Abstract
Introduction
The aim of this study was to identify patients with malignant hand lesions, establish the proportions of those that were metastases and review their clinical course.
Methods
A retrospective search of a prospective tumour database was carried out to identify all patients treated at our unit with hand metastases. Patient demographics were recorded including site of primary malignancy, region of the hand involved, management of their metastasis and clinical outcome.
Results
Overall, 149 patients were identified with a malignant tumour of the hand. Ten had a metastatic lesion. There were 3 women and 7 men with a median age of 68 years (range: 28–91 years) at presentation. All presented with non-mechanical hand pain while four had pain and swelling. The median interval from symptom onset to diagnosis was eight weeks. The minimum follow-up duration was four months.
Three patients had no history of malignancy. Of the remaining seven patients, three had other known metastases. Six patients underwent solely palliative radiotherapy. Three patients had amputation. One was treated with surgical excision and radiotherapy. One had an amputation and axillary node clearance.
All but one patient had died by the time of the latest follow-up appointment. The median time to death following identification of acrometastases was 18 months. Sites of primary disease were skin (n=4), lung (n=3), kidney (n=2) and neuroendocrine system (n=1). The thumb was the most commonly affected location.
Conclusions
This study demonstrates that patients presenting with non-mechanical hand pain should be considered to have a malignant process until proved otherwise, particularly in patients with thumb symptoms and a history of prior malignancy.
Keywords: Hand, Neoplasm, Metastasis, Oncology, Finger phalanges, Thumb, Metacarpal bones
Bone has been reported as one of the most common sites of metastasis.1,2 The most common primary malignancy to metastasise to bone is breast followed by lung and prostate.2 The incidence of bone metastasis varies according to the tumour type and the stage at diagnosis. In a large epidemiological study of over 382,000 patients with solid tumours, 60.4% of stage IV prostate cancer patients developed bone metastases at 5 years, with 49.9% of breast and 25.0% of lung cancer patients.3 It has been shown that patients who develop bone disease following initial primary diagnosis of malignancy go on to consume progressively more hospital resources.4,5
Metastasis to the hand is rare with a reported incidence of 0.1%.6–9 It is associated with a poor prognosis, which largely depends on the behaviour of the primary tumour.10–12 The median survival following development of symptomatic hand metastasis has been reported as between five and six months.4,7,13 It is the rarity of the condition that can lead to a delay in diagnosis and treatment as symptoms can often be overlooked or attributed to another cause. The aim of this study was to identify patients treated at our centre with metastatic hand lesions, and review their clinical course and outcomes.
Methods
For the past 30 years, our centre has maintained a tumour database that holds prospectively collected epidemiological and clinical data on all patients presenting to our unit, amounting to over 35,000 patients. The catchment area of our unit includes referrals from across the whole of England and from Northern Ireland. All patients are diagnosed after multidisciplinary team review with radiological and histological assessment following either open or core biopsy. As part of our standard clinical record keeping, demographics, histological diagnosis, stage, grade and site of primary disease are recorded.
Our database was interrogated retrospectively to identify patients who had presented with metastatic hand lesions. These patients were referred with hand lesions rather than having these lesions detected during staging for other site disease. The location of the disease in the hand as well as the subsequent treatment and outcomes were assessed. This was performed in conjunction with local ethics committee approval.
Results
Our database held records of 2,801 patients with bone metastasis and 149 patients with a malignant tumour involving the hand. The earliest of these received their diagnosis in 1988 and the most recent in 2014. One hundred and thirty-nine patients had a variety of primary tumours of both soft tissue and bone. These included 66 primary soft tissue sarcomas, 54 chondrosarcomas, 5 osteosarcomas, 4 lymphomas, 4 carcinomas, 2 Ewing’s sarcomas, 2 malignant giant cell tumours, 1 plasmacytoma and 1 malignant melanoma.
Ten patients (6.7%) had a metastatic lesion (Table 1). This group comprised seven men and three women. The median age at presentation was 68 years (range: 28–91 years). The symptoms had been present for a median of 8 weeks (range: 1–33 weeks) prior to diagnosis. The main presenting symptom in all patients was intractable, non-mechanical hand pain. Four patients also had associated hand swelling, with one patient having a pathological fracture through the metastatic lesion (Fig 1).
Table 1.
Summary of the ten patients with a metastatic lesion
| Case | Age / sex | Diagnosis | Clinical presentation | Symptoms | Metastasis at diagnosis | Management | Survival |
| 1 | 76 M | Lung | Thumb pain | 33 wks | No | Radiotherapy | 11 mths |
| 2 | 61 M | Axillary adnexal tumour | Thumb pain and swelling | 7 wks | No | Excision and radiotherapy | 138 mths |
| 3 | 91 F | SCC | Thumb pain | 12 wks | No | Radiotherapy | 18 mths |
| 4 | 65 M | SCC | Pain | 8 wks | Forearm, axilla | Amputation, radiotherapy | 38 mths |
| 5 | 28 F | PNET | Hand pain and swelling | 6 wks | No | Humeral disarticulation, radiotherapy | 24 mths |
| 6 | 57 M | Lung | Thumb pain and swelling | 12 wks | No | Radiotherapy | 6 mths |
| 7 | 58 F | Lung | Index finger pain and swelling | 16 wks | No | Radiotherapy | 5 mths |
| 8 | 71 M | Kidney | Pathological fracture (thumb metacarpal) | 1 wk | Rib, lung | Radiotherapy | 22 mths |
| 9 | 74 M | Kidney | Thumb pain | 6 wks | No | Amputation, radiotherapy | 6 mths |
| 10 | 76 M | Lung | Index finger pain | 3 wks | Skull | Radiotherapy | Alive at 5 months |
PNET = peripheral neuroendocrine tumour; SCC = squamous cell carcinoma
Figure 1.
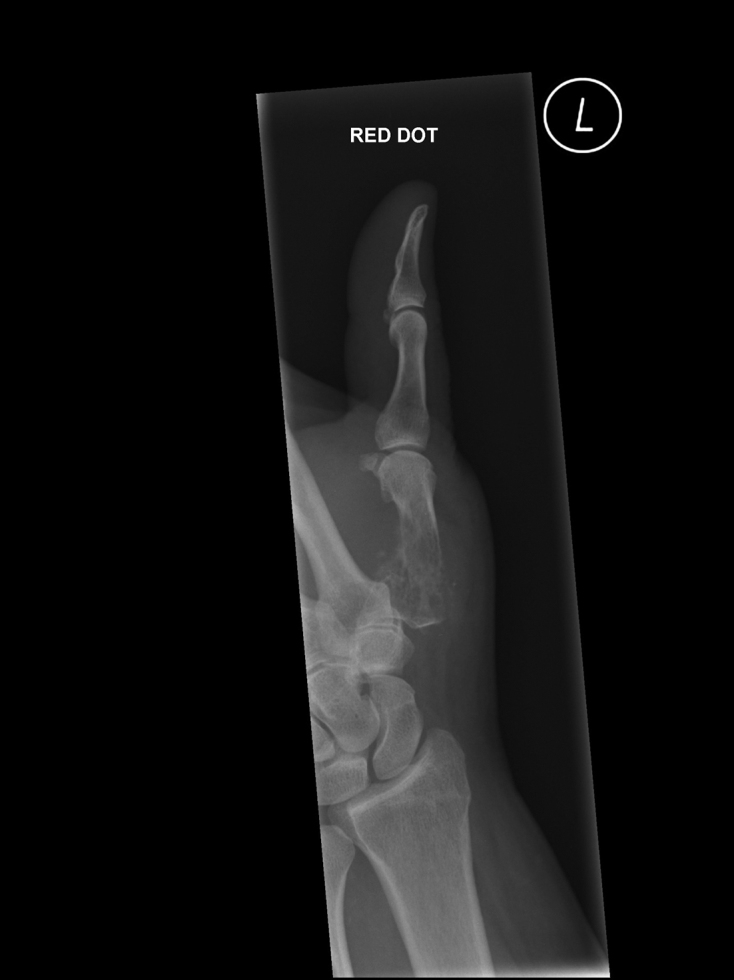
Lateral radiography showing a pathological fracture through a lesion in the thumb metacarpal
There was a history of treated malignancy in seven patients. Additional sites of metastasis, other than the presenting hand complaint, were found in three patients. The patients who had a history of malignancy had previous malignancies of skin (n=3), lung (n=2), kidney (n=1) and neuroendocrine system (n=1). In three patients (30%), the investigation of their hand lesion led to their first diagnosis of malignancy: two had primary tumours in the lung and one in the kidney. The only clinical manifestations of metastatic disease were the symptoms of the presenting hand lesion in seven (70%) of the ten patients.
When reviewing the treatment course, six patients (60%) had solely palliative radiotherapy for advanced disease. Three patients (30%) underwent amputation for disease control; all of these had further postoperative palliative radiotherapy. One patient (10%) was treated with surgical excision and radiotherapy. One amputation involved shoulder disarticulation and axillary node clearance owing to involvement of the ipsilateral axilla. The median time to death after diagnosis was 18 months (mean: 29.7 months, range: 5–138 months) (Table 1). One patient remains alive at the time of writing, five months from diagnosis.
The primary sites of malignancy were diverse. They comprised lung (n=4), skin (n=3), kidney (n=2) and neuroendocrine system (n=1).
Further study of anatomical metastasis location revealed four patients had involvement of a metacarpal only, three had involvement of a phalanx only (Fig 2), two had involvement of a metacarpal and phalanx, and one had involvement of a metacarpal and carpal bone. Six of the patients had thumb involvement and this was seen more commonly in the men.
Figure 2.
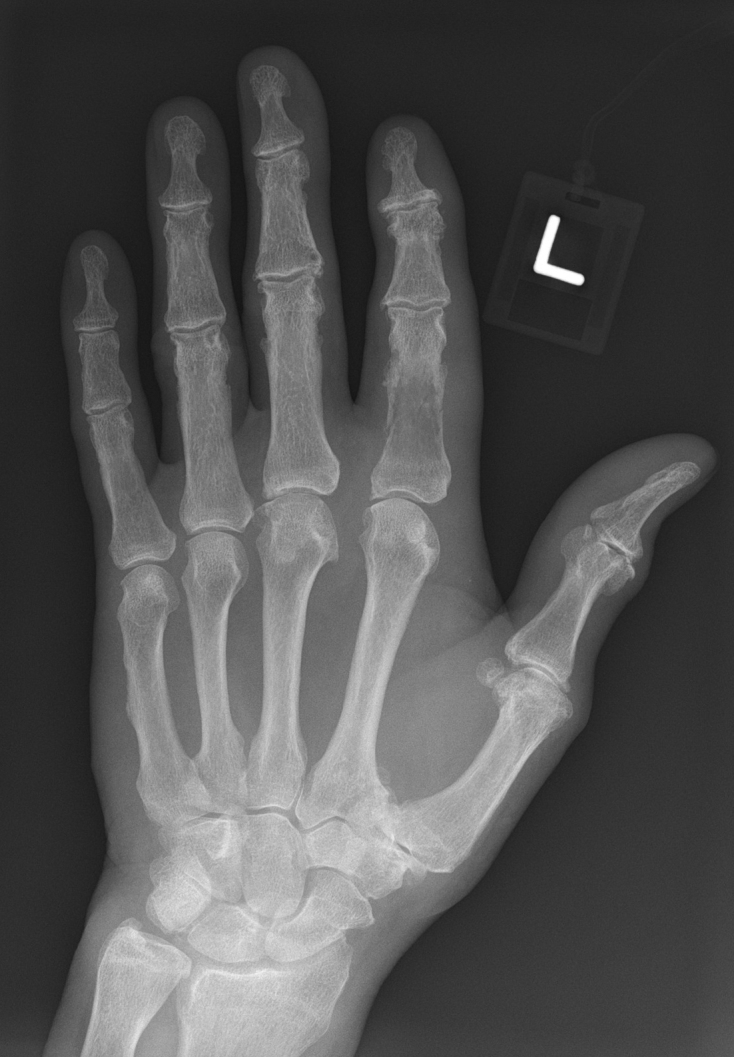
Radiography showing a lesion in the proximal phalanx of the index finger
Discussion
Metastatic disease affecting the hand is reported as a rare occurrence.14 On review of our database, it was found that 6.1% of all malignant tumours of the hand are due to metastatic disease and the total incidence of hand metastasis is 0.42%. It is possible that the reported incidence of acral metastases is low owing to a lack of clinical suspicion and therefore subsequent underdiagnosis.15 Patients with metastatic lesions are frequently misdiagnosed with a variety of other more common hand conditions ranging from a simple felon to Paget’s disease.15–23
Even in patients with known malignant disease, whole body imaging for staging may not reveal disease affecting the hands or feet as they are often not included in the scanning fields.24 It has been recommended that local views of extremities are obtained on standard whole body imaging (Fig 3).25 However, a high index of suspicion must be maintained in order to correctly identify a pathological lesion as there have been reports of an overlooked thumb lesion dismissed as artefact on bone imaging.21 Furthermore, metastases are commonly only diagnosed at autopsy but the extremities may not be examined then and so lesions at these sites may not be detected.1
Figure 3.
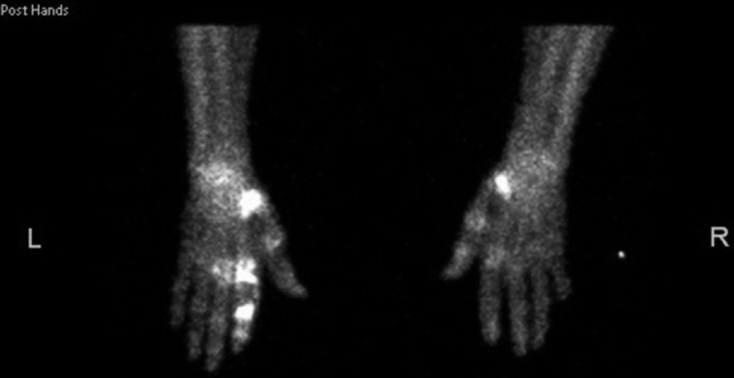
Positron emission tomography combined with computed tomography of the patient in Figure 2 revealing additional metastases not visible on plain radiography
A symptomatic lesion in the hand may be the first clinical sign of either malignancy or progression to metastatic disease. A study looking at 29 cases of acrometastasis found that acral symptoms were the first sign of malignancy in 4 patients (14%).22 In a literature review by Afshar et al, it was noted that 30% of patients had hand metastasis as their first diagnosis of malignancy.5 The distal phalanx was the most frequently involved bone and the thumb was the most frequently involved digit, which reflects our experience as thumb involvement was seen in 66% of our patients. In addition, three (30%) of our patients had no prior diagnosis of malignancy and seven had pre-existing malignant disease but were considered disease free at presentation. Ahlmann et al reported similar findings in two of their three patients with hand metastasis.23
From our results and on reviewing the available literature, the most frequently presenting symptom of hand metastatic disease is that of pain. It is well known that pain is the first and predominant feature of skeletal metastases.26,27 With this in mind, it is vital to ensure a timely diagnosis and prompt treatment in patients presenting with unexplained progressive pain; especially those with a history of prior malignancy warrant further investigation to exclude metastasis (Fig 4).26,28 The three most common primary malignancies that resulted in acrometastasis were lung, gastrointestinal tract and kidney. In our series, the majority of primary malignancies were of supradiaphragmatic origin. Lung and skin primary tumours were the most common, occurring in 40% and 30% of cases respectively. This concurs with high recorded incidence rates of these malignancies in the UK (with lung and non-melanoma skin cancer recorded at 79.3 and 223.6 per 100,000 people respectively in 2014).29
Figure 4.
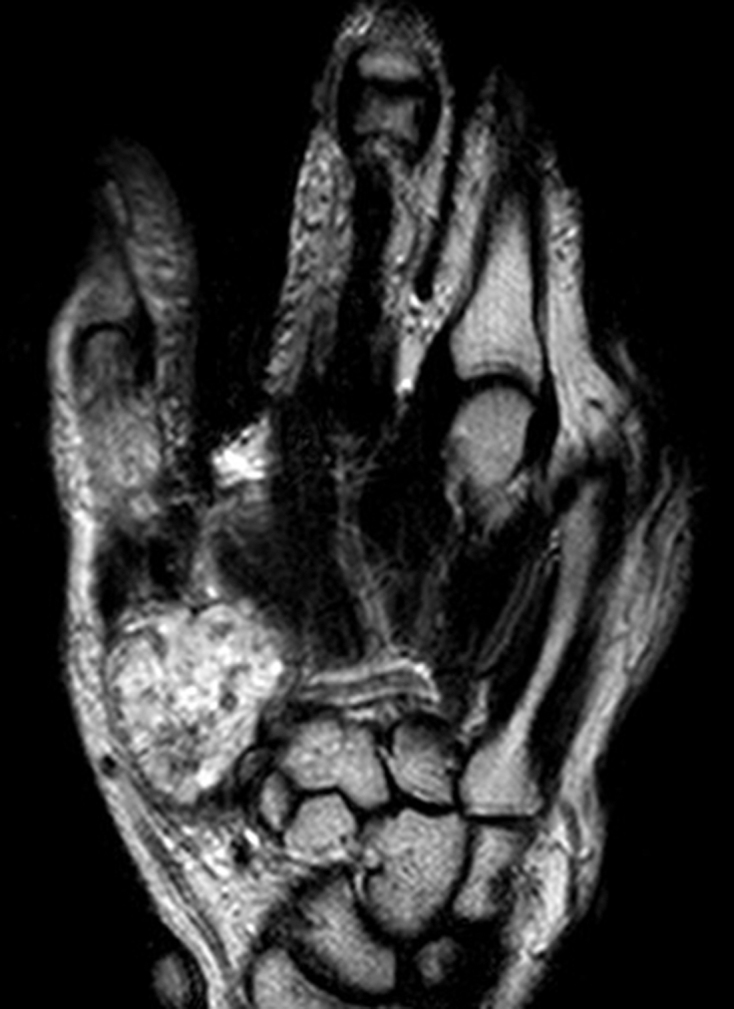
Magnetic resonance imaging of the thumb revealing the extent of bone and soft tissue involvement of the lesion in Figure 1
Acral metastasis is recognised as a poor prognostic indicator. Metastasis to the hand is often present in disseminated disease and consequently carries with it a short life expectancy of five to six months.4,7,13 Our patients waited a median of 8 weeks from symptom onset before diagnosis. The median survival following diagnosis was 18 months, with a mean of 14.5 months. This is after adjusting for the extended survival time of a patient who survived 138 months with a metastatic axillary adnexal tumour (sweat gland tumour).
As this highlights the possibility of extended survival beyond conventional concepts, we would suggest that early investigation with appropriate imaging modalities is imperative. Plain radiography is the most affordable and attainable, and should be considered as first-line investigation despite its poor sensitivity.30 Further cross-sectional imaging is necessary. Magnetic resonance imaging has the benefits of avoiding ionising radiation as well as having a high sensitivity and specificity for the detection of metastatic disease (91% and 95% respectively).31 This allows the identification of suspicious lesions and subsequent referral to the local orthopaedic oncology multidisciplinary team for further specific investigations and management.
The definitive diagnostic tool (where appropriate) is a biopsy and histological examination, which should be carried out by a dedicated oncology centre. This is in accordance with the seminal paper by Mankin et al, which revealed that errors, complications and changes in the outcome of patients were 12–14 times greater when biopsy was performed in the referring institution rather than the treatment centre.32 This also remains the standard of care that should be met according to the British Orthopaedic Oncology Society and British Orthopaedic Association’s guide to good practice for metastatic bone disease.27 Biopsy to establish histological diagnosis, even in the presence of previous malignancy, is vital as the suspicious lesion may in fact be a primary soft tissue or bone tumour. If this were the case the treatment and prognosis would be vastly different to that of metastatic disease.
Currently, there is no standardised treatment for hand metastasis as each tumour type will behave uniquely; options range from analgesia to radiotherapy or amputation and treatment is usually palliative.9,14,15,26,33,34 Isolated bone lesions from thyroid or renal cancer should be considered for resection.33,35 In renal cancer, en bloc excision has been associated with a fourfold increase in survival time.36 Each case is handled on an individual basis, the aim being symptom control and maintenance of function.14 Surgical intervention should only be considered if it confers a survival advantage or when symptoms are not controlled by other means. As limited life expectancy is the rule, any surgical treatment should be modified to reduce surgical morbidity and unnecessary hospitalisation.15 Alternative palliative treatments such as radiotherapy or chemotherapy can aid in symptom control by reducing the tumour load and consequently also pain.14,26,34
Conclusions
While it is accepted that the incidence of benign hand conditions far outweighs that of metastatic hand disease, it is important to maintain a high index of suspicion for unexplained protracted hand pain as this can be the first presentation of malignant, metastatic disease. This suspicion must be heightened further for patients with a known prior or current malignancy. This should prompt early radiological investigation and appropriate referral based on clinical history and radiological findings. As hand metastasis indicates terminal disease with limited life expectancy, early recognition is vital to ensure adequate treatment is initiated as soon as possible to maintain a patient’s quality of life.
References
- 1.diSibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med 2008; : 931–939. [DOI] [PubMed] [Google Scholar]
- 2.Singh VA, Haseeb A, Alkubaisi AA. Incidence and outcome of bone metastatic disease at University Malaya Medical Centre. Singapore Med J 2014; : 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernandez RK, Wade SW, Reich A et al. Incidence of bone metastases in US patients with solid tumors. J Clin Oncol 2016; (Suppl): e13099. [Google Scholar]
- 4.Morris DM, House HC. The significance of metastasis to the bones and soft tissues of the hand. J Surg Oncol 1985; : 146–150. [DOI] [PubMed] [Google Scholar]
- 5.Afshar A, Farhadnia P, Khalkhali H. Metastases to the hand and wrist: an analysis of 221 cases. J Hand Surg Am 2014; : 923–932. [DOI] [PubMed] [Google Scholar]
- 6.Kerin R. The hand in metastatic disease. J Hand Surg Am 1987; : 77–83. [DOI] [PubMed] [Google Scholar]
- 7.Amadio PC, Lombardi RM. Metastatic tumors of the hand. Hand Surg Am 1987; : 311–316. [DOI] [PubMed] [Google Scholar]
- 8.Hayden RJ, Sullivan LG, Jebson PJ. The hand in metastatic disease and acral manifestations of paraneoplastic syndromes. Hand Clin 2004; : 335–343. [DOI] [PubMed] [Google Scholar]
- 9.Sur YJ, Kang YK, Bahk WJ et al. Metastatic malignant tumour in the hand. J Plast Surg Hand Surg 2011; : 90–95. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh CY, Bai LY, Lo WC et al. Esophageal squamous cell carcinoma with a solitary phalangeal metastasis. South Med J 2008; : 1,159–1,160. [DOI] [PubMed] [Google Scholar]
- 11.Sonoda LI, Halim MY, Balan KK. Solitary phalangeal metastasis of renal cell carcinoma on bone scintigram. Clin Nucl Med 2011; : 237–239. [DOI] [PubMed] [Google Scholar]
- 12.Daly B, Granata J, Mayerson J. Acrometastasis: first metatarsophalangeal joint pain due to metastatic lung cancer. JBJS Case Connect 2012; : e4. [DOI] [PubMed] [Google Scholar]
- 13.Flynn CJ, Danjoux C, Wong J et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol 2008; : 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randall RL. Metastatic Bone Disease. New York: Springer; 2016. [Google Scholar]
- 15.Hsu CS, Hentz VR, Yao J. Tumours of the hand. Lancet Oncol 2007; : 157–166. [DOI] [PubMed] [Google Scholar]
- 16.Karten I, Bartfeld H. Bronchogenic carcinoma simulating early rheumatoid arthritis. Metastases to the fingers. JAMA 1962; : 162–164. [DOI] [PubMed] [Google Scholar]
- 17.Sneddon J. Painless metastatic deposit in a finger presenting as a pulp infection with osteitis. Br J Clin Pract 1969; : 511–513. [PubMed] [Google Scholar]
- 18.Bevan DA, Ehrlich GE, Gupta VP. Metastatic carcinoma simulating gout. JAMA 1977; : 2,746–2,747. [PubMed] [Google Scholar]
- 19.Wu KK, Guise ER. Metastatic tumors of the hand: a report of six cases. J Hand Surg Am 1978; : 271–276. [DOI] [PubMed] [Google Scholar]
- 20.Rose BA, Wood FM. Metastatic bronchogenic carcinoma masquerading as a felon. J Hand Surg Am 1983; : 325–328. [DOI] [PubMed] [Google Scholar]
- 21.Cross AB. Bronchogenic carcinoma presenting as an injured thumb. Arch Emerg Med 1985; : 93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am 1986; : 743–746. [PubMed] [Google Scholar]
- 23.Ahlmann ER, Greene NW, Menendez LR, Stevanovic MV. Unusual locations for metastatic malignancy of the hand: a report of three cases. J Surg Orthop Adv 2008; : 267–270. [PubMed] [Google Scholar]
- 24.Hattrup SJ, Amadio PC, Sim FH, Lombardi RM. Metastatic tumors of the foot and ankle. Foot Ankle 1988; : 243–247. [DOI] [PubMed] [Google Scholar]
- 25.Krishnamurthy GT, Tubis M, Hiss J, Blahd WH. Distribution pattern of metastatic bone disease. A need for total body skeletal image. JAMA 1977; : 2,504–2,506. [PubMed] [Google Scholar]
- 26.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001; : 165–176. [DOI] [PubMed] [Google Scholar]
- 27.British Orthopaedic Oncology Society, British Orthopaedic Association Metastatic Bone Disease: A Guide to Good Practice. London: BOA; 2015. [Google Scholar]
- 28.British Association of Surgical Oncology Guidelines: The management of metastatic bone disease in the United Kingdom. Eur J Surg Oncol 1999; : 3–23. [PubMed] [Google Scholar]
- 29.Cancer Research UK Cancer Statistics for the UK. http://www.cancerresearchuk.org/health-professional/cancer-statistics/ (cited May 2017).
- 30.Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med 2001; : 53–64. [PubMed] [Google Scholar]
- 31.Yang HL, Liu T, Wang XM et al. Diagnosis of bone metastases: a meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur Radiol 2011; : 2604–2617. [DOI] [PubMed] [Google Scholar]
- 32.Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. J Bone Joint Surg Am 1996; : 656–663. [DOI] [PubMed] [Google Scholar]
- 33.Jung ST, Ghert MA, Harrelson JM, Scully SP. Treatment of osseous metastases in patients with renal cell carcinoma. Clin Orthop Relat Res 2003; : 223–231. [DOI] [PubMed] [Google Scholar]
- 34.Soyfer V, Corn BW, Kollender Y et al. Radiation therapy for palliation of sarcoma metastases: a unique and uniform hypofractionation experience. Sarcoma 2010; 927972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghert MA, Harrelson JM, Scully SP. Solitary renal cell carcinoma metastasis to the hand: the need for wide excision or amputation. J Hand Surg Am 2001; : 156–160. [DOI] [PubMed] [Google Scholar]
- 36.Ratasvuori M, Wedin R, Hansen BH et al. Prognostic role of en-bloc resection and late onset of bone metastasis in patients with bone-seeking carcinomas of the kidney, breast, lung, and prostate: SSG study on 672 operated skeletal metastases. J Surg Oncol 2014; : 360–365. [DOI] [PubMed] [Google Scholar]


