Abstract
INTRODUCTION
Many studies have addressed the accuracy of prognostic scoring systems in the treatment of differentiated thyroid cancers as a whole but few have addressed this issue in patients with follicular thyroid cancer (FTC) alone. The aim of this study was to establish the accuracy of the various scoring systems in determining the overall and disease free survival of FTC patients in Singapore.
METHODS
Retrospective review was undertaken of 82 patients with FTC treated at a single tertiary institution between January 2000 and December 2014. Demographic, clinical, pathological and treatment outcomes were analysed. Prognostic scoring systems evaluated for the cohort included TNM (Tumour, Nodes, Metastases), AGES (Age, Grade, Extent, Size), MACIS (Metastases, Age, Completeness of resection, Invasion, Size), AMES (Age, Metastases, Extent, Sex) and EORTC (European Organisation for Research and Treatment of Cancer). Statistical analysis was performed by plotting Kaplan–Meier survival curves and using the Cox proportional hazards model.
RESULTS
There were 29 male and 53 female patients with a mean age of 48 years. The mean follow-up duration was 88 months and there were 7 deaths (9%). The ten-year overall survival rate was 90%. Factors predictive of survival on univariate analysis were age, size of tumour, invasiveness, completeness of resection, metastasis, external beam radiotherapy, and risk scores using the AGES and MACIS scoring systems (p<0.05). On multivariate analysis, AGES and MACIS provided the best prognostic information.
CONCLUSIONS
MACIS is the best prognostic scoring system currently available for FTC and it is superior to other scoring systems in term of guiding management. The scoring systems require further development to accommodate variations in clinical practice globally and to improve the prognostic accuracy.
Keywords: Follicular, Thyroid, Cancer, Staging
Follicular thyroid cancer (FTC) is the second most common form of thyroid cancer and accounts for about 10–15% of differentiated thyroid cancers.1 The incidence of FTC has remained stable worldwide.2 Nevertheless, in regions where iodine deficiency is endemic, the incidence is around 40%3 and in Singapore it accounts for a fifth of well differentiated thyroid carcinomas. It is considered a more aggressive disease with poorer prognosis owing to its propensity for vascular invasion and distant metastases at initial presentation.4,5
The importance of prognostication as a determinant of treatment has been demonstrated in numerous articles, and scoring systems have been developed by a number of institutions and validated for use with thyroid cancers. These include the TNM (Tumour, Nodes, Metastases),6 AGES (Age, Grade, Extent, Size),7 MACIS (Metastases, Age, Completeness of resection, Invasion, Size),8 AMES (Age, Metastases, Extent, Size)9 and EORTC (European Organisation for Research and Treatment of Cancer)10 systems. Other prognostic factors often used are age at time of diagnosis, sex, size of tumour, isthmic invasion, vascular invasion, capsular invasion, recurrence after treatment and use of postoperative radioactive iodine therapy.11 However, these indices have been developed in heterogeneous groups of thyroid cancer patients or solely for those with papillary thyroid cancer (PTC) and the specific prognostic factors for FTC have not yet been validated.
A limited number of studies have been carried out on the accuracy of prognostic scoring systems in FTC, with results varying widely.11–13 To date, no study of this nature has been attempted in the local setting. The aim of our study was to establish whether commonly described prognostic factors and scoring systems were significant predictors of overall survival of FTC patients in Singapore.
Methods
This was a retrospective cohort study of patients diagnosed with FTC at a tertiary referral hospital between January 2000 and December 2014. The inclusion criteria comprised histopathological diagnosis of follicular carcinoma, follow-up duration of at least five years (except patients who died of the disease) and complete surgical treatment. Exclusion criteria were distant metastasis at presentation, concomitant cancers and non-follicular or Hürthle cell thyroid cancers.
Clinical, demographic and epidemiological data were gathered from electronic medical records as well as hard copy case notes. The demographic data included sex and age at diagnosis, operative details (type of operation and date of completion thyroidectomy), histopathological features (size of tumour, local invasion, vascular invasion, capsular invasion and whether the tumour was widely or minimally invasive), postoperative status (use of adjuvant radioactive iodine therapy), and presence and site of recurrence as well as subsequent treatment.
The prognostic scoring systems studied comprised TNM, AGES, MACIS, AMES and EORTC. Table 1 shows the formulas used to calculate the various scores as well as the risk stratification for the different scoring systems.
Table 1.
The various prognostic scoring systems and risk stratification used in the study
| Scoring system | Formula | Risk stratification |
| TNM | T0 = no tumour detected T1 = intrathyroidal tumour <1cm T2 = intrathyroidal tumour 1–4cm T3 = intrathyroidal tumour >4cm T4 = locally invasive tumour of any size T4a = moderately advanced T4b = very advanced N0 = no regional lymph node involvement N1 = regional lymph node metastasis N1a = metastasis to level VI nodes N1b = metastasis to all other cervical nodes M0 = no distant metastasis M1 = distant metastasis |
5-year survival Stage I <45 years: any T, any N, M0 = 100% Stage I ≥45 years: T1, N0, M0 = 100% Stage II <45 years: any T, any N, M1 = 100% Stage II >45 years: T2, N0, M0 = 100% Stage III: T3, N0, M0 or T1–3, N1a, M0 = 71% Stage IVA: T1–3, N1b, M0 or T4a, any N, M0 = 50% Stage IVB: T4b, any N, M0 = 50% Stage IVC: any T, any N, M1 = 50% |
| AGES | 0.05 x age in years (if age is ≥40 years) or +0 (if age is <40 years) +1 if tumour is grade 2 +3 if tumour is grade 3 or 4 +3 if distant metastases +0.2 x tumour size in cm |
20-year survival ≤3.99 = 99% 4–4.99 = 80% 5–5.99 = 67% ≥6 = 13% |
| MACIS | 3.1 (if age <40 years) or 0.08 x age in years (if age ≥40 years) +0.3 x tumour size in cm +1 if incompletely resected +1 if locally invasive +3 if distant metastases |
20-year survival <6 = 99% 6–6.99 = 89% 7–7.99 = 56% ≥8 = 24% |
| AMES | Low risk: Men ≤40 years or women ≤50 years with no metastases, or older patients with intrathyroidal lesions, minor capsular invasion, tumour size <5cm and no distant metastases High risk: All patients with distant metastases, extrathyroidal lesions or major capsular invasion, or tumour size ≥5cm in older patients |
20-year survival Low risk = 99% High risk = 61% |
| EORTC | +12 if male, +10 if follicular lesion is poorly differentiated, +10 if ≥T3, +15 if there is one distant metastasis, +15 for each additional metastasis | 5-year survival <50 = 95% 50–65 = 80% 66–83 = 51% 84–108 = 33% >108 = 5% |
AGES = Age, Grade, Extent, Size; AMES = Age, Metastases, Extent, Sex; EORTC = European Organisation for Research and Treatment of Cancer; TNM = Tumour, Nodes, Metastases; MACIS = Metastases, Age, Completeness of resection, Invasion, Size
Statistical analysis
Data were analysed using SPSS® version 21.0 (IBM, New York, US). Univariate analysis was performed using Kaplan–Meier survival curves, significance testing using a logrank test and multivariate analysis using the Cox proportional hazards model. A p-value of <0.05 was considered statistically significant.
Cox proportional hazards analysis was employed to determine the proportion of variation in survival time explained (PVE), which calculates the relative importance of each staging system.14 PVE can range from 0% to 100%, with higher numbers indicating better cancer specific survival predictability.
Results
There were 29 male and 43 female patients with a mean age of 47.9 years (standard deviation: 18.9 years, range: 13–86 years). Total thyroidectomy was performed in 25 cases (30%) as the first operation and lobectomy followed by completion thyroidectomy in 57 (70%). The mean follow-up duration was 88 months and 7 patients (8.5%) died during the study period. Adjuvant radioiodine ablation was administered in 52 cases (63%) and external beam radiotherapy in 3 (4%). Disease progression was seen in six individuals (7%). The baseline characteristics of the study population are summarised in Table 2.
Table 2.
Baseline characteristics of follicular thyroid cancer patients (n=82)
| Characteristic | Number of patients |
| Mean age in years | 47.9 (SD: 18.9, range: 13–89) |
| Female sex | 53 (65%) |
| Type of surgery Hemithyroidectomy with completion Total thyroidectomy |
57 (70%) 25 (30%) |
| Pathological details Mean tumour size in cm Gross invasion Vascular invasion Capsular invasion Completeness of resection |
3.79 (SD: 2.01) 37 (45%) 54 (66%) 70 (85%) 76 (93%) |
| Postoperative details Adjuvant radioactive iodine Recurrence External beam radiotherapy Distant metastasis |
52 (63%) 11 (13%) 3 (4%) 20 (24%) |
| Mean follow-up duration in months | 88.2 (SD: 46.5) |
| Deaths | 7 (9%) |
| TNM I II III IV |
7(9%) 7 (9%) 66 (80%) 2 (2%) |
| AGES ≤3.99 4–4.99 5–5.99 ≥6 |
42 (51%) 15 (18%) 6 (7%) 19 (24%) |
| MACIS <6 6–6.99 7–7.99 ≥8 |
40 (49%) 12 (15%) 6 (7%) 24 (29%) |
| AMES Low risk High risk |
17 (21%) 65 (79%) |
| EORTC <50 50–65 66–83 84–108 >108 |
24 (30%) 17 (20%) 20 (24%) 20 (24%) 1 (1%) |
AGES = Age, Grade, Extent, Size; AMES = Age, Metastases, Extent, Sex; EORTC = European Organisation for Research and Treatment of Cancer; TNM = Tumour, Nodes, Metastases; MACIS = Metastases, Age, Completeness of resection, Invasion, Size; SD = standard deviation
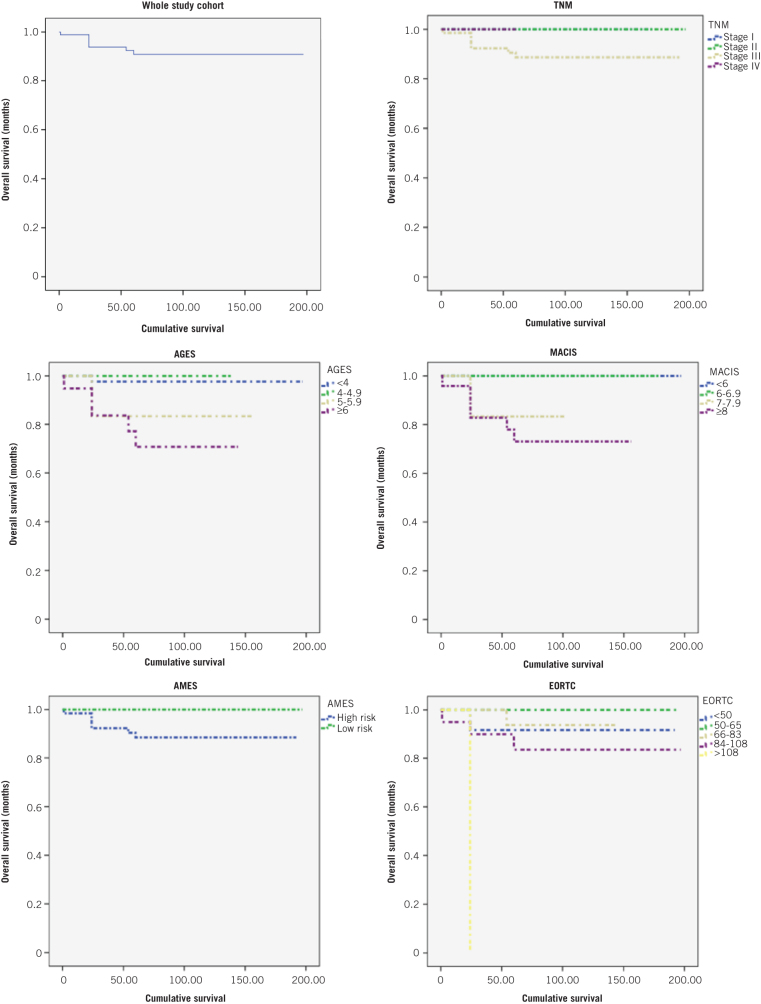
The results of the Kaplan–Meier analysis are shown in Table 3. On univariate analysis, gross invasion, extrathyroidal extension, capsular invasion and completeness of resection correlated with poor survival. Following surgical excision, factors that correlated with poor prognosis included adjuvant radioiodine ablation, external beam radiotherapy, metastasis and recurrence. Of the various scoring systems, AGES and MACIS correlated with a poorer overall survival. The overall survival rates at five and ten years for the whole cohort were 91% and 90% respectively. The Kaplan–Meier survival curves for the different risk groups of the various scoring systems are illustrated in Figure 1.
Table 3.
Univariate analysis of prognostic factors for overall survival
| Prognostic factor | χ2 | p-value |
| Age | 45.310 | 0.159 |
| Sex | 0.044 | 0.663 |
| Pathological details Tumour size Gross invasion Extrathyroidal extension Vascular invasion Capsular invasion Completeness of resection |
3.710 1.147 0.310 0.064 0.617 0.970 |
0.354 0.024 0.048 0.617 0.027 0.009 |
| Postoperative details Adjuvant radioactive iodine Metastasis Recurrence External beam radiotherapy |
1.066 4.404 0.348 0.476 |
0.036 0.009 0.024 0.009 |
| Scoring systems TNM AGES MACIS AMES EORTC |
1.855 11.638 13.693 0.360 3.587 |
0.315 0.001 0.009 0.161 0.121 |
AGES = Age, Grade, Extent, Size; AMES = Age, Metastases, Extent, Sex; EORTC = European Organisation for Research and Treatment of Cancer; TNM = Tumour, Nodes, Metastases; MACIS = Metastases, Age, Completeness of resection, Invasion, Size
Figure 1.
Kaplan–Meier survival curves
The results of the Cox proportional hazards analysis are shown in Table 4. Of the scoring systems, only AGES and MACIS were good predictors of survival. On comparison of the systems using PVE, MACIS was the most accurate scoring system, followed by AGES, EORTC, AMES and TNM (Table 5).
Table 4.
Multivariate analysis of prognostic factors for overall survival
| Prognostic factor | χ2 | p-value |
| Type of surgery | 3.357 | 0.030 |
| Pathological details Gross invasion Extrathyroidal extension Capsular invasion Completeness of resection |
4.681 0.310 0.617 0.970 |
0.030 0.048 0.027 0.009 |
| Postoperative details Adjuvant radioactive iodine Metastasis Recurrence External beam radiotherapy |
1.066 4.404 0.348 0.476 |
0.044 0.009 0.038 0.001 |
| Scoring systems TNM AGES MACIS AMES EORTC |
1.021 11.090 14.766 2.002 2.455 |
0.635 0.008 0.003 0.170 0.113 |
AGES = Age, Grade, Extent, Size; AMES = Age, Metastases, Extent, Sex; EORTC = European Organisation for Research and Treatment of Cancer; TNM = Tumour, Nodes, Metastases; MACIS = Metastases, Age, Completeness of resection, Invasion, Size
Table 5.
Comparison of the various prognostic scoring systems
| Scoring system | Number of patients | Deaths | Logrank statistic | G2/n | PVE | Rank |
| TNM I II III IV |
82 33 12 28 9 |
7 (8.5%) 0 (0%) 0 (0%) 5 (17.8%) 2 (22.2%) |
1.021 | 0.012 | 1.19 | 5 |
| AGES ≤3.99 4–4.99 5–5.99 ≥6 |
82 42 15 6 19 |
7 (8.5%) 1 (2.4%) 0 (0%) 1 (16.6%) 5 (26.3%) |
11.090 | 1.499 | 77.66 | 2 |
| MACIS <6 6–6.99 7–7.99 ≥8 |
82 40 12 6 24 |
7 (8.5%) 0 (0%) 0 (0%) 1 (16.6%) 6 (25.0%) |
14.776 | 2.658 | 92.99 | 1 |
| AMES Low risk High risk |
82 17 65 |
7 (8.5%) 0 (0%) 7 (10.8%) |
2.002 | 0.048 | 4.76 | 4 |
| EORTC <50 50–65 66–83 84–108 >108 |
82 27 17 20 20 1 |
7 (8.5%) 2 (8.3%) 0 (0%) 1 (5.0%) 3 (15.0%) 1 (100%) |
2.455 | 0.073 | 7.03 | 3 |
AGES = Age, Grade, Extent, Size; AMES = Age, Metastases, Extent, Sex; EORTC = European Organisation for Research and Treatment of Cancer; TNM = Tumour, Nodes, Metastases; MACIS = Metastases, Age, Completeness of resection, Invasion, Size; PVE = proportion of variation in survival time explained
Discussion
FTC is the second most common differentiated thyroid cancer; it has a more aggressive biological behaviour than PTC.15 Despite this, the majority of studies on prognostication have included a combination of PTC and FTC cases. As such, their results reflect the prognostic factors associated with PTC rather than FTC.16 There are only a handful of studies that have looked purely at prognostic scoring in FTC, with varied results.11,13 In the local setting, prognostic scoring has not been well characterised.
The ten-year overall survival rate among our patients was 90%. Factors that have been described as predictors of survival are age,17 tumour size,18 vascular invasion,13,17 tumour invasiveness19 and distant metastasis.17,20,21 In our cohort, aside from these factors, completeness of resection, recurrence and external beam radiotherapy were also prognostic for survival.
A study in Egypt arrived at a similar overall survival rate of 89% for patients with FTC22 and this is comparable with studies in other regions. Our study spanned a total of 14 years, the mean follow-up duration being 88 months. This corresponds with the lengthy follow-up period in numerous studies on FTC conducted in Italy (104 months),23 Brazil (113 months)15 and Slovenia (117 months).24 Our sample size and demographics also correlated well with studies on FTC in populations worldwide, such as in California and Italy.13,23
Various prognostic systems have been designed to identify high risk patients who require more aggressive treatment to reduce morbidity and mortality.25 These include the TNM,6 AGES,7 MACIS,8 AMES9 and EORTC10 scoring systems. In our study, MACIS was the most accurate prognostic system once PVE had been determined, followed by AGES, EORTC, AMES and TNM.
The MACIS system was developed to predict survival in papillary thyroid cancer following evaluation in 1,779 patients over a 50-year period.8 MACIS has been shown to have the best predictice value in papillary thyroid cancer;8,26,27 the results are similar in FTC.12,13 While TNM is the most widely accepted staging system,11,12 it was found to be the least useful in terms of predictive survival in our cohort.
The predictive value of the various prognostic scoring systems seems to vary in different populations.12,13,19 This inconcistency may be due to treatment bias from the different institutions, either in the surgical approach or in the use of adjuvant radioactive iodine therapy and external beam radiotherapy.28 Each of the scoring systems was developed by different institutions using a specific database of their patient population. This could also explain why a single scoring system may not be applicable across the board.28
Study limitations
It is acknowledged that our study is limited owing to its retrospective nature. Furthermore, the relatively small sample size could limit the strength of any conclusions that can be drawn from the results.
Conclusions
Of the scoring systems studied, MACIS appears to be the most accurate prognostic system currently available for FTC. However, in order to best guide patient management, the scoring systems require further development to accommodate variations in clinical practice and improve the prognostic accuracy.
References
- 1.Dralle H, Machens A, Basa J et al. . Follicular cell-derived thyroid cancer. Nat Rev Dis Primers 2015; : 15077. [DOI] [PubMed] [Google Scholar]
- 2.Albores-Saavedra J, Henson DE, Glazer E, Schwartz AM. Changing patterns in the incidence and survival of thyroid cancer with follicular phenotype – papillary, follicular, and anaplastic: a morphological and epidemiological study. Endocr Pathol 2007; : 1–7. [DOI] [PubMed] [Google Scholar]
- 3.Williams ED, Doniach I, Bjarnason O, Michie W. Thyroid cancer in an iodide rich area: a histopathological study. Cancer 1977; : 215–222. [DOI] [PubMed] [Google Scholar]
- 4.Passler C, Scheuba C, Prager G et al. . Prognostic factors of papillary and follicular thyroid cancer: differences in an iodine-replete endemic goiter region. Endocr Relat Cancer 2004; : 131–139. [DOI] [PubMed] [Google Scholar]
- 5.DeGroot LJ, Kaplan EL, Shukla MS et al. . Morbidity and mortality in follicular thyroid cancer. J Clin Endocrinol Metab 1995; : 2,946–2,953. [DOI] [PubMed] [Google Scholar]
- 6.Compton CC, Byrd DR, Garcia-Aguilar J et al. . AJCC Cancer Staging Atlas. 2nd edn New York: Springer; 2012. [Google Scholar]
- 7.Sanders LE, Silverman M. Follicular and Hürthle cell carcinoma: predicting outcome and directing therapy. Surgery 1998; : 967–974. [PubMed] [Google Scholar]
- 8.Hay ID, Bergstralh EJ, Goellner JR et al. . Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 1993; 114: 1,050–1,057. [PubMed] [Google Scholar]
- 9.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 1988; : 947–953. [PubMed] [Google Scholar]
- 10.Byar DP, Green SB, Dor P et al. . A prognostic index for thyroid carcinoma. A study of the EORTC Thyroid Cancer Cooperative Group. Eur J Cancer 1979; : 1,033–1,041. [DOI] [PubMed] [Google Scholar]
- 11.Lo CY, Chan WF, Lam KY, Wan KY. Follicular thyroid carcinoma: the role of histology and staging systems in predicting survival. Ann Surg 2005; : 708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ríos A, Rodríguez JM, Ferri B et al. . Are prognostic scoring systems of value in patients with follicular thyroid carcinoma? Eur J Endocrinol 2013; : 821–827. [DOI] [PubMed] [Google Scholar]
- 13.D’Avanzo A, Ituarte P, Treseler P et al. . Prognostic scoring systems in patients with follicular thyroid cancer: a comparison of different staging systems in predicting the patient outcome. Thyroid 2004; : 453–458. [DOI] [PubMed] [Google Scholar]
- 14.Schemper M, Stare J. Explained variation in survival analysis. Stat Med 1996; : 1,999–2,012. [DOI] [PubMed] [Google Scholar]
- 15.de Melo TG, Zantut-Wittmann DE, Ficher E, da Assumpção LV. Factors related to mortality in patients with papillary and follicular thyroid cancer in long-term follow-up. J Endocrinol Invest 2014; : 1,195–1,200. [DOI] [PubMed] [Google Scholar]
- 16.Cooper DS, Schneyer CR. Follicular and Hürthle cell carcinoma of the thyroid. Endocrinol Metab Clin North Am 1990; : 577–591. [PubMed] [Google Scholar]
- 17.Brennan MD, Bergstralh EJ, van Heerden JA, McConahey WM. Follicular thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clinic Proc 1991; 66: 11–22. [DOI] [PubMed] [Google Scholar]
- 18.Zidan J, Kassem S, Kuten A. Follicular carcinoma of the thyroid gland: prognostic factors, treatment, and survival. Am J Clin Oncol 2000; : 1–5. [DOI] [PubMed] [Google Scholar]
- 19.Gemsenjäger E, Heitz PU, Martina B. Selective treatment of differentiated thyroid carcinoma. World J Surg 1997; : 546–551. [DOI] [PubMed] [Google Scholar]
- 20.van Heerden JA, Hay ID, Goellner JR et al. . Follicular thyroid carcinoma with capsular invasion alone: a nonthreatening malignancy. Surgery 1992; : 1,130–1,136. [PubMed] [Google Scholar]
- 21.Simpson WJ, Panzarella T, Carruthers JS et al. . Papillary and follicular thyroid cancer: impact of treatment in 1578 patients. Int J Radiat Oncol Biol Phys 1988; : 1,063–1,075. [DOI] [PubMed] [Google Scholar]
- 22.Aboelnaga EM, Ahmed RA. Difference between papillary and follicular thyroid carcinoma outcomes: an experience from Egyptian institution. Cancer Biol Med 2015; : 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Crea C, Raffaelli M, Sessa L et al. . Actual incidence and clinical behaviour of follicular thyroid carcinoma: an institutional experience. ScientificWorldJournal 2014; 952095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petric R, Gazic B, Besic N. Prognostic factors for disease-specific survival in 108 patients with Hürthle cell thyroid carcinoma: a single-institution experience. BMC Cancer 2014; : 777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherman SI, Brierley JD, Sperling M et al. . Prospective multicenter study of thyroid carcinoma treatment: initial analysis of staging and outcome. Cancer 1998; : 1,012–1,021. [DOI] [PubMed] [Google Scholar]
- 26.Londero SC, Krogdahl A, Bastholt L et al. . Papillary thyroid carcinoma in Denmark, 1996–2008: outcome and evaluation of established prognostic scoring systems in a prospective national cohort. Thyroid 2015; 25: 78–84. [DOI] [PubMed] [Google Scholar]
- 27.Passler C, Prager G, Scheuba C et al. . Application of staging systems for differentiated thyroid carcinoma in an endemic goiter region with iodine substitution. Ann Surg 2003; : 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong RM, Bresee C, Braunstein GD. Comparison with published systems of a new staging system for papillary and follicular thyroid carcinoma. Thyroid 2013; : 566–574. [DOI] [PubMed] [Google Scholar]