 94% in the domain of data completeness in the National Hip Fracture Database.9 Audit data are strictly confidential and managed in accordance with national data protection (Caldecott) guidelines. During the study period, there was no change in the definitions used by the audit for collected data. This was a pragmatic, clinical audit with diagnosis based on clinical history, examination and retrospective review of medical records.
Information was extracted from the database on pertinent patient demographics (eg age and sex), physical functioning (mobility status, independence both within and outside the house, and ability to perform activities of daily living [ADLs] such as washing, dressing, cleaning, feeding and toileting), social circumstances (type of residence, cohabitation and requirement for additional carers), co-morbidities and medications, and admission characteristics (fracture type, admission haemoglobin). The specific co-morbidities assessed comprised cardiovascular disease (CVD), cerebrovascular accident (CVA), chronic obstructive pulmonary disease (COPD), renal disease, diabetes mellitus, rheumatoid arthritis, Parkinson’s disease and malignancy. Information was also available relating to the use of antiplatelet agents and/or anticoagulants (clopidogrel/warfarin) as well as polypharmacy (≥4 regular medications). The abbreviated mental test score (AMTS) was used to assess cognitive capacity.
Following treatment, a patient can take a number of paths that may or may not lead to return to home. This study specifically investigated patients who were living at home prior to their injury and who were subsequently discharged home directly from the acute ward following treatment. Interim rehabilitation stays either in the admitting hospital or in community facilities (eg community hospitals, residential rehabilitation units or interim residential home placements) prior to an eventual discharge home were not considered as DHD.
Statistical analysis
Initial comparative analysis was undertaken to determine the differences in the factors described above between patients admitted from their own home and those admitted from another residence. Having identified a cohort of patients admitted from their own home, factors associated with DHD vs DAL were analysed. Data were initially compared using Student’s t-test (parametric continuous data), the Mann–Whitney U test (non-parametric continuous data), and Fisher’s and chi-squared tests (categorical data) as appropriate.
Univariate and multivariate logistic analyses from binomial regression were conducted to establish which factors influence DHD and to quantify their relative influence using odds ratios (ORs). Multivariate models were derived using both forward stepwise regression from the null model and backward stepwise regression from the full model. Within the models, a p-value of <0.01 was considered to be significant. The same model was derived from forward and backward regression.
ORs were produced for each factor to determine the odds of DHD compared with the reference category for that factor. For example, a patient aged <65 years had an OR of 9.2 of DHD compared with the reference group of patients aged >85 years, meaning a patient in the younger group was 9.2 times more likely to be discharged home than the reference category. An OR of >1 indicates a greater chance of discharge home relative to the reference category whereas an OR of <1 indicates a decreased chance of discharge home relative to the reference category. Data generated and utilised previously1 were analysed with Excel® 2011 (Microsoft, Redmond, WA, US) and SPSS® version 19 (IBM, New York, US).
Results
The study population consisted of 10,044 patients admitted between January 2000 and December 2012. Of these, 6,742 were admitted from their own home.
Patients admitted from their own home
The demographics of the cohort living in their own home as well as of the patient group not living in their own home are shown in Table 1. Patients admitted from their own home tended to be younger, were more likely to be male and had a significantly higher median AMTS (all p<0.001). This group was more independent physically and socially, with higher levels of walking ability, lower reliance on walking aids and a lower requirement for assistance with activities of daily living (ADLs) (all p<0.001). Patients living at home had a significantly lower rate of previous CVAs (p<0.001) although rates of other co-morbidities were similar between the two groups. The group living at home also had a lower rate of polypharmacy than those admitted from another location (p<0.001).
Table 1.
Patient demographics by place of residence prior to admission
| Admission residence | p-value | ||
| Own home (n=6,742) | Other (n=3,302) | ||
| Median age | 81 yrs (IQR: 73–86 yrs) | 86 yrs (IQR: 81–90 yrs) | <0.001 |
| Sex Male Female |
1,977 (29%) 4,765 (71%) |
649 (20%) 2,653 (80%) |
<0.001 |
| Median preinjury AMTS | 10 (IQR: 7–10) | 3 (IQR: 0–8) | <0.001 |
| Preinjury co-morbidities Cardiovascular disease Cerebrovascular accident COPD Renal disease Diabetes Rheumatoid arthritis Parkinson’s disease Malignancy |
3,282 (49%) 864 (13%) 1,185 (18%) 411 (6%) 851 (13%) 259 (4%) 198 (3%) 858 (13%) |
1,569 (48%) 578 (18%) 541 (16%) 203 (6%) 391 (12%) 91 (3%) 127 (4%) 325 (10%) |
0.14 <0.001 0.07 0.48 0.14 0.003 0.009 <0.001 |
| Smokers | 946 (14%) | 211 (6%) | <0.001 |
| Preinjury medication Clopidogrel Warfarin Polypharmacy (≥4 medications) |
292 (4%) 105 (2%) 2,365 (35%) |
83 (3%) 38 (1%) 1541 (47%) |
<0.001 0.06 <0.001 |
| Preinjury walking ability Alone outdoors Alone indoors Accompanied outdoors Accompanied indoors Unable / transfers only Unknown |
4,262 (63%) 1,146 (17%) 864 (13%) 147 (2%) 96 (1%) 227 (3%) |
765 (23%) 1,297 (39%) 438 (13%) 414 (13%) 165 (5%) 223 (7%) |
<0.001 |
| Walking aids used prior to injury No aids One aid Two aids Frame / walker Wheelchair / bed bound Unknown |
3,216 (48%) 2,098 (31%) 199 (3%) 978 (15%) 132 (2%) 119 (2%) |
1,218 (37%) 727 (22%) 62 (2%) 1031 (31%) 161 (5%) 103 (3%) |
<0.001 |
| Preinjury assistance with basic care ADLs (washing, dressing, feeding, toileting etc) | 920 (14%) | 1,678 (51%) | <0.001 |
ADLs = activities of daily living; AMTS = abbreviated mental test score; COPD = chronic obstructive pulmonary disease; IQR = interquartile range
Patients discharged back to their own home
A total of 3,509 (52%) of the 6,742 patients admitted from their own home underwent DHD. Of the remaining patients, 593 (9%) died, 1,995 (30%) went to rehabilitation facilities (eg community hospitals, residential rehabilitation units or facilities for care of the elderly such as acute healthcare of older people [HCOP] wards), 186 (3%) went to an acute hospital bed (eg transfer to an acute non-HCOP bed in the same hospital or repatriation to the patient’s local acute hospital), 147 (2%) went to a nursing or residential home institution and 312 (5%) went to other locations (Fig 1).
Figure 1.
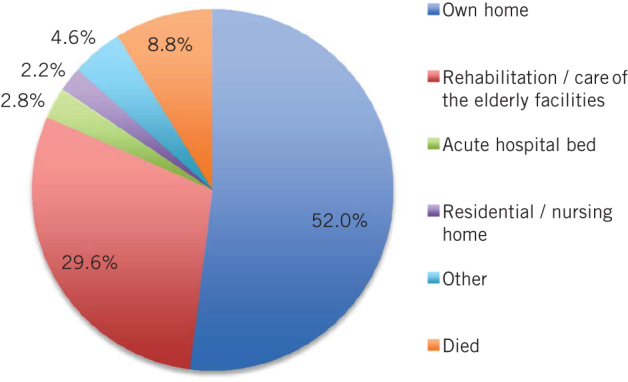
Discharge location for 6,742 patients admitted from their own home following fractured neck of femur
A summary of the demographics of those patients directly discharged to their own home (DHD) versus those discharged to another location (DAL) is given in Table 2. Patients who underwent DHD were younger, had a higher AMTS, a lower incidence of specific co-morbidities, and a lower incidence of use of medication including antiplatelets and anticoagulants (all p<0.001). In addition, they had a higher level of physical functioning and required less assistance with their mobility.
Table 2.
Demographics for the 6,742 patients admitted from their own home by discharge destination
| Discharge destination | p-value | ||
| Own home (n=3,509) | Other (n=3,233) | ||
| Median age | 77 yrs (IQR: 68–83 yrs) | 84 yrs (IQR: 78–88 yrs) | <0.001 |
| Sex Male Female |
1,018 (29%) 2,491 (71%) |
959 (30%) 2,274 (70%) |
0.28 |
| Median preinjury AMTS | 10 (IQR: 9–10) | 9 (IQR: 5–10) | <0.001 |
| Preinjury co-morbidities Cardiovascular disease Cerebrovascular accident COPD Renal disease Diabetes Rheumatoid arthritis Parkinson’s disease Malignancy |
1,506 (43%) 335 (10%) 547 (16%) 131 (4%) 384 (11%) 139 (4%) 65 (2%) 434 (12%) |
1,776 (55%) 529 (16%) 638 (20%) 280 (9%) 467 (14%) 120 (4%) 133 (4%) 424 (13%) |
<0.001 <0.001 <0.001 <0.001 <0.001 0.32 <0.001 0.19 |
| Smokers | 604 (17%) | 342 (11%) | <0.001 |
| Preinjury medication Clopidogrel Warfarin Polypharmacy (≥4 medications) |
122 (4%) 35 (1%) 1,047 (30%) |
170 (5%) 70 (2%) 1,318 (41%) |
<0.001 <0.001 <0.001 |
| Living alone prior to injury | 1,449 (41%) | 1,767 (55%) | <0.001 |
| Preinjury walking ability Alone outdoors Alone indoors Accompanied outdoors Accompanied indoors Unable / transfers only Unknown |
2,621 (75%) 373 (11%) 346 (10%) 48 (1%) 46 (1%) 75 (2%) |
1,641 (51%) 773 (24%) 518 (16%) 99 (3%) 50 (2%) 152 (5%) |
<0.001 |
| Walking aids used prior to injury No aids One aid Two aids Frame / walker Wheelchair / bed bound Unknown |
2,088 (60%) 912 (26%) 82 (2%) 316 (9%) 69 (2%) 42 (1%) |
1,128 (35%) 1,186 (37%) 117 (4%) 662 (21%) 63 (2%) 77 (2%) |
<0.001 |
| Preinjury assistance with basic care ADLs (washing, dressing, feeding, toileting etc) | 302 (9%) | 618 (19%) | <0.001 |
ADLs = activities of daily living; AMTS = abbreviated mental test score; COPD = chronic obstructive pulmonary disease; IQR = interquartile range
Table 3 compares the different discharge locations (DHD vs DAL) for the different fracture types. There was a higher proportion of intracapsular fractures in the DHD group than in the DAL group (60% vs 52%, p<0.001).
Table 3.
Fracture type by discharge destination for the 6,742 patients admitted from their own home
| Fracture type | Discharge destination | p-value | |
| Own home (n=3,509) | Other (n=3,233) | ||
| Intracapsular (undisplaced or displaced) Basicervical Trochanteric (2–4-part fractures) Subtrochanteric / reverse oblique Other / unknown |
2,120 (60%) 137 (4%) 932 (27%) 265 (8%) 53 (2%) |
1,687 (52%) 127 (4%) 1,114 (35%) 270 (8%) 35 (1%) |
<0.001 |
Univariate and multivariate analysis
The results of the univariate and multivariate regression analyses are shown in Table 4. Univariate analysis demonstrated that when considered in isolation, most of the factors had an influence of the odds of DHD. However, once these factors were considered simultaneously in the multivariate analysis, patients were seen to have an increased chance of DHD if they: were younger; were female; had an AMTS of 10; did not have either CVA, COPD, renal disease or Parkinson’s disease; cohabited; walked independently outdoors prior to injury; did not use a walking aid; did not require assistance with basic ADLs; had an intracapsular fracture; and had an admission haemoglobin level of ≥12g/dl (all p<0.01).
Table 4.
Univariate and multivariate regression models predicting DHD. Results are presented as ORs where an OR of >1 indicates an increased chance of DHD compared with the reference category and an OR of <1 indicates a reduced chance of DHD. All results from the univariate analysis are presented but for clarity, only the statistically significant results from the multivariate regression are presented. Non-significant results (p>0.05) are represented by an asterisk.
| Univariate analysis | Multivariate analysis | |||
| OR | p-value | OR | p-value | |
| Patient / co-morbid characteristics | ||||
| Age group <65 years 65–75 years 75.01–85 years >85 years |
9.2 (95% CI: 7.6–11.1) 3.9 (95% CI: 3.3–4.5) 2.1 (95% CI: 1.9–2.4) Reference |
<0.001 <0.001 <0.001 – |
5.5 (95% CI: 4.4–6.8) 2.7 (95% CI: 2.3–3.2) 1.7 (95% CI: 1.5–1.9) Reference |
<0.001 <0.001 <0.001 – |
| Sex Male Female |
Reference 1.0 (95% CI: 0.9–1.1) |
– 0.56 |
Reference 1.3 (95% CI: 1.1–1.5) |
– <0.001 |
| Preinjury AMTS 10 ≤9 |
2.6 (95% CI: 2.4–2.9) Reference |
<0.001 – |
1.7 (95% CI: 1.5–1.9) Reference |
<0.001 – |
| Preinjury co-morbidities (Reference = Yes) CVD = No CVA = No COPD = No Renal disease = No Diabetes = No Rheumatoid arthritis = No Parkinson’s disease = No Malignancy = No |
1.6 (95% CI: 1.5–1.8) 1.9 (95% CI: 1.6–2.1) 1.3 (95% CI: 1.2–1.5) 2.4 (95% CI: 2.0–3.0) 1.4 (95% CI: 1.2–1.6) 0.9 (95% CI: 0.7–1.2) 2.3 (95% CI: 1.7–3.1) 1.1 (95% CI: 0.9–1.2) |
<0.001 <0.001 <0.001 <0.001 <0.001 0.59 <0.001 0.36 |
* 1.4 (95% CI: 1.1–1.6) 1.2 (95% CI: 1.1–1.4) 1.7 (95% CI: 1.3–2.1) * * 2.0 (95% CI: 1.5–2.8) * |
* <0.001 0.003 <0.004 * * <0.001 * |
| Smoking Yes No |
Reference 0.6 (95% CI: 0.5–0.7) |
– <0.001 |
* |
* |
| Preinjury medication (Reference = Yes) Clopidogrel = No Warfarin = No Polypharmacy (≥4 medications) = No |
1.5 (95% CI: 1.2–2.0) 2.2 (95% CI: 1.5–3.3) 1.6 (95% CI: 1.5–1.8) |
<0.001 <0.001 <0.001 |
* * * |
* * * |
| Social characteristics | ||||
| Living alone prior to injury Yes No Unknown |
Reference 1.7 (95% CI: 1.5–1.9) 3.0 (95% CI: 1.5–6.1) |
– <0.001 0.002 |
Reference 1.5 (95% CI: 1.3–1.6) 1.5 (95% CI: 0.7–3.3) |
– <0.001 0.29 |
| Preinjury walking ability Alone outdoors Alone indoors Accompanied outdoors Accompanied indoors Unable / transfers only Unknown |
Reference 0.3 (95% CI: 0.3–0.3) 0.4 (95% CI: 0.4–0.5) 0.3 (95% CI: 0.2–0.4) 0.3 (95% CI: 0.2–0.4) 0.6 (95% CI: 0.4–0.9) |
– <0.001 <0.001 <0.001 0.008 <0.001 |
Reference 0.6 (95% CI: 0.5–0.7) 0.7 (95% CI: 0.6–0.9) 0.7 (95% CI: 0.4–1.0) 0.7 (95% CI: 0.4–1.3) 0.6 (59% CI: 0.4–0.8) |
– <0.001 0.003 0.040 0.31 0.002 |
| Walking aids used prior to injury No aids One aid Two aids Frame / walker Wheelchair / bed bound Unknown |
Reference 0.4 (95% CI: 0.4–0.5) 0.4 (95% CI: 0.3–0.5) 0.3 (95% CI: 0.2–0.3) 0.6 (95% CI: 0.4–0.8) 0.3 (95% CI: 0.2–0.4) |
– <0.001 <0.001 <0.001 0.003 <0.001 |
Reference 0.7 (95% CI: 0.6–0.8) 0.6 (95% CI: 0.4–0.8) 0.6 (95% CI: 0.5–0.8) 1.0 (95% CI: 0.6–1.8) 0.7 (95% CI: 0.5–1.2) |
– <0.001 0.001 <0.001 0.90 0.21 |
| Preinjury assistance with basic care ADLs
(washing, dressing, feeding, toileting etc) Yes No |
Reference 2.5 (95% CI: 2.2–2.9) |
– <0.001 |
Reference 1.3 (95% CI: 1.1–1.6) |
– 0.002 |
| Admission characteristics | ||||
| Fracture type Intracapsular Basicervical Intertrochanteric Subtrochanteric / reverse oblique Other / unknown |
1.3 (95% CI: 1.1–1.5) 1.1 (95% CI: 0.8–1.5) 0.9 (95% CI: 0.7–1.0) Reference 1.6 (95% CI: 1.0–2.5) |
0.007 0.53 0.10 – 0.040 |
1.5 (95% CI: 1.2–1.9) 1.2 (95% CI: 0.9–1.7) 1.1 (95% CI: 0.9–1.4) Reference 1.4 (95% CI: 0.8–2.3) |
<0.001 0.31 0.26 – 0.22 |
| Haemoglobin <12g/dl ≥12g/dl |
Reference 1.6 (95% CI: 1.4–1.8) |
– <0.001 |
Reference 1.2 (95% CI: 1.1–1.3) |
– 0.001 |
ADLs = activities of daily living; AMTS = abbreviated mental test score; CI = confidence interval; DHD = direct home discharge; OR = odds ratio
Discussion
Our study demonstrates that there are key identifiable preoperative patient parameters that could help stratify the hip fracture population and enable prediction of which patients admitted from their own home are most likely to undergo DHD. In our study, patients had an increased chance of DHD if they: were younger; were female; had an AMTS of 10; did not have either CVA, COPD, renal disease or Parkinson’s disease; cohabited; walked independently outdoors prior to the injury; did not use a walking aid; did not require assistance with basic ADLs; had an intracapsular fracture; and had an admission haemoglobin level of ≥12g/dl (all p<0.01). These findings are in line with the contemporary literature.7,10
Scoring systems have been developed for risk of morbidity and mortality following hip fracture surgery.11–13 In addition to showing strong predictive value for mortality at 30 days, the Nottingham Hip Fracture Score (NHFS) also has a strong predictive value for DHD for patients at both 14 and 21 days.14 Indeed, Moppett et al have demonstrated that patients with a NHFS of 0, 1 or 2 have a greater than 70% chance of returning home and a greater than 50% chance of returning home within 15 days of admission.14 Patients in this group were identified as being younger (<66 years) and either male with no co-morbidities or female with minor co-morbidities.
The NHFS system itself identifies the following as key prognostic factors: older age, male sex, ≥2 co-morbidities, mini-mental test score ≤6/10, admission haemoglobin ≤10g/dl, living in an institution and malignant disease.14 The present study’s findings also reflect the risk factors implicated in the scoring system of the NHFS but we nevertheless note the absence of functional and mobility status in the NHFS, which were shown in our study to be predictors of DAL.
Vochteloo et al identified older age, female sex, dementia, living alone and a lower level of mobility as risk factors for DAL.15 They proposed a scoring system based on age and its stratification, sex (female sex conferring a higher risk of DAL), presence of dementia, absence of partner and mobility levels at admission. Although our study corroborates the prognostic ability of certain factors, it should be pointed out that no individual co-morbidities were analysed by Vochteloo et al, who instead favoured the analysis of ASA (American Society of Anesthesiologists) grade. We find the latter alone to be liable to more subjectivity and confounding variables than individual co-morbidities.
Other studies have also investigated independent factors associated with DAL.3,16–18 Deakin et al stated that lack of physical independence, increasing age, male sex and sustaining a fracture while an inpatient are all predictors of DAL.3 Again, we note this study does not implicate a lack of functional status as a factor associated with DAL. Similar to Deakin et al, we also observed a statistically significant difference regarding sex, which implied a negative association between male sex and DHD. As well as being a risk factor for DAL and reduced chance of DHD, male sex has also been linked with increased mortality and morbidity following hip fracture.10
Our study confirms there is a ‘downward drift’ in discharge destination in that approximately 40% of patients admitted from their own home subsequently undergo DAL. A large cohort study by Nanjayan et al in 2014 suggested that increasing ASA grade, delay in surgical treatment due to medical conditions requiring specialist review, and delay in surgery due to medical problems and high ASA grade are an accurate overall representation of the medical condition of the patient, and (as a combination) increase the chance of DAL 15-fold.7 In addition, the authors stated that male sex, increasing age (>80 years), poor preoperative mobility and poor cognitive function were all independent risk factors for DAL.
On multivariate analysis, our results support the findings of Nanjayan et al.7 Nevertheless, we feel that individual co-morbidities known to be implicated in increased mortality and morbidity better represent the risk of DAL as there is likely to be an amplification effect when combining ASA grade, delay to surgery for specialist medical condition review, and delay to surgery due to medical conditions and high ASA grade as a triad.
Historically, research on hip fractures has always concentrated on mortality, morbidity and the technical aspects of surgical technique.10,19 As society enhances its ability to rehabilitate an ageing population, the burden on healthcare will necessarily increase. Successful sustenance of community management of the ageing population is critical to a sustainable healthcare system. As such, it may be of surprise that very few papers have focused on the population most relevant to this, namely patients who are admitted from their own home. Indeed, DAL has been shown to be related to an increased length of stay in hospital,3 which increases the already significant yearly cost (approximately £12,000)20 of treating a hip fracture.
Study strengths and limitations
The strengths and limitations of this dataset have been described previously.1 Principally, this study benefits from the size of the cohort, the 13-year study period, consistent data collection and the range of data collected. The hospital serves a well defined urban/rural population with no alternative hip fracture service in the geographical location. Audit completeness as assessed by the National Hip Fracture Database annual report in 2013 was 94%,9 which suggests a high level of compliance with the data recording fields.
Inevitable inaccuracies in coding and inter-recorder variability are potential sources of discrepancy, which may have an effect over a period of time. Furthermore, as stated previously,1 we believe that the hip fracture population as a whole has evolved in terms of its demographics and co-morbidities because of a number of national directives. As a result, a retrospective analysis of a 13-year period of a hip fracture population (which may have changed considerably from one end of the time period to the other) may skew interpretation of any static derivations/analysis. For example, the prevalence of CVD in this cohort was lower prior to 2005.1 This is possibly a result of the implementation of national guidelines around that time. Despite this, the entire cohort of patients, admitted between 2000 and 2012, has been considered as one whole entity.
Community support systems also changed over the study period, limiting or enhancing the possibility of rehabilitating less mobile and more dependent patients in their own home. The contemporary need for rapid throughput of patients through acute services may have increased the proportion who undergo DAL to placements such as rehabilitation facilities if not quite ready for DHD.
Any projections from a single institution and single population being extrapolated to a national population may be liable to regional discrepancy, and may not be applicable to local hip fracture populations where prevalence of co-morbidity, for example, may be different. We nevertheless feel that it is possible to generalise the findings of this study to national practice. In addition, while we have attempted to include all relevant factors in the multivariate analysis, there are of course a number of confounding variables that have not been accounted for (eg time/delay to surgery, ASA grade, perioperative morbidity and type of operation/prosthesis).
Critically, the factors predictive of DHD are all fixed on admission and non-modifiable in an acute stay. This could therefore allow the development of a discharge planning scoring and stratification system on admission that could assist in the discharge planning process and potential fast track systems. Being the largest cohort analysis on hip fracture discharge location for those patients admitted from their own home that is available in the contemporary literature, we hope this work will instigate further research on a validated admission DHD scoring system.
Conclusions
The hip fracture population comprises a spectrum of patients ranging from those who are independent and likely to be discharged within a few days after surgery to those for whom a hip fracture represents a preterminal event. As well as supporting work that has preceded this paper regarding risk factors for DHD following hip fracture, the results of our study suggest that particular substrata exist within the group of patients admitted from home and that these subgroups can be readily established on admission.
Much effort has been directed to identifying key parameters applicable to the population as a whole that could augment the chances of an expedited return to prefracture function. These efforts have culminated in the development of NICE guidance, the National Service Framework for Older People and reporting via the National Hip Fracture Database annual report. Despite this, there is currently no guidance on a fast track system to identify those likely to undergo DHD (or indeed those at risk of DAL) having been admitted from home to allow early and appropriate discharge planning.
In an era of incentivised practice driven by outcomes, both preoperative demographic variables and co-morbidities as well as fracture pattern should be considered individually as risk factors for DAL. Furthermore, we would suggest the next step in optimisation of hip fracture care should be the development of a validated scoring system for patients admitted from home based on the current literature and adaptation of the NHFS.
References
- 1.Baker PN, Salar O, Ollivere BJ et al. Evolution of the hip fracture population: time to consider the future? A retrospective observational analysis. BMJ Open 2014; : e004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White SM, Griffiths R. Projected incidence of proximal femoral fracture in England: a report from the NHS Hip Fracture Anaesthesia Network (HIPFAN). Injury 2011; : 1,230–1,233. [DOI] [PubMed] [Google Scholar]
- 3.Deakin DE, Wenn RT, Moran CG. Factors influencing discharge location following hip fracture. Injury 2008; : 213–218. [DOI] [PubMed] [Google Scholar]
- 4.Koval KJ, Skovron ML, Aharonoff GB et al. Ambulatory ability after hip fracture. A prospective study in geriatric patients. Clin Orthop Relat Res 1995; : 150–159. [PubMed] [Google Scholar]
- 5.Department of Health. National Service Framework for Older People. London: DH; 2001. [Google Scholar]
- 6.National Institute for Health and Clinical Excellence. Hip Fracture: Management. London: NICE; 2011. [Google Scholar]
- 7.Nanjayan SK, John J, Swamy G et al. Predictors of change in ‘discharge destination’ following treatment for fracture neck of femur. Injury 2014; : 1,080–1,084. [DOI] [PubMed] [Google Scholar]
- 8.Cree AK, Nade S. How to predict return to the community after fractured proximal femur in the elderly. Aust N Z J Surg 1999; : 723–725. [DOI] [PubMed] [Google Scholar]
- 9.National Hip Fracture Database. National Hip Fracture Database: National Report 2013. London: NHFD; 2013. [Google Scholar]
- 10.Deakin DE, Boulton C, Moran CG. Mortality and causes of death among patients with isolated limb and pelvic fractures. Injury 2007; : 312–317. [DOI] [PubMed] [Google Scholar]
- 11.Moppett IK, Parker M, Griffiths R et al. Nottingham Hip Fracture Score: longitudinal and multi-centre assessment. Br J Anaesth 2012; : 546–550. [DOI] [PubMed] [Google Scholar]
- 12.Burgos E, Gómez-Arnau JI, Díez R et al. Predictive value of six risk scores for outcome after surgical repair of hip fracture in elderly patients. Acta Anaesthesiol Scand 2008; : 125–131. [DOI] [PubMed] [Google Scholar]
- 13.Souza RC, Pinheiro RS, Coeli CM, Camargo KR. The Charlson comorbidity index (CCI) for adjustment of hip fracture mortality in the elderly: analysis of the importance of recording secondary diagnoses. Cad Saude Publica 2008; : 315–322. [DOI] [PubMed] [Google Scholar]
- 14.Moppett IK, Wiles MD, Moran CG, Sahota O. The Nottingham Hip Fracture Score as a predictor of early discharge following fractured neck of femur. Age Ageing 2012; : 322–326. [DOI] [PubMed] [Google Scholar]
- 15.Vochteloo AJ, Tuinebreijer WE, Maier AB et al. Predicting discharge location of hip fracture patients; the new discharge of hip fracture patients score. Int Orthop 2012; : 1,709–1,714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vochteloo AJ, van Vliet-Koppert ST, Maier AB et al. Risk factors for failure to return to the pre-fracture place of residence after hip fracture: a prospective longitudinal study of 444 patients. Arch Orthop Trauma Surg 2012; : 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagino T, Ochiai S, Sato E et al. Prognostic prediction in patients with hip fracture: risk factors predicting difficulties with discharge to own home. J Orthop Traumatol 2011; : 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heikkinen T, Parker M, Jalovaara P. Hip fractures in Finland and Great Britain – a comparison of patient characteristics and outcomes. Int Orthop 2001; : 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldacre MJ, Roberts SE, Yeates D. Mortality after admission to hospital with fractured neck of femur: database study. BMJ 2002; : 868–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrence TM, White CT, Wenn R, Moran CG. The current hospital costs of treating hip fractures. Injury 2005; : 88–91. [DOI] [PubMed] [Google Scholar]


