Abstract
The skin graft was born in 1869 and since then, surgeons have been using split skin grafts for wound repair. Nevertheless, this asset fails the big burn patient, who deserves an elastic, mobile and robust outcome but who receives the poorest possible outcome based on donor site paucity. Negating the need for the skin graft requires an autologous composite cultured skin and a material capable of temporising the burn wound for four weeks until the composite is produced. A novel, biodegradable polyurethane chemistry has been used to create two such products.
This paper describes the design, production, optimisation and evaluation of several iterations of these products. The evaluation has occurred in a variety of models, both in vitro and in vivo, employing Hunterian scientific principles, and embracing Hunter’s love and appreciation of comparative anatomy. The process has culminated in significant human experience in complex wounds and extensive burn injury. Used serially, the products offer robust and elastic healing in deep burns of any size within 6 weeks of injury.
Keywords: Skin graft replacement, Composite cultured skin, Bioreactor, Biodegradable temporising matrix, Biodegradable polyurethane
In 1869, 76 years after the death of John Hunter, Jacques-Louis Reverdin performed the first ‘fresh skin’ allograft at the Hôpital Necker in Paris.1 This effort, effectively pinch grafting with allogeneic skin, was no more informed of the principles of tissue rejection than Hunter’s own work with the human tooth implanted into a cock’s comb a century earlier.2 Advancement of the principle of skin grafting occurred in 1872 (Ollier)3 and 1886 (Thiersch).4 Other than improvements in harvesting graft and Tanner’s introduction of graft meshing (expansion) in 1964,5 the graft has remained relatively unchanged for nearly 150 years.
Considering the many downsides of the skin graft and its donor site,6 perhaps we should ask why. Technology to replace this useful asset has not needed to progress since those whom the skin graft failed (like individuals with extensive burn injuries) invariably died. The advances in burn injury knowledge, multidisciplinary and critical care, and pharmacological developments mean that these patients now frequently survive the initial insult. Our onus has shifted from mere survival to rapid wound closure, improved scar quality and functional outcome. Now the full extent of the graft’s limitations become obvious. The big burn patient, who most needs the best graft outcome in terms of suppleness, mobility, robustness and cosmesis, receives the poorest outcome as scant donor sites limit us to thinner, widely meshed grafts from repeatedly harvested (and ultimately poorly healing and badly scarring) donor sites.7 This has finally prompted research into technologies to replace the autograft.
A viable replacement for the skin graft mandates in-depth understanding of skin structure. The skin is not one organ, but two, originating from differing embryological germ lines (cellular epidermis from ectoderm, molecular dermis from mesoderm), with a natural and weak cleavage plane between, strengthened by the papilla/crypt structure of the dermoepidermal interface and the anchoring basement membrane. While the in vitro culture of epidermal cells (keratinocytes) is relatively easy and expansion reasonably rapid, the creation of a dermis in culture requires a scaffold, to encourage fibroblast colonisation as well as collagen production and deposition. Co-culture of dermis and epidermis to create a composite cultured skin (CCS) in a clinically useful quantity (the surface area of an average human male is ∼1.8m) is both difficult and slow.
These issues are compounded by the need for early burn excision (and therefore also wound creation, and the early and aggressive onset of wound contraction, which is potentially crippling by the time a CCS can be readied). This necessitates a means to ‘temporise’ the created wound to prevent (or at least minimise) contraction.
Materials to provide this function exist, and can be roughly divided into ‘passive’ and ‘active’ temporisers. Passive temporisers are little better than dressings, minimally altering the wound bed and requiring (or spontaneously undergoing) removal before definitive closure of the wound, and include cadaver allograft. Active temporisers, on the other hand, contribute to the wound bed, allowing tissue in-growth and creating a ‘neodermis’, which will better sustain subsequent definitive wound closure. The best known of these materials is Integra® Dermal Regeneration Template (Integra LifeSciences, Plainsboro, NJ, US),8–10 created by Jack Burke and Ionnas Yannas, and in clinical use since the 1980s. Unfortunately misnamed (the molecular dermis is not capable of regeneration, only repair), Integra® has received limited uptake by surgeons, discouraged by its high cost11 and reported ‘loss to infection’ risk.12,13
In considering the properties of the ideal scaffold for CCS production in vitro and the in vivo dermal component of an active temporiser, several desirable attributes were identified, as described below.
CCS scaffold ideals
The CCS scaffold must be non-cytotoxic and structurally robust to tolerate 28 days of in vitro culture at 37°C submerged in feeding media without interfering with its constituents or its nutritional, stimulant or protective properties. It must allow serial cellular seeding (fibroblasts initially, followed by keratinocytes) and support in vitro growth to create a bilayer ‘skin’ in 28 days, with the dermoepidermal junction reinforced by a basement membrane. The resulting CCS must be robust enough to handle, cut and fix.
Additional properties (pertaining to supply and demand) include that the material has to be light, inexpensive, mass producible (to cope with global demand), rapidly producible (to cope with surge demand following global mass burn casualty incidents), easily sterilised, stored and transported, and easy to use (handling, cutting and fixing).
Biological agents fail to meet several criteria. Collagen and glycosaminoglycans (like hyaluronic acid) are animal derived and costly as well as being slow to produce and incapable of ‘surge’ production. They are ‘wet’ packed and heavy, require refrigeration/freezing and need preparation time in theatre prior to use. Their biological composition makes them prone to infection and loss. Commonly used synthetic materials such as poly(lactic acid) and poly(glycolic acid) or copolymers like poly(lactic-co-glycolic acid) degrade to acidic monomers and produce an unfavourable culture environment.
In 2004 PolyNovo Biomaterials was established to exploit and commercialise a biodegradable polyurethane developed by Thilak Gunitillake at the Commonwealth Scientific and Industrial Research Organisation. I was fortunate to meet the executives of the company and pitch my concept for a cultured skin equivalent. They agreed to provide material for evaluation.
Stage 1: In vitro culture in the presence of NovoSorb™
In order to create a CCS, it would be necessary to culture appropriate skin cells in a scaffold made of biodegradable polyurethane (NovoSorb™; PolyNovo, Melbourne, Australia), since both the material and its degradation products could potentially kill cells or inhibit their replication in culture. Three varieties of the NovoSorb™ polymer (each with slightly different properties) were provided to us as extruded fibres, 50–100μm in diameter, and as film sheets. The cell types of interest (human keratinocytes, fibroblasts and microvascular endothelial cells) were cultured individually either on the sheets or in the presence of fibres. The three polymer variants allowed growth of the three cell types individually.14
A spun mat of NovoSorb™ fibres was provided (∼50μm fibres, ∼200μm pores; 6 alternate layers at 90° to the layer above and below). Fibroblasts were seeded into the matrix, where they adhered, proliferated and migrated to fill the pores. Once ‘happy’, they began to produce collagen. Keratinocytes were subsequently seeded and formed a layer superficial to the fibroblasts, producing our first CCS.
Three problems became apparent. First, the polymer was less dense than water and therefore buoyant in culture. Second, the spun ‘matrix’ was time consuming to create (6 layers were still only 0.3mm thick), and the cross-fibre structure was stiff and contained a relatively large amount of polymer, meaning it was costly as well as producing a high volume of degradation products. Finally, the basic CCS took three weeks to create and a more mature form (with confluent epidermis and basement membrane) took four weeks. After immediate burn excision, four weeks would allow significant wound granulation and contraction before the CCS was ready for transplantation.
Despite ‘proof of concept’, changes to the matrix were necessary. More significantly, a further product had to be developed to ‘temporise’ the wound after burn excision and before CCS application: a biodegradable temporising matrix (BTM). This would require a different set of ideal properties.
Temporising scaffold ideals
The material must integrate into the implanted wound, allowing invasion by fibroblasts, other cells and neoangiogenetic vessels from the wound bed. The environment created must be conducive for fibroblasts to produce autologous collagen and the scaffold should ‘compartmentalise’ in-growing tissue to discourage formation of long stretches of linear collagen, which might facilitate subsequent wound contraction. The scaffold must resist infection, and be able to survive, persist and reconstitute a neodermal structure even if the wound environment is not ideal (eg hypoxia). The scaffold must biodegrade, retaining mechanical properties for 3 months (maximally strong during the most significant phase of scarring) before beginning to break down and being completely absorbed by 12 months. Furthermore, it must be non-allergenic, biotolerated and non-toxic so that neither it nor its degradation products have adverse effects in biosystems.
The neodermis developed in the temporising scaffold must be flexible and mildly elastic, able to resist shear and offer long-term wound stability.
Stage 2: NovoSorb™ and degradation products in biological systems
While human skin cells were happy in vitro in the presence of NovoSorb™, how the material and its degradation products would interact with biological systems (organ function, biochemical and haematological systems, immune system etc) was not known. Implantation of NovoSorb™ fibres subcutaneously in rats demonstrated no systemic effects.14 The foreign body reaction to NovoSorb™ was no greater than that to absorbable suture materials. These findings were confirmed subsequently by independent ISO 10993 studies, where large volumes of NovoSorb™ were subcutaneously implanted.
Stage 3: In vivo skin wound implantation in a contractile model
A hair bearing (panniculus carnosus present), mammalian skin model (sheep) was chosen to compare wound contracture with the original spun matrix against a commercially available collagen/glycosaminoglycan matrix, demonstrating that NovoSorb™ matrices integrated into wounds. Wound contracture with the two materials was very similar.14
As CCS development was underway and NovoSorb™ was confirmed to be biologically compatible, attention shifted in 2007 to the BTM. The spun matrix structure was obviously not optimal where rapid, large scale, inexpensive production of large pieces of matrix was necessary. I conceived that these problems could be overcome if the polymer could be produced as a ‘foam’. Open-cell foam sheets of 1mm thickness (based on average dermal thickness) were produced for use in porcine studies. During evolution, the pig has replaced its panniculus carnosus with a panniculus adiposus and is an excellent model for skin wound research.
Stage 4: In vivo porcine wound implantation
With seed funding provided by BioInnovationSA (a South Australian government affiliated grant funding body), 11 porcine studies were performed in 2010 and 2011, the results of each determining the structure of the next. The 1mm foam showed excellent and consistent integration into porcine wounds, being completely integrated 11 days following implantation and allowing definitive closure by skin grafting after this time.15 However, temporisation of the wound was necessary until CCS was ready at day 28. After day 11, tissue growing into the matrix continued through the superficial border of the foam, generating a 7–8mm thick scar block, which then allowed aggressive wound contraction. A way to prevent this overgrowth was needed.
A seal capable of preventing or minimising evaporative water loss might stop overgrowth either by contact inhibition with the seal, or by ‘mimicking’ wound closure (since evaporative water loss is a major factor in wound detection). The first seals were biodegradable polyurethane. Tissue in-growth stopped at the seal but fragmentation of the biodegradable polymer allowed blebs of granulation tissue to grow through the seal and contract. The sealed matrix resisted infection compared with a biological collagen/glycosaminoglycan competitor (Integra®). None of the matrices were lost to infection.16 Nevertheless, the fragmentation of the biodegradable seal meant that the next iteration would have to be more robust and not degradable.
Stage 5: Seal optimisation
Although the new seal had to be non-biodegradable, how thick it should be or whether it should have perforations to allow fluid escape in the event of seroma was unknown. Four seal variants were bonded to the foam matrix, which had itself been increased to 2mm thick for adult human use. The variants were either ‘thin’ or ‘thick’ and ‘porous’ or ‘non-porous’. Comparison of the four variants yielded confusing results and investigation revealed that only the method of bonding the seal to the foam was important.17 We thus moved to a 50μm thick seal without pores. In the porcine model, this BTM iteration consistently demonstrated complete integration with no wound contraction over three weeks.
Stage 6: Short-term implantation
Pigs seldom have allergic reactions whereas humans do with increasing frequency. Since the implantable portion of the BTM was the NovoSorb™ foam, the foam could be used as a negative pressure wound therapy interface.18 In 2012 foam blocks 3cm thick were produced and compared against a commercially available reticulated foam for efficacy while providing the opportunity to assess the surrounding skin and wound for signs of reaction to the presence of the NovoSorb™ in the wound. The dressings were changed three times a week. No ill effect was observed and the NovoSorb™ demonstrated some advantages over the reticulated comparator.18
Stage 7: Long-term implantation in complex surgical wounds
Late in 2012 and prior to assessing the BTM in burn injuries, a more controllable surgical wound was chosen to assess long-term implantation. Free flap harvest and inset is specialised work performed by expert surgeons whereas the repair of the donor site is often left to the junior surgeon, with variable (and frequently disappointing) cosmetic and functional outcomes. The second pilot trial employed BTM in three anterolateral thigh flap, three fibular flap and four radial/ulnar forearm flap donor site reconstructions.19 The BTM took longer to integrate in these patients, many of whom were older, had multiple medical co-morbidities and underwent prolonged anaesthesia for major surgical procedures. Once familiar with this different physiology, the results were surprisingly good.
There were two important lessons: the seal was too flimsy and came off piecemeal (not acceptable in major burn injury where surgery is time critical to maintain patient temperature), and seromas formed in some radial forearm situations, where fluid from the muscular compartment could not escape through the fascial hole because of the impermeability of the seal. Although this did not result in loss of the material, infection did require us to remove some of the seal to treat infection topically. The results were still impressive. Nevertheless, following this pilot study, a more robust seal, with perforations, was bonded to the NovoSorb™ foam in preparation for the pilot burn trial. The new material has also been used in a further 25 free flap donor site reconstructions to date (Fig 1).20
Figure 1.
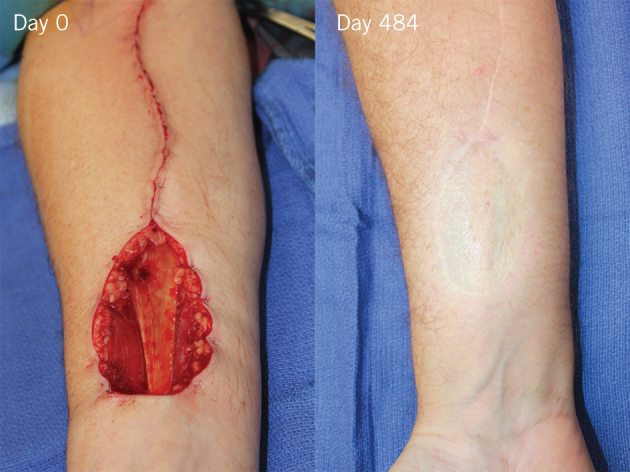
Day 0 (prior to biodegradable temporising matrix [BTM] insertion): Radial forearm free flap donor site just after flap harvest. The pedicle wound has been closed (proximal). The tendon of the flexor carpi radialis muscle runs across the wound bed from proximal to distal. Day 484: After BTM integration and skin graft application at day 36, the result at 16 months is shown.
Stage 8: Pilot human burn trial
In 2014 and 2015 a pilot human burn trial was undertaken with four Caucasians and one Indigenous Australian who had suffered injuries ranging between 21% and 46% total body surface area (TBSA) full-thickness burns. Several salient lessons were learned. The first patient demonstrated that areas of infection (confirmed by swab) under the BTM seal could be expressed and treated topically without losing the matrix or adversely affecting integration. The material did not spontaneously delaminate and areas retained their seal until the graft was available for definitive closure seven weeks after the burn. Graft adherence was rapid and the graft was immovable by the first check on day 4. Excellent long-term results followed. Punch biopsies during integration showed initial invasion by inflammatory cells followed by appearance, proliferation and invasion by fibroblasts.
The third study patient was Indigenous Australian. This Aboriginal patient enjoyed soft, supple and flat graft result in BTM areas with disappearance of the mesh pattern and excellent pigmentation compared with a raised and dysaesthetic mesh graft pattern elsewhere, particularly the back.
The fourth patient had sustained fourth-degree injuries to right lateral flank, chest, total axilla, medial arm, volar forearm and hand, exposing rib and necessitating excision of heat destroyed muscles in the volar compartment of the forearm. The functional result in the axilla is excellent, with full ranges of lateral and forward flexion possible. This patient also showed us that exposed, periosteum-denuded bone could be covered by BTM, and allow integration and grafting rather than flap repair. Additionally, a large area of posterior trunk burn had BTM applied with complete integration despite the patient lying on it.
In the fifth patient, the entire posterior trunk and bilateral buttocks received BTM, which integrated despite supine positioning on the intensive care unit. This left an outstanding graft result (Fig 2).
Figure 2.

Patient 5 sustained complete full-thickness back burns and biodegradable temporising matrix (BTM) was applied. The patient was nursed supine on the intensive care unit. BTM integration was followed by delamination and graft application when donor sites became available. The result at 10 months was excellent.
Since the conclusion of the trial, a further 7 significant burns cases have been treated including a 66-year-old man with 75% TBSA full-thickness burns and exposure of the entire calvarium where the outer skull table was removed and the BTM applied to diploe, followed by integration and successful grafting (Fig 3).21
Figure 3.

A 66-year-old, 75% full-thickness burn patient whose head and neck were burned sparing only the midface. Gentle debridement resulted in a clean wound by day 40 but complete exposure of the calvarium. The calvarial outer table was burred to diploe, and biodegradable temporising matrix (BTM) was applied to the whole burn and diploe (like a bathing cap). BTM delamination and graft application followed on day 89. Successful integration and grafting is clear by day 115.
A late middle-aged, female patient with necrotising fasciitis of the neck following infective parotitis recently received BTM following serial debridement and negative pressure wound therapy to her neck musculature. Results at 86 days after grafting over the integrated BTM demonstrated full neck movement in all directions with a soft and pliable result (Fig 4).22 (See also video available online as supplementary material.)
Figure 4.

A: Necrotising fasciitis of the neck following infection in the left parotid gland resulted in aggressive debridement with muscle and salivary gland exposure. By day 11, after negative pressure wound therapy, biodegradable temporising matrix (BTM) was applied. B: Integration and delamination was followed by application of a fenestrated sheet graft. C: At 86 days following grafting, the result is flexible, soft, mobile and robust. (See also video available online.)
The characteristic ‘lizard skin’ mesh pattern seen with widely meshed skin graft applied to fat was not observed in our patients when the same graft was applied over integrated BTM.
Stage 9: Development of the CCS
Progress on the development of the CCS ceased once it was possible to produce 10cm × 10cm pieces of autologous porcine CCS that could be used to definitively close over integrated BTM and fresh wounds.17,23 A 21-day-old CCS displayed basement membrane formation by day 7 after application. To produce enough CCS for a major burn would require the development of a specialised culture environment.
Stage 10: Creation of the bioreactor prototype
A second grant from BioInnovationSA allowed computer aided design and subsequent construction of a compact bioreactor, the prototype of which is capable of producing 20 pieces of CCS, each measuring 25cm × 25cm (1.25m in total), in 28 days from a skin biopsy (10cm × 10cm) (Fig 5). The only human contact once the CCS is inside the bioreactor occurs at day 7 when the keratinocyte seeding occurs. The bioreactor will be housed in a clean room filtered to 150ppm during human production, where all non-bioreactor seed culture will occur. CCS production in large sheets has been successful, with neodermis covered by confluent epidermis (Fig 6). Grant funding to complete validation of the bioreactor and CCS has been secured, and human pilot trials will begin late in 2017.
Figure 5.

Complete bioreactor in incubator with software controlled pump array and media fridge. A mock up of a bioreactor tower (10 cassettes, each capable of containing a 25cm × 25cm composite cultured skin, are divided between two ‘shoes’ in the full tower) is shown in the foreground.
Figure 6.

With 9 days of culture left before day 28 is reached, epidermal confluence is nearly complete over the cultured neodermis.
Conclusions
Using this two-stage strategy (immediate burn excision on arrival with BTM application and biopsy harvest for CCS production, followed by BTM delamination and CCS application at day 28) should allow burns of any size to be definitively closed by six weeks after injury. The early iatrogenic physiological insult sustained by our patients as a result of skin graft harvesting will not be necessary and our reliance as surgeons on the split skin graft will become optional rather than mandatory.
Acknowledgements
The material in this paper was presented as a Hunterian lecture at the Winter Meeting of the British Association of Plastic, Reconstructive and Aesthetic Surgeons in London in November 2016.
The author would like to acknowledge the ongoing contributions of others in the development of the BTM (Tim Moore at PolyNovo, and Marcus Wagstaff and Yugesh Caplash at the Royal Adelaide Hospital) and the CCS (Bronwyn Dearman and Amy Li of the Skin Engineering Laboratory at the Royal Adelaide Hospital, David Robertson at Icarus Design and Andre Mielke at Apexus Engineering) as well as thanking BioInnovationSA (now TechInSA) and the South Australian Lifetime Support Authority for pivotal funding support over the years. Thanks also go to Tim Kuchel, Sue Porter, Loren Matthews and other staff at the Preclinical, Imaging and Research Laboratories, and to SA Pathology’s John Finnie (for pathology reporting), and Jim Manovus and Sophie Grabol (for tissue preparation).
References
- 1.Davis JS. The story of plastic surgery. Ann Surg 1994; : 641–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore W. The Knife Man. London: Bantam; 2005. [Google Scholar]
- 3.Smahel J. The healing of skin grafts. Clin Plast Surg 1977; : 409–424. [PubMed] [Google Scholar]
- 4.Pollack SV. Wound healing: a review. IV. Systemic medications affecting wound healing. J Dermatol Surg Oncol 1982; : 667–672. [DOI] [PubMed] [Google Scholar]
- 5.Tanner JC, Vandeput J, Olley JF. The mesh skin graft. Plast Reconstr Surg 1964; : 287–292. [PubMed] [Google Scholar]
- 6.Greenwood JE. Hybrid Biomaterials for Skin Tissue Engineering. In: Albanna MZ, Holmes JH. Skin Tissue Engineering and Regenerative Medicine. London: Academic Press; 2016. pp185. –210. [Google Scholar]
- 7.Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 1995; : 243–248. [DOI] [PubMed] [Google Scholar]
- 8.Yannas IV, Burke JF. Design of an artificial skin. I. Basic design principles. J Biomed Mater Res 1980; : 65–81. [DOI] [PubMed] [Google Scholar]
- 9.Yannas IV, Burke JF, Gordon PL et al. Design of an artificial skin. II. Control of chemical composition. J Biomed Mater Res 1980; : 107–132. [DOI] [PubMed] [Google Scholar]
- 10.Dagalakis N, Flink J, Stasikelis P et al. Design of an artificial skin. III. Control of pore structure. J Biomed Mater Res 1980; : 511–528. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen DQ, Potokar TS, Price P. An objective long-term evaluation of Integra (a dermal skin substitute) and split thickness skin grafts, in acute burns and reconstructive surgery. Burns 2010; : 23–28. [DOI] [PubMed] [Google Scholar]
- 12.Heimbach DM, Warden GD, Luterman A et al. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil 2003; : 42–48. [DOI] [PubMed] [Google Scholar]
- 13.Peck MD, Kessler M, Meyer AA, Bonham Morris PA. A trial of the effectiveness of artificial dermis in the treatment of patients with burns greater than 45% total body surface area. J Trauma 2002; : 971–978. [DOI] [PubMed] [Google Scholar]
- 14.Li A, Dearman BL, Crompton KE et al. Evaluation of a novel biodegradable polymer for the generation of a dermal matrix. J Burn Care Res 2009; : 717–728. [DOI] [PubMed] [Google Scholar]
- 15.Greenwood JE, Dearman BL. Split skin graft application over an integrating, biodegradable temporizing polymer matrix: immediate and delayed. J Burn Care Res 2012; : 7–19. [DOI] [PubMed] [Google Scholar]
- 16.Greenwood JE, Dearman BL. Comparison of a sealed, polymer foam biodegradable temporizing matrix against Integra® dermal regeneration template in a porcine wound model. J Burn Care Res 2012; : 163–173. [DOI] [PubMed] [Google Scholar]
- 17.Dearman BL, Li A, Greenwood JE. Optimisation of a polyurethane dermal matrix and experience with a polymer-based cultured composite skin. J Burn Care Res 2014; : 437–448. [DOI] [PubMed] [Google Scholar]
- 18.Wagstaff MJ, Driver S, Coghlan P, Greenwood JE. A randomized, controlled trial of negative pressure wound therapy of pressure ulcers via a novel polyurethane foam. Wound Repair Regen 2014; : 205–211. [DOI] [PubMed] [Google Scholar]
- 19.Wagstaff MJ, Schmitt BJ, Coghlan P et al. A biodegradable polyurethane dermal matrix in reconstruction of free flap donor sites: a pilot study. Eplasty 2015; : e13. [PMC free article] [PubMed] [Google Scholar]
- 20.Wagstaff MJ, Schmitt BJ, Caplash Y, Greenwood JE. Free flap donor site reconstruction: a prospective case series using an optimized polyurethane biodegradable temporizing matrix. Eplasty 2015; : e27. [PMC free article] [PubMed] [Google Scholar]
- 21.Greenwood JE, Wagstaff MJ, Rooke M, Caplash Y. Reconstruction of extensive calvarial exposure after major burn injury in 2 stages using a biodegradable polyurethane matrix. Eplasty 2016; : e17. [PMC free article] [PubMed] [Google Scholar]
- 22.Wagstaff MJ, Caplash Y, Greenwood JE. Reconstruction of an anterior cervical necrotizing fasciitis defect using a biodegradable polyurethane dermal substitute. Eplasty 2017; : e3. [PMC free article] [PubMed] [Google Scholar]
- 23.Dearman BL, Stefani K, Li A, Greenwood JE. ‘Take’ of a polymer-based autologous cultured composite ‘skin’ on an integrated temporizing dermal matrix: proof of concept. J Burn Care Res 2013; : 151–160. [DOI] [PubMed] [Google Scholar]


