Abstract
AngioVac is a vacuum-based device approved in 2014 for percutaneous removal of undesirable materials from the intravascular system. Although numerous reports exist with regard to the use of the AngioVac device in aspiration of iliocaval, pulmonary, upper extremity, and right-sided heart chamber thrombi, very few data are present demonstrating its use in treatment of right-sided endocarditis. In this case report, we describe the novel device used in debulking a large right-sided tricuspid valve vegetation reducing the occurrence of septic embolisation and enhancing the efficacy of antibiotics in clearance of bloodstream infection. Further research is needed in larger RSIE patient populations to confirm the benefits and the potential of improved outcomes associated with the AngioVac device as well as identify its potential complications.
1. Case Presentation and Procedure Description
A 33-year-old female with a history of hepatitis C and active intravenous heroin use presented to an outside facility with complaints of worsening shortness of breath. A transthoracic echocardiogram (TTE) was done and showed tricuspid valve (TV) vegetation measuring 3 × 1.5 cm with severe tricuspid regurgitation (TR). CT scan of the chest revealed signs concerning septic pulmonary emboli. She was also found to have severe sepsis and was transferred to the coronary care unit for further management. Blood cultures on admission yielded Streptococcus pyogenes, and she was empirically started on vancomycin, linezolid, and cefepime until organisms were shown to be pan-sensitive after which antibiotic coverage was narrowed to penicillin G. Clindamycin was added for synergism, given her extensive septic pulmonary emboli. Repeat blood cultures revealed the same organism for 3 consecutive days. Her respiratory status continued to worsen, and she was consequently intubated. No source other than intravenous drug use (IVDU) was found to be the cause of the bacteremia. Due to the size of the vegetation and persistent bacteremia despite appropriate medical therapy, cardiothoracic surgery was consulted for the possibility of surgical intervention, but the patient was deemed a poor surgical candidate due to her poor nutritional state and hemodynamic instability. After discussion with the family regarding the patient's critically ill state and poor prognosis, a decision was made to transfer her to our facility for debulking of the tricuspid valve vegetation using the AngioVac system. A repeat TTE at our facility confirmed prior findings of TV vegetation measuring 3 × 1.5 cm and moderate TR (Figure 1). Blood cultures repeated at our facility revealed no growth. The patient was immediately taken to the catheterization laboratory for vegetation debulking using the AngioVac system.
Figure 1.
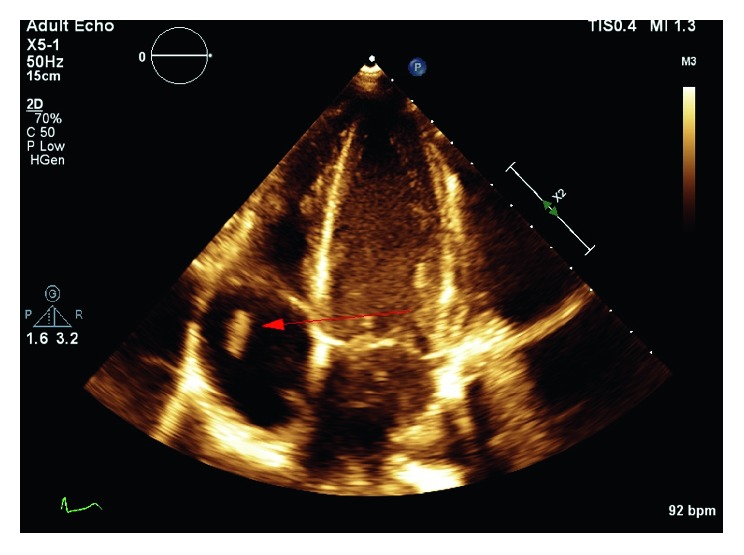
Preprocedural transthoracic echocardiogram (TTE). Apical 4-chamber view revealing a 3 × 1.5 cm tricuspid valve vegetation (red arrow).
The patient arrived in the catheterization laboratory intubated and sedated. Bilateral femoral veins were accessed using the modified Seldinger technique. A 6-French (Fr) precision sheath was placed in the right common femoral vein, and an 8-French sheath was inserted into the left common femoral vein. A 6-French sheath was inserted into the right internal jugular vein (IJ). The right IJ was then progressively dilated with escalating size sheaths and, subsequently, a 24-French sheath was placed over a Lunderquist wire. The left common femoral vein sheath was escalated to a 16-French outlet sheath. The AngioVac system was advanced through the right IJ—a 24-French inlet sheath over a Wholey wire. Under transesophageal echocardiography (TEE) guidance, the system was further advanced via the superior vena cava and right atrium towards the TV, where the vegetation was then repeatedly suctioned. Two pieces of the vegetation were successfully extracted. The intraprocedural TEE revealed a 50–60% reduction in the size of the TV vegetation (2.1 cm decrease in the longest dimension) (Figures 2 and 3). The AngioVac system was removed, and hemostasis was achieved in both the right IJ and the left common femoral venous accesses via purse-string sutures. The patient tolerated the procedure well. Heparin was used for anticoagulation during the procedure.
Figure 2.
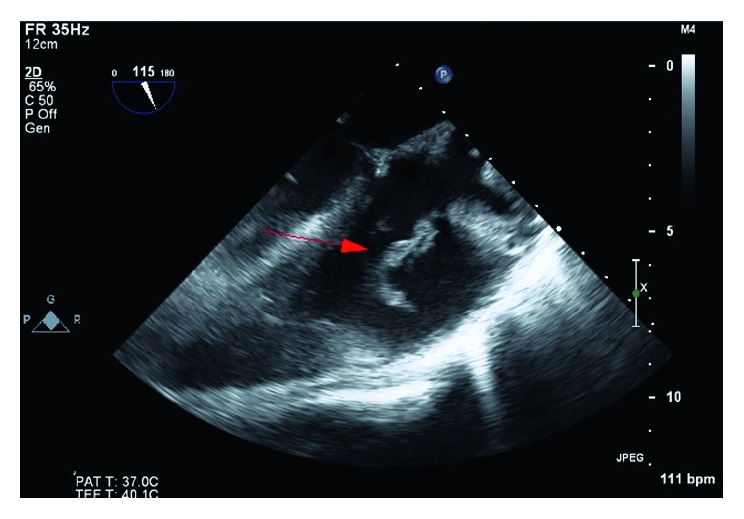
Preprocedural transesophageal echocardiogram (TEE). Midesophageal longitudinal-axis 115-degree view revealing the tricuspid valve vegetation 4.2 cm in its largest diameter (red arrow).
Figure 3.
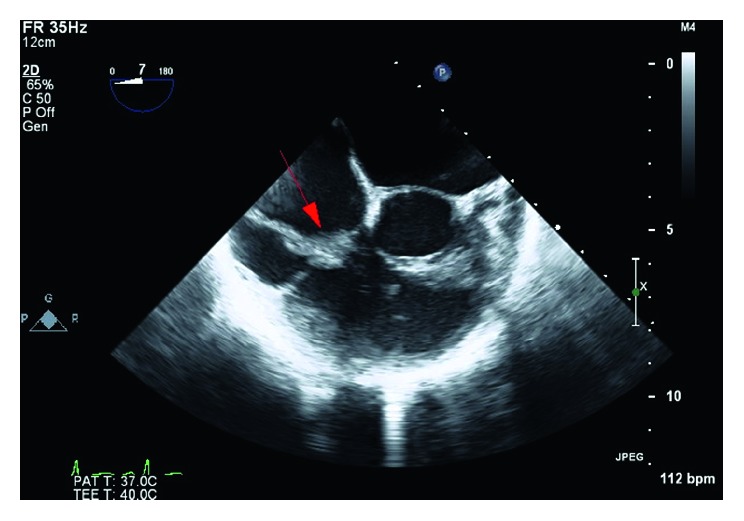
Postprocedural transesophageal echocardiogram (TEE). Midesophageal short-axis 0-degree view revealing the tricuspid valve vegetation reduced in size to 2.1 cm from its largest diameter (red arrow).
The patient's condition improved, and she was extubated on the second day after the procedure. Antibiotics therapy continued. Subsequent blood cultures remained negative, and she became afebrile with a steady decline in her white blood cell count. Seven days after the AngioVac procedure, she was discharged to a rehabilitation facility to finish six weeks of intravenous penicillin G therapy. During a follow-up appointment at an infectious disease clinic one month after hospital discharge, the patient was seen with no symptoms or signs of reinfection.
2. Discussion
The vast majority of cases of right-sided infective endocarditis (RSIE) are in the intravenous drug use (IVDU) population [1]. Epidemiological studies have shown that, among IVDU patients who present with fever, 13% will have echocardiographic evidence of IE [2] and 41% of bacteremic IVDU patients will have evidence of IE, the majority of which is RSIE [3]. Clinical features are distinct from those of left-sided endocarditis. The most common manifestations are fever and sepsis in addition to symptoms related to septic pulmonary emboli including chest pain, dyspnea, cough, and hemoptysis [4]. Although the Duke criteria have their theoretical limitations when applied in diagnosing RSIE, they remain the most widely utilized criteria for diagnosis [4]. The close association of the disease to IVDU creates obstacles to successful treatment. These challenges include nonadherence to in-hospital treatment and noncompliance to follow-up, creating scarcity of data on long-term outcomes [1]. Despite these challenges, RSIE is shown to have a relatively more favorable prognosis when compared to left-sided disease with an in-hospital mortality of less than 10% [1].
Management is multidisciplinary and involves the involvement of a general cardiologist, an infectious disease specialist, a cardiac surgeon, and, occasionally, an interventional cardiologist. There is sufficient evidence that, in uncomplicated cases of RSIE caused by Staphylococcus aureus, two weeks of antibiotic therapy is sufficient [5]. Antibiotic treatment of nonstaphylococcal RSIE, as in our case, is analogous to that of left-sided disease and includes an extended treatment course of 4–6 weeks [6]. While indications of surgery for left-sided disease are well established, the role of surgery in RSIE is less clear. Nevertheless, there are complications where surgery is agreed to be reasonable. These include right heart failure secondary to severe tricuspid regurgitation with poor response to medical therapy, sustained infection caused by difficult-to-treat organisms (i.e., fungi and multidrug-resistant bacteria) or lack of response to appropriate antimicrobial therapy, and tricuspid valve vegetations that are ≥ 20 mm in diameter and recurrent pulmonary embolism despite antimicrobial therapy [6]. When surgery is warranted, valve repair rather than replacement is preferred especially in IVDU patients due to the risk of recurrence of infection in prosthetic valves [6]. Our patient had a tricuspid valve vegetation with a diameter of >30 mm, which led to the consideration of surgical intervention. Due to the patient's poor nutritional status, respiratory failure due to multiple septic emboli, and early signs of DIC, she was deemed an unsuitable candidate for open cardiac surgery.
A noninvasive approach utilizing the AngioVac device was justified, given our patient's critically ill state and high perioperative risk. The US Food and Drug Administration approved the AngioVac system for removal of unwanted intravascular materials (thrombi and emboli) in 2014 [7]. The device is composed of a venous drainage cannula and a reinfusion (venous return) cannula which are connected to an extracorporeal circuit and a commercially available pump head and bubble trap. The venous drainage component is a 22 Fr cannula with a funnel-shaped distal tip that can be advanced through a 26 Fr sheath over a guidewire into the venous system percutaneously. When the bypass pump is started, a suction force is created that facilitates aspiration of blood and thrombotic materials into the tip of the AngioVac cannula, circulating the blood through a filter. After filtration, the drained blood is returned to the patient via a second percutaneously placed reinfusion venous cannula through the internal jugular or femoral vein [8]. This recirculation of venous blood minimizes intraprocedural blood loss and the need of blood transfusion. There are numerous reports on the use of the AngioVac device in aspiration of iliocaval, pulmonary, upper extremity, and right-sided heart chamber thrombi [8]. In contrast to alternative percutaneous thrombectomy devices that require prior thrombus fragmentation and/or thrombolysis to facilitate aspiration, the AngioVac device has the advantage of aspirating the whole intact thrombus. This eliminates the need of preaspiration thrombolysis and reduces the consequent risk of embolisation [9].
Very few data are present with regard to the use of the AngioVac device management of RSIE. The biggest study to date by George et al. reviewed the periprocedural course of 33 patients with tricuspid valve (TV) endocarditis who underwent TV vegetation debulking using the AngioVac device. Average preprocedural vegetation size was 2.1 ± 0.7 cm and average vegetation size on postprocedural echocardiography was 0.82 ± 0.5 cm, demonstrating a 61% average reduction in vegetation size that is in parallel to that seen in our patient. All patients in the study survived the procedure, and 90.9% survived the hospitalization with no further reinfection. We searched two literature databases (PubMed and Embase) to identify publications reporting the use of the AngioVac device for treatment of RSIE (results in Table 1). Most reported indications for the use of the AngioVac device for vegetation debulking in RSIE are consistent with AHA proposed indications of surgery [6]. However, there are reports on utilization of the AngioVac system to debulk lead vegetations as a bridge to percutaneous lead removal in patients with cardiovascular implantable electronic device- (CIED-) related endocarditis [10–12]. Despite data that demonstrate the safety of percutaneous lead removal in patients with vegetations >1 cm [13], the risk of septic pulmonary embolism remains significant at 34–55% in this subset of patients [14, 15]. This predisposes to further infectious complications including pulmonary abscesses and refractory sepsis. Although large population data are lacking, Patel et al. proposed that the AngioVac device used for large vegetation debulking prior to percutaneous lead removal may reduce the incidence of septic pulmonary embolism in patients with CIED-related endocarditis [11]. The use of the novel technique has also been reported as a bridge to reduce lag time and perioperative complications of cardiac surgery [7, 16]. Diminishing intracardiac vegetation size and the associated bacterial load may increase the efficacy of antibiotics in clearing of the bloodstream infection. This concept is supported by studies investigating the efficacy of antibiotics on different bacterial inoculums [17]. High bacterial inoculums are associated with higher antibiotic resistance and reduced penetration, a phenomenon known as the inoculum effect [18]. Through vegetation debulking, the AngioVac allows for reduction of the bacterial inoculum and enhancement of antibiotic activity. This hastens resolution of the septic state and consequent hemodynamic compromise which is a key determinant of operative and postoperative mortality associated with cardiac surgery [19].
Table 1.
Reports on the use of the AngioVac device in treatment of RSIE.
| Author/year | Type of publication | Age/sex | Organism | Location of vegetation | Indication for procedure | Preprocedural vegetation size | Reduction in vegetation size | Postprocedural bacteremia | Tricuspid regurgitation (TR) progression | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Todoran et al. [20] (2011) | Case report | 53 y/o, M | Haemophilus parainfluenzae | SVC/RA junction | Lack of response to appropriate antimicrobial therapy | 1.7 cm | 100% removal | Resolved | Not reported | Improvement with no further sequel |
|
| ||||||||||
| Jones et al. [21] (2017) | Case report | 25 y/o, F | Candida albicans | (i) SV/RA junction attached to ICD RV lead | (i) Persistent fungemia despite appropriate antimicrobial therapy | SV/RA junction: 6.1 cm × 1.65 cm | Removal of 6 cm vegetation (residual vegetation size not reported) | Resolved | Not reported | Improvement with no further sequel |
| (ii) RA | (ii) Recurrent septic emboli | RA: −2.1 cm × 1.6 cm | ||||||||
|
| ||||||||||
| Schaerf et al. [10] (2016) | Retrospective study (20 patients) | Mean age: 76 ± 11 | 8 coagulase-negative SA | 13 ICD; 7 pacemaker | (i) Lack of response to appropriate antimicrobial therapy | Average size: 3.6 cm ± 1.2 cm | Not mentioned | Resolved in 19/20 patients | Not reported | Not reported clearly |
| 3 MSSA | ||||||||||
| 4 MRSA | ||||||||||
| 3 Streptococci | ||||||||||
| Sex not reported | ||||||||||
| 1 Enterococcus | ||||||||||
| 1 Polymicrobial | ||||||||||
| (ii) Bridge to percutaneous lead removal | ||||||||||
|
| ||||||||||
| Thiagaraj et al. [22] (2017) | Case series | Patient 1: 35 y/o, M | MSSA | SVC/RA junction extending into TV | (i) Lack of response to appropriate antimicrobial therapy | 4.5 cm | 100% removal | Resolved | Not reported | Improvement with no further sequel |
| (ii) Vegetation size ≥ 20 mm | ||||||||||
| Patient 2: 28 y/o, F | MRSA | TV | (i) Lack of response to appropriate antimicrobial therapy | 2.2 × 1.7 cm | 100% removal | Resolved | Not reported | MRSA bacteremia recurrence, cardiac arrest, and death 5 days post procedure | ||
| (ii) Vegetation size ≥ 20 mm | ||||||||||
| Patient 3: 53 y/o, F | Enterococcus faecalis | Bioprosthetic TV | (i) Vegetation size ≥ 20 mm | 3.2 cm | 25–50% reduction in size | Resolved | Improvement from moderate to mild | Improvement with mild worsening of TR | ||
| (ii) Worsening of TV regurgitation | ||||||||||
|
| ||||||||||
| Divekar et al. [7] (2013) | Case report | 17 y/o, M | MSSA | Pulmonary valve | Recurrent pulmonary embolism despite antimicrobial therapy | 3.5 cm × 1.5 cm | Significant reduction (residual vegetation size not reported) | Resolved | Not reported | Clinical improvement with no further sequel |
|
| ||||||||||
| George et al. [23] (2017) | Retrospective study (33 patients) | Mean age: 37 ± 12 | 14 MRSA | TV | Lack of response to appropriate antimicrobial therapy | 2.1 cm ± 0.7 cm | Average of 61% reduction in size | Resolved in 28/33 patients | 14 patients: worsening of TR (3 required elective TV repair) | 28 patients: improvement with no further sequel |
| 11 MSSA | ||||||||||
| 12, M | 3 polymicrobial | 1 patient: developed postprocedural cardiac tamponade requiring pericardiocentesis | ||||||||
| 21, F | 5 Candida | 3 patients: death | ||||||||
|
| ||||||||||
| Makdisi et al. [16] (2016) | Case report | 24 y/o, M | MRSA | TV | Lack of response to appropriate antimicrobial therapy | 0.9 cm × 0.7 cm | 80% reduction in size | Resolved | No change | Clinical improvement with no further sequel |
| 0.7 cm × 1 cm | ||||||||||
|
| ||||||||||
| Patel et al. [11] (2013) | Case series | Patient 1: 59 y/o, M | SA | ICD lead | (i) Vegetation size ≥ 20 mm | 3 cm × 2 cm | Significant reduction (residual vegetation size not reported) | Resolved | Improvement in degree of TR | Clinical improvement with no further sequel |
| (ii) Bridge to percutaneous lead removal | ||||||||||
| Patient 2: 82 y/o, M | Group B Streptococcus | (i) Pacemaker lead | (i) Vegetation size ≥ 20 mm | (i) Pacemaker lead: 4 cm × 1.5 cm | Significant reduction (residual vegetation size not reported) | Not reported | Not reported | Not reported | ||
| (ii) TV | (ii) Bridge to percutaneous lead removal | (ii) TV: 0.5 cm × 1.1 cm | ||||||||
| Patient 3: 56 y/o, F | MRSA | Pacemaker lead | (i) Vegetation size ≥ 20 mm | 3.5 cm × 1.7 cm | Significant reduction (residual vegetation size not reported) | Persistent bacteremia | Worsening of TR | Formation of new vegetation with severe TR that required TV repair | ||
| (ii) Bridge to percutaneous lead removal | ||||||||||
|
| ||||||||||
| Dalia et al. [12] (2016) | Case report | 26 y/o, F | Not reported | TV | Bridge to pulmonary artery aneurysm repair | 1.6 cm × 0.8 cm | Significant reduction (residual vegetation size not reported) | Not reported | Not reported | Underwent pulmonary artery aneurysm repair successfully; clinical improvement with no further sequel |
|
| ||||||||||
| Hosoba et al. [24] (2015) | Case series | Patient 1: 67 y/o, F | MRSA | RA | Not reported | 1.5 cm × 1.5 cm | Not reported | Resolved | Not reported | Clinical improvement with no further sequel |
| Patient 2: 33 y/o, M | Enterobacter cloacae | RA near Chiari network | Not reported | 2.2 cm × 0.6 cm | Not reported | Resolved | Not reported | Clinical improvement with no further sequel | ||
| Patient 3: 70 y/o, M | MSSA | SVC/RA junction | Vegetation size 20 mm | 3.4 cm × 1.3 cm | Not reported | Resolved | Not reported | Clinical improvement with no further sequel | ||
M: male, F: female, y/o: years old, SVC: superior vena cava, RA: right atrium, ICD: implantable cardioverter defibrillator, RV: right ventricle, SA: Staphylococcus aureus, MSSA: methicillin-sensitive Staphylococcus aureus, MRSA: methicillin-resistant Staphylococcus aureus, TV: tricuspid valve, TR: tricuspid regurgitation.
3. Conclusion
Our case describes a novel modality of treatment in RSIE. Although currently approved for removal of intravascular thrombi and emboli, the AngioVac device may be a promising noninvasive treatment option for RSIE for a multitude of indications. The novel modality may be used as a substitute for surgery where surgery is indicated but not possible due to associated perioperative risk. The device may also be utilized as a bridge to surgery and percutaneous CIED removal by reducing the perioperative risk through hastening clearance of infection, improving hemodynamics, and reducing the risk of periprocedural septic pulmonary embolism. Further research is needed in larger RSIE patient populations to confirm the benefits and the potential of improved outcomes associated with the AngioVac device as well as identify its potential complications.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Moss R., Munt B. Injection drug use and right sided endocarditis. Heart. 2003;89(5):577–581. doi: 10.1136/heart.89.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weisse A. B., Heller D. R., Schimenti R. J., Montgomery R. L., Kapila R. The febrile parenteral drug user: a prospective study in 121 patients. American Journal of Medicine. 1993;94(3):274–280. doi: 10.1016/0002-9343(93)90059-x. [DOI] [PubMed] [Google Scholar]
- 3.Crane L. R., Levine D. P., Zervos M. J., Cummings G. Bacteremia in narcotic addicts at the Detroit Medical Center. I. Microbiology, epidemiology, risk factors, and empiric therapy. Reviews of Infectious Diseases. 1986;8(3):364–373. doi: 10.1093/clinids/8.3.364. [DOI] [PubMed] [Google Scholar]
- 4.Cahill T. J., Prendergast B. D. Infective endocarditis. Lancet. 2016;387(10021):882–893. doi: 10.1016/S0140-6736(15)00067-7. [DOI] [PubMed] [Google Scholar]
- 5.DiNubile M. J. Short-course antibiotic therapy for right-sided endocarditis caused by Staphylococcus aureus in injection drug users. Annals of Internal Medicine. 1994;121(11):873–876. doi: 10.7326/0003-4819-121-11-199412010-00009. [DOI] [PubMed] [Google Scholar]
- 6.Baddour L. M., Wilson W. R., Bayer A. S., et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 7.Divekar A. A., Scholz T., Fernandez J. D. Novel percutaneous transcatheter intervention for refractory active endocarditis as a bridge to surgery-AngioVac aspiration system. Catheterization and Cardiovascular Interventions. 2013;81(6):1008–1012. doi: 10.1002/ccd.24593. [DOI] [PubMed] [Google Scholar]
- 8.Worku B., Salemi A., D’Ayala M. D., Tranbaugh R. F., Girardi L. N., Gulkarov I. M. The AngioVac device: understanding the failures on the road to success. Innovations. 2016;11(6):430–433. doi: 10.1097/IMI.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 9.Resnick S. A., O’Brien D., Strain D., et al. Single-center experience using AngioVac with extracorporeal bypass for mechanical thrombectomy of atrial and central vein thrombi. Journal of Vascular and Interventional Radiology. 2016;27(5):723–729.e1. doi: 10.1016/j.jvir.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Schaerf R. H. M., Najibi S., Conrad J. Percutaneous vacuum-assisted thrombectomy device used for removal of large vegetations on infected pacemaker and defibrillator leads as an adjunct to lead extraction. Journal of Atrial Fibrillation. 2016;9(3):p. 1455. doi: 10.4022/jafib.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel N., Azemi T., Zaeem F., et al. Vacuum assisted vegetation extraction for the management of large lead vegetations. Journal of Cardiac Surgery. 2013;28(3):321–324. doi: 10.1111/jocs.12087. [DOI] [PubMed] [Google Scholar]
- 12.Dalia A. A., Bamira D., Albaghdadi M., Essandoh M., Rosenfield K., Dudzinski D. Four-dimensional transesophageal echocardiography-guided AngioVac debulking of a tricuspid valve vegetation. Journal of Cardiothoracic and Vascular Anesthesia. 2016;31(5):1713–1716. doi: 10.1053/j.jvca.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 13.Grammes J. A., Schulze C. M., Al-Bataineh M., et al. Percutaneous pacemaker and implantable cardioverter-defibrillator lead extraction in 100 patients with intracardiac vegetations defined by transesophageal echocardiogram. Journal of the American College of Cardiology. 2010;55(9):886–894. doi: 10.1016/j.jacc.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 14.Klug D., Lacroix D., Savoye C., et al. Systemic infection related to endocarditis on pacemaker leads: clinical presentation and management. Circulation. 1997;95(8):2098–2107. doi: 10.1161/01.cir.95.8.2098. [DOI] [PubMed] [Google Scholar]
- 15.Meier-Ewert H. K., Gray M.-E., John R. M. Endocardial pacemaker or defibrillator leads with infected vegetations: a single-center experience and consequences of transvenous extraction. American Heart Journal. 2003;146(2):339–344. doi: 10.1016/S0002-8703(03)00188-1. [DOI] [PubMed] [Google Scholar]
- 16.Makdisi G., Casciani T., Wozniak T. C., Roe D. W., Hashmi Z. A. A successful percutaneous mechanical vegetation debulking used as a bridge to surgery in acute tricuspid valve endocarditis. Journal of Thoracic Disease. 2016;8(1):E137–E139. doi: 10.3978/j.issn.2072-1439.2016.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaPlante K. L., Rybak M. J. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrobial Agents and Chemotherapy. 2004;48(12):4665–4672. doi: 10.1128/aac.48.12.4665-4672.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose W. E., Leonard S. N., Rossi K. L., Kaatz G. W., Rybak M. J. Impact of inoculum size and heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) on vancomycin activity and emergence of VISA in an in vitro pharmacodynamic model. Antimicrobial Agents and Chemotherapy. 2009;53(2):805–807. doi: 10.1128/aac.01009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Symbas P. N., Vlasis S. E., Zacharopoulos L., Hatcher C. R., Jr., Arensberg D. Immediate and long-term outlook for valve replacement in acute bacterial endocarditis. Annals of Surgery. 1982;195(6):721–725. doi: 10.1097/00000658-198206000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todoran T. M., Sobieszczyk P. S., Levy M. S., et al. Percutaneous extraction of right atrial mass using the AngioVac aspiration system. Journal of Vascular and Interventional Radiology. 2011;22(9):1345–1347. doi: 10.1016/j.jvir.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Jones B. M., Wazni O., Rehm S. J., Shishehbor M. H. Fighting fungus with a laser and a hose: Management of a giant Candida albicans implantable cardioverter-defibrillator lead vegetation with simultaneous AngioVac aspiration and laser sheath lead extraction. Catheterization and Cardiovascular Interventions. 2017:1–4. doi: 10.1002/ccd.27153. [DOI] [PubMed] [Google Scholar]
- 22.Thiagaraj A. K., Malviya M., Htun W. W., et al. A novel approach in the management of right-sided endocarditis: percutaneous vegectomy using the AngioVac cannula. Future Cardiology. 2017;13(3):211–217. doi: 10.2217/fca-2016-0076. [DOI] [PubMed] [Google Scholar]
- 23.George B., Voelkel A., Kotter J., Leventhal A., Gurley J. A novel approach to percutaneous removal of large tricuspid valve vegetations using suction filtration and veno-venous bypass: a single center experience. Catheterization and Cardiovascular Interventions. 2017 doi: 10.1002/ccd.27097. [DOI] [PubMed] [Google Scholar]
- 24.Hosoba S., Mori M., Furtado A. D., Lattouf O. M. Extraction of right-sided vegetation with use of an aspiration catheter system. Innovations. 2015;10(5):357–359. doi: 10.1097/imi.0000000000000189. [DOI] [PubMed] [Google Scholar]


