Abstract
Introduction
Malignant osseous foot tumours are uncommon. Their oncological outcomes have been poorly documented in the literature so far. The aim of this study was to establish the incidence and to evaluate the oncological outcomes of such patients.
Methods
Our large orthopaedic oncology database was used to review 70 malignant osseous foot tumour patients.
Results
The age at diagnosis of malignant osseous foot tumours demonstrated a bimodal distribution peaking in the second and eighth decades of life. Overall, 55 primary malignant bone tumours of the foot (79%) were identified. The median duration from onset of symptoms to diagnosis was 52 weeks (interquartile range [IQR]: 17–104). Eight primary tumours (15%) underwent an accidental excision (ie intralesional excision of a malignant bone tumour where some of the tumour has been left behind, also known as a ‘whoops procedure’) prior to referral to our unit. Forty-six patients (84%) underwent surgery overall and thirteen of these developed recurrence or metastases. Seven of eight patients with a previous accidental excision underwent amputation.
Fifteen osseous metastatic foot lesions were identified. The median length of foot symptoms to diagnosis was 24 weeks (IQR: 20–36 weeks). The median time to death following diagnosis of osseous foot metastases was 20.1 months (IQR: 11.3–27.8 months).
Conclusions
A high index of suspicion and awareness of clinical features of malignant osseous foot tumours are both essential to avoid diagnostic delays. Amputation is associated with a respectable outcome for patients who have undergone previous accidental excisions.
Keywords: Bone tumour, Foot, Malignancy, Primary, Metastasis
Bone tumours of the foot are uncommon, constituting less than 4% of all neoplasms.1,2 Only 15–25% of bony foot lesions are malignant,3 making it easy for clinicians to underestimate the malignant potential of an abnormality of the foot. Delays in diagnosis of malignant bony foot tumours may worsen the prognosis and late presentation potentially modifies treatment strategies towards amputation rather than limb salvage.4,5
There have been few reports of large series documenting malignant foot tumours in the literature so their incidence and clinical outcomes remain poorly established. Most studies published so far have focused on soft tissue sarcomas of the foot rather than osseous malignancies.6–8
The present study reviewed our experience with malignant bone tumours of the foot seen at our supraregional sarcoma unit over a 30-year period. The primary objective was to establish the presenting features and incidence as well as evaluating the clinical course and oncological outcomes of malignant osseous foot tumours.
Methods
Our unit maintains a prospective tumour database containing over 40,000 patients, including 4,000 patients with a bone sarcoma. Using this database, a retrospective search of a 30-year period (1985–2015) was conducted to identify patients with a malignant tumour of the foot. Patients with metastatic lesions to the feet were also included in the study. All haematological malignancies were excluded. Patients’ age and sex were recorded, alongside anatomical location, histology, treatment modality, treatment response, complications, previous accidental excisions (ie intralesional excision of a malignant bone tumour where some of the tumour has been left behind, also known as a ‘whoops procedure’), disease recurrence and length of survival.
Overall survival was determined as the interval in months from diagnosis to death or to the date of the last clinic visit. It was assessed using the Kaplan–Meier method. Statistical interpretation was performed for categorical data using the chi-squared test. An alpha value of 0.05 was considered statistically significant.
Results
A total of 239 patients with foot malignancy were diagnosed and treated at our unit over the 30-year study period. There was an equal distribution of male and female patients. Of the 70 patients (29%) who had a malignant tumour involving bones of the foot, 55 (79%) had primary malignant lesions and 15 (21%) had metastatic lesions. The remaining 169 patients had a soft tissue sarcoma affecting the foot. Our institution serves a population of 15 million. This implies that the overall incidence of primary osseous foot malignancy was 0.12 cases per year per million people.
The age at diagnosis of malignant bone tumours demonstrated a bimodal distribution peaking in the second and eighth decades of life (Fig 1). The median age of presentation for primary bone tumours was 37 years (interquartile range [IQR]: 16–57 years). Ewing’s sarcoma most commonly affected young patients, with a median age at diagnosis of 15 years (IQR: 12–26 years). Comparatively, chondrosarcomas, osteosarcomas and bone metastases affecting the foot were diagnosed later in life (Table 1).
Figure 1.

Age at diagnosis of malignant bone tumours.
Table 1.
Average age at presentation, duration from onset of symptoms to diagnosis and survival rates by tumour type
| Tumour type | Median age at presentation | Median duration of symptoms | Survival rates |
| Ewing’s sarcoma | 15 yrs (range: 1–45 yrs) | 26 wks | 58% at 5 and 10 yrs |
| Chondrosarcoma | 56 yrs (range: 19–89 yrs) | 104 wks | 74% at 5 yrs, 64% at 10 yrs |
| Osteosarcoma | 50 yrs (range: 14–70 yrs) | 44 wks | 68% at 5 and 10 yrs |
| Metastases | 67 yrs (range: 14–83 yrs) | 48 wks | N/A |
Primary malignant bone tumours of the foot
Primary malignant tumours were found in 27 female and 28 male patients. Eight patients were referred to our unit following an accidental excision at their referring hospitals. Overall, 20 primary tumours (36%) were Ewing’s sarcomas, 25 (46%) were chondrosarcomas (6 low grade, 17 intermediate grade, 1 high grade, 1 dedifferentiated) and 10 (18%) were osteosarcomas (2 low grade central, 4 parosteal, 4 high grade). Grouped according to regions of the foot, 15 primary bone tumours (27%) were located in the hallux, 22 (40%) in the metatarsals, 4 (7%) in the midfoot and 14 (25%) in the hindfoot (Table 2). The forefoot was affected statistically more significantly than the mid or hindfoot (p=0.04).
Table 2.
Frequency of tumours by tumour type and region of the foot
| Site | Ewing’s sarcoma (n=20) | Chondrosarcoma (n=25) | Osteosarcoma (n=10) | Metastases (n=15) |
| Hallux (n=15) | 3 | 10 | 2 | 0 |
| Rays (n=25) | 8 | 9 | 5 | 3 |
| Midfoot (n=12) | 2 | 1 | 1 | 8 |
| Hindfoot (n=18) | 6 | 5 | 3 | 4 |
The predominant symptoms of primary osseous foot tumours were non-mechanical pain. Some patients had associated swelling, irrespective of tumour site or histology. The median interval between onset of symptoms and diagnosis of primary malignant bone tumours of the foot was 52 weeks (IQR: 17–104 weeks). Chondrosarcomas presented with the longest duration of symptoms prior to diagnosis (median: 104 weeks, IQR: 52–156 weeks), followed by osteosarcomas (median: 44 weeks, IQR: 28–78 weeks) and Ewing’s sarcomas (median: 26 weeks, IQR: 12–30 weeks).
Four (20%) of twenty cases of Ewing’s sarcoma presented with metastases at diagnosis (2 to lungs, 1 to lung and other bones, 1 to multiple sites). Only one case of osteosarcoma was metastatic at diagnosis. No chondrosarcomas presented with metastases.
The management of all primary malignant tumours of the foot was decided following multidisciplinary discussion. Neoadjuvant and adjuvant chemotherapy as well as radiotherapy were administered according to standard protocols for Ewing’s sarcoma and osteosarcoma. Overall, 46 patients (84%) underwent surgery. This was combined with chemotherapy in nine cases (20%). Eighteen patients had hallux amputations, eighteen had below-knee amputations, five had ray amputations, two underwent resections with biological reconstruction and three underwent intralesional curettage for low grade tumours. Subsequently, six patients (4 Ewing’s sarcomas, 1 chondrosarcoma, 1 osteosarcoma) developed local recurrence and a further seven patients (13%) developed metastases. Overall, 8 of these 13 patients with disease recurrence died.
Of the patients who developed local recurrence, three had primary lesions in the metatarsals, one in the cuboid and two in the calcaneus. Interestingly, two (33%) of these patients underwent ray amputations with wide margins before developing recurrence and one had a below-knee amputation. Of the seven patients who developed metastases, two had primary lesions in the hallux, one in the navicular, two in the talus and three in the calcaneus. Three of these patients initially underwent below-knee amputations for their primary lesions, two had hallux amputations and the remaining patients received chemotherapy only.
Of the eight patients who initially underwent an accidental excision, three had ray amputations, three had below-knee amputations, one had a toe amputation and one received only chemotherapy owing to being too frail for surgery. The latter patient subsequently developed local recurrence and another patient who had a ray amputation developed metastasis. These 2 patients (25%) eventually died at 38 months and 55 months respectively following diagnosis but the remaining 6 patients have survived at least 5 years following amputation.
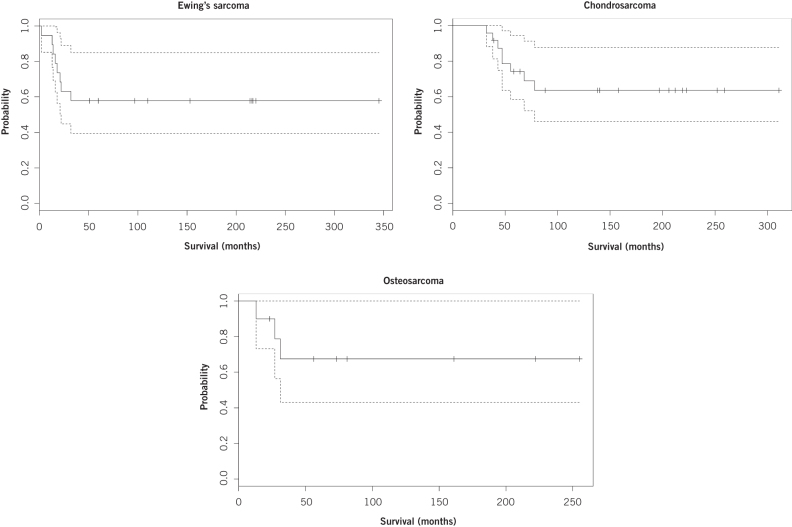
Of all patients who died, the median time to death was 19 months (IQR: 17–25 months) for Ewing’s sarcoma, 47 months (IQR: 44–68 months) for chondrosarcoma and 27 months (IQR: 24–31 months) for osteosarcoma. The Kaplan–Meier survival estimate for both five and ten years for Ewing’s sarcoma was 57.9% (95% CI: 39.5–85.0%). For chondrosarcoma, the Kaplan–Meier five-year estimate was 74.2% (95% CI: 58.4–94.3%) while the ten-year estimate was 63.6% (95% CI: 46.1–87.7%). For osteosarcoma, the survival estimate for both five and ten years was 67.5% (95% CI: 43.0–100%). The Kaplan–Meier survival curves are shown in Figure 2.
Figure 2.
Kaplan–Meier survival curves for primary bone tumours of the foot.
Metastatic bone tumours of the foot
Of the 15 patients (9 female, 6 male) with osseous metastatic lesions to the foot, 12 had metastases in the mid or hindfoot while the remaining 3 patients had lesions in the forefoot (Table 2). The median duration of foot symptoms to diagnosis was 24 weeks (IQR: 20–36 weeks). The disease free period from diagnosis of primary malignancy to the detection of bony foot metastases ranged from 7 to 64 months (mean: 19.3 months).
Only four patients (27%) with osseous foot metastases had primary lung carcinoma. Two patients (13%) had primary breast malignancy and nine (60%) had infradiaphragmatic primary lesions (2 in bladder, 2 melanomas, 1 uterine, 1 anal, 1 vaginal, 1 rhabdomyosarcoma). Three patients (20%) with bony foot metastases presented with no history of previous malignancy and the sites of their primary tumours were only determined following biopsy. In 12 patients (80%), foot metastasis was the only metastasis apparent at the time of presentation. Only three patients (20%) were found to have additional metastases in sites other than the feet. Nine patients (60%) underwent palliative radiotherapy to the feet, three (20%) underwent amputation and three (20%) were managed palliatively. The median time to death following diagnosis of osseous foot metastases was 20.1 months (IQR: 11.3–27.8 months).
Discussion
Malignant bone tumours of the foot are rare, and the epidemiology and clinical outcomes have not been extensively studied in the literature. This study reviewed the clinical course of 70 malignant bone tumours of the foot over a period of 30 years in our supraregional sarcoma unit. In our experience, 79% of malignant bone lesions affecting the foot were primary tumours and the remaining 21% were bone metastases. Our institution has a catchment population of 15 million and the incidence of primary bone tumours of the foot is 0.12 cases per year per million people.
All malignant bone tumours of the foot presented with non-mechanical pain and some with associated swelling. Chondrosarcomas were the most frequent primary tumour to affect the foot (46%) and osteosarcomas were the least frequent (18%) at our unit. The majority (67%) of primary tumours were located in the forefoot whereas the majority (80%) of metastases were located in the mid or hindfoot. This highlights that symptoms of non-mechanical pain in any region of the foot could potentially indicate osseous malignancy.
An increasing number of studies have shown that otherwise healthy young individuals are presenting with malignant foot tumours.9–11 In our cohort, the age of diagnosis of malignant bone tumours of the foot peaked in the second and eighth decades of life. This was also found in a review by Khan et al.5 Furthermore, our findings that Ewing’s sarcomas commonly present in the second decade of life whereas chondrosarcomas and osteosarcomas usually present between the fourth and sixth decades are supported by those of Bos et al.4
This age of presentation of osteosarcomas of the foot is contrary to the usual age of presentation in the rest of the appendicular skeleton (second and third decades of life).12 We cannot explain this observation although Choong et al reported similar findings.13 It is therefore important to raise the clinical suspicion of foot malignancy in patients of any age in order to reduce the risks of a delayed diagnosis.
Aside from their relatively low prevalence, malignant bone tumours of the foot can also often be misdiagnosed owing to the variety of potential differential diagnoses. Biscaglia et al described a series where there was initial misdiagnosis of 6 out of 12 osteosarcoma cases.12 In addition, a study by Adkins et al found that 11 of 16 Ewing’s sarcoma patients had initial misdiagnoses.14 The most common symptoms of malignant bone tumours of the foot include pain or a palpable swelling.15,16 However, these are frequently misdiagnosed as a benign condition such as osteoarthritis, rheumatoid arthritis or a benign tumour.15 Clinical suspicion may only increase if a swelling that has remained dormant for many years suddenly becomes symptomatic or starts growing; this can result in a delayed diagnosis.17
Our series confirmed that patients with either a primary tumour or metastasis often suffer from protracted symptoms before establishing a correct diagnosis. The overall duration of symptoms prior to diagnosis of malignant bone tumours of the foot ranged from 4 to 780 weeks. The median duration was 52 weeks. Chondrosarcomas are the most slow growing of these tumours4 and therefore have the most prolonged duration of symptoms.
Worryingly, 15% of the primary bone malignancies in the foot had undergone accidental excision prior to being referred to our unit. Six of the eight patients who received an accidental excision had a chondrosarcoma of the forefoot. This reflects the difficulty in distinguishing between benign and malignant lesions in the foot region based on clinical history and examination alone. Many studies have reported that differentiation of chondrosarcomas from benign cartilage tumours may even prove difficult on histology for an experienced pathologist.18–21 Despite this, our study shows that chondrosarcoma patients still have the longest average length of survival compared with those with any other bony foot malignancy.
Surgical amputation was performed in 73% of our cohort of patients with primary malignant bone tumours of the foot, regardless of tumour histology or site. Amputation may be in the form of below-knee amputation for midfoot or hindfoot involvement, or ray amputation for toes or metatarsals. The majority (82%) of patients who underwent amputation had no local recurrence or metastases. The remaining 18% may have had occult metastases that were not detected or removed at the time of surgery.
Amputation is particularly appropriate for Ewing’s sarcoma and osteosarcoma as these tumours are less amenable to limb sparing surgery because of poor tumour compartmentalisation in the foot and difficulty in achieving adequate resection margins.2,5,13 In our experience, amputation was an appropriate management option for patients with accidental excision. Seven of the eight patients who were referred with initial accidental excision subsequently underwent amputation. This achieved a five-year survival rate of 75% with no local recurrence or metastases.
A third (31%) of our cohort of patients with osseous foot malignancy died within five years of diagnosis. The mean time to death overall was 28.2 months. Local recurrence developed in 10.9% of patients and a further 12.7% developed metastases. Comparatively, 14–25% of patients with soft tissue sarcomas of the foot die within ten years of diagnosis.22–24 The recurrence rate can be up to 12%.24 This reflects a worse prognosis for patients with osseous sarcomas of the foot than for those with soft tissue sarcomas.
Our finding that Ewing’s sarcoma has the worst prognosis of malignant osseous foot tumours (despite most frequently affecting the youngest patients) concurs with that of O’Connor and Pritchard.25 Our study reflects that this tumour has the shortest median time to death compared with any other osseous foot malignancy. Patients with Ewing’s sarcoma had the highest occurrence of distal metastases at diagnosis (especially to the lungs and bones) compared with other primary foot tumours. Shirley et al reported similar findings.26 In our cohort, the local recurrence rate was also highest in Ewing’s sarcoma cases.
The reported average time to death for osseous foot metastases is 9.9–14.7 months.27 The overall mean time to death in our series was slightly longer at 20.1 months. Moreover, the duration of symptoms prior to diagnosis of foot metastases (median: 24 weeks) was shorter than that for primary bone tumours (median: 52 weeks). The delayed diagnosis of primary bone tumours relative to diagnosis of metastatic lesions of the foot may be related to the physician not considering primary malignant neoplasm as a possible differential diagnosis. The diagnosis of metastatic lesions could still prove difficult, however, as 80% of our patients had no other metastasis at presentation and 20% did not have a known existing primary malignancy. Consequently, new symptoms (such as pain or swelling) in any part of the foot should be investigated promptly using further imaging as they may be the only indication of a disseminated malignancy.
Conclusions
Our study included the largest number of malignant bone tumours that were categorised to various anatomical areas of the foot. It has demonstrated that patients with osseous foot malignancy often have a protracted length of symptoms. A high index of suspicion as well as an awareness of the epidemiological and clinical features of malignant bony foot lesions are therefore required to avoid diagnostic delays. Surgical amputation achieved respectable survival in patients with primary malignant bone tumours of the foot, including those who had originally undergone accidental excision. Early diagnosis and appropriate management of malignant bone tumours of the foot are key factors in improving oncological outcomes for patients.
References
- 1.Dahlin DC, Unni KK. Bone Tumors: General Aspects and Data on 8,542 Cases. 4th edn. Springfield, IL: Thomas; 1986. [Google Scholar]
- 2.Azevedo CP, Casanova JM, Guerra MG et al. Tumors of the foot and ankle: a single-institution experience. J Foot Ankle Surg 2013; : 147–152. [DOI] [PubMed] [Google Scholar]
- 3.Zeytoonjian T, Mankin HJ, Gebhardt MC, Hornicek FJ. Distal lower extremity sarcomas: frequency of occurrence and patient survival rate. Foot Ankle Int 2004; : 325–330. [DOI] [PubMed] [Google Scholar]
- 4.Bos GD, Esther RJ, Woll TS. Foot tumors: diagnosis and treatment. J Am Acad Orthop Surg 2002; : 259–270. [DOI] [PubMed] [Google Scholar]
- 5.Khan Z, Hussain S, Carter SR. Tumours of the foot and ankle. Foot 2015; : 164–172. [DOI] [PubMed] [Google Scholar]
- 6.Scully SP, Temple HT, Harrelson JM. Synovial sarcoma of the foot and ankle. Clin Orthop Relat Res 1999; : 220–226. [DOI] [PubMed] [Google Scholar]
- 7.Bakotik BW, Borkowski P. Primary soft-tissue neoplasms of the foot: the clinicopathologic features of 401 cases. J Foot Ankle Surg 2001; : 28–35. [DOI] [PubMed] [Google Scholar]
- 8.DeGroot H. Approach to the management of soft tissue tumors of the foot and ankle. Foot Ankle Spec 2008; : 168–176. [DOI] [PubMed] [Google Scholar]
- 9.Pritchard DJ, Soule EH, Taylor WF, Ivins JC. Fibrosarcoma – a clinicopathologic and statistical study of 199 tumors of the soft tissues of the extremities and trunk. Cancer 1974; : 888–897. [DOI] [PubMed] [Google Scholar]
- 10.Hwang JS, Fitzhugh VA, Kaushal N, Beebe KS. Epithelioid sarcoma: an unusual presentation in the distal phalanx of the toe. Am J Orthop 2012; : 223–227. [PubMed] [Google Scholar]
- 11.Ruggieri P, Angelini A, Jorge FD et al. Review of foot tumors seen in a university tumor institute. J Foot Ankle Surg 2014; : 282–285. [DOI] [PubMed] [Google Scholar]
- 12.Biscaglia R, Gasbarrini A, Böhling T et al. Osteosarcoma of the bones of the foot – an easily misdiagnosed malignant tumor. Mayo Clin Proc 1998; : 842–847. [DOI] [PubMed] [Google Scholar]
- 13.Choong PF, Qureshi AA, Sim FH, Unni KK. Osteosarcoma of the foot: a review of 52 patients at the Mayo Clinic. Acta Orthop Scand 1999; : 361–364. [DOI] [PubMed] [Google Scholar]
- 14.Adkins CD, Kitaoka HB, Seidl RK, Pritchard DJ. Ewing’s sarcoma of the foot. Clin Orthop Relat Res 1997; : 173–182. [PubMed] [Google Scholar]
- 15.Simon MA, Springfield DS. Surgery for Bone and Soft-tissue Tumors. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 16.Evans S, Ramasamy A, Jeys L, Grimer R. Delayed diagnosis in metastatic lesions of the foot. Ann R Coll Surg Engl 2014; : 536–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campanacci M. Bone and Soft Tissue Tumors. 2nd edn Vienna: Springer; 1999. [Google Scholar]
- 18.Ogose A, Unni KK, Swee RG et al. Chondrosarcoma of small bones of the hands and feet. Cancer 1997; : 50–59. [PubMed] [Google Scholar]
- 19.Murphey MD, Flemming DJ, Boyea SR et al. Enchondroma versus chondrosarcoma in the appendicular skeleton: differentiating features. Radiographics 1998; : 1213–1237. [DOI] [PubMed] [Google Scholar]
- 20.Lee FY, Mankin HJ, Fondren G et al. Chondrosarcoma of bone: an assessment of outcome. J Bone Joint Surg Am 1999; : 326–338. [DOI] [PubMed] [Google Scholar]
- 21.Wang XL, De Beuckeleer LH, De Schepper AM, Van Marck E. Low-grade chondrosarcoma vs enchondroma: challenges in diagnosis and management. Eur Radiol 2001; : 1054–1057. [DOI] [PubMed] [Google Scholar]
- 22.Brennan MF. Management of extremity soft-tissue sarcoma. Am J Surg 1989; : 71–78. [DOI] [PubMed] [Google Scholar]
- 23.Singer >S, Corson JM, Gonin R et al. Prognostic factors predictive of survival and local recurrence for extremity soft tissue sarcoma. Ann Surg 1994; : 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin PP, Guzel VB, Pisters PW et al. Surgical management of soft tissue sarcomas of the hand and foot. Cancer 2002; : 852–861. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor MI, Pritchard DJ. Ewing’s sarcoma. Prognostic factors, disease control, and the reemerging role of surgical treatment. Clin Orthop Relat Res 1991; : 78–87. [PubMed] [Google Scholar]
- 26.Shirley SK, Askin FB, Gilula LA et al. Ewing’s sarcoma in bones of the hands and feet: a clinicopathologic study and review of the literature. J Clin Oncol 1985; : 686–697. [DOI] [PubMed] [Google Scholar]
- 27.Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am 1986; : 743–746. [PubMed] [Google Scholar]