Abstract
Introduction
Medication overuse headache (MOH) is a clinical concern in the management of migraine headache. MOH arises from the frequent use of medications used for the treatment of a primary headache. Medications that can cause MOH include opioid analgesics as well as formulations designed for the treatment of migraine, such as triptans, ergot alkaloids, or drug combinations that include caffeine and barbiturates.
Literature review
Gathering evidence indicates that migraine patients are more susceptible to development of MOH, and that prolonged use of these medications increases the prognosis for development of chronic migraine, leading to the suggestion that similar underlying mechanisms may drive both migraine headache and MOH. In this review, we examine the link between several mechanisms that have been linked to migraine headache and a potential role in MOH. For example, cortical spreading depression (CSD), associated with migraine development, is increased in frequency with prolonged use of topiramate or paracetamol.
Conclusions
Increased CGRP levels in the blood have been linked to migraine and elevated CGRP can be casued by prolonged sumatriptan exposure. Possible mechanisms that may be common to both migraine and MOH include increased endogenous facilitation of pain and/or diminished diminished endogenous pain inhibition. Neuroanatomical pathways mediating these effects are examined.
Keywords: Medication overuse headache, allodynia, pain, headache, opioids, triptans, sensitization
Introduction
Medication overuse headache (MOH) (previously referred to as rebound headache, drug-induced headache or drug-misuse headache) is a secondary cause of chronic daily headache (≥15 headache days per month) that occurs in patients with a primary headache disorder who overuse acute medications (1). The prevalence of MOH in the general population worldwide is estimated to be at least 1% in adults and 0.5% in adolescents (2–4). Approximately one-third of individuals with chronic daily headache in the general population meet the criteria for medication overuse (MO) (2,5–7); the rates are higher in specialty and tertiary care centers (8,9). Data from a physician survey that included neurologists, headache specialists and general practitioners suggested that MOH may be the third most frequent type of headache after migraine and tension-type headaches (10).
Most patients with MOH in the population and clinical practice have migraine. While migraine is a complex paroxysmal neurological disorder with multiple symptoms, headache pain is the most disabling feature. More than 95% of migraine sufferers regularly use acute medications which include analgesics, migraine-specific medications (triptans, ergot medications), opioids, or a combination thereof. Emerging evidence suggests that the choice of acute treatment and the frequency of use have a major influence on the prognosis of migraine (11,12). Clinic-based and case-control studies evaluating the influence of acute medication use in migraine and non-migraine sufferers have suggested that individuals with migraine are uniquely vulnerable to the development of chronic daily headache (chronic migraine) and that certain acute medications, particularly opioids, barbiturate-containing combination analgesics, as well as triptans and ergots, potentiate this risk of progression (13–18).
There is considerable variability in the propensity and dose-relationship with which certain acute medications can lead to migraine progression. The speed with which certain medications result in the progression of migraine and the durability of this transformation also appears to differ between acute medications. The frequent use of triptans has been shown to result in migraine progression with fewer doses compared with ergots and analgesics (19). In a prospective German study (n = 96), the interval between first intake and daily headache was 1.7 years for triptans, 2.7 years for ergots, and 4.8 years for analgesics (19). Moreover, the phenotype of the daily headache differed based on the acute medication being used; patients with triptan-induced migraine progression were more likely to describe a daily migraine-like headache or an increase in migraine frequency. This suggests that the biology of migraine headache, and the biology of migraine progression caused by the overuse of triptans, is probably similar. In a prospective study of 95 patients, the same authors investigated the duration and severity of withdrawal headache after overuse of various headache drugs, including single and combination analgesics, ergots and triptans (20). The duration of withdrawal headache was less severe and shorter in patients overusing triptans (4.1 days) than in patients overusing ergots (6.7 days) or analgesics (9.5 days; p < 0.002). This study demonstrated that just as the time interval to the development of daily headache differs depending on the acute medication used, so too does the duration and severity of withdrawal headache and other symptoms upon discontinuation of the medication.
As migraineurs are most susceptible to development of MOH, some common neural mechanisms between migraine and MOH might be expected. A review of migraine mechanisms and neuroplastic changes that occur with chronic medication exposure reveals considerable overlap between the two. In the migraineur, the trigeminal system is likely to be in a state of hyperexcitability. Preclinically, chronic exposure to analgesics (e.g. triptans, opioids) has been shown to induce an up-regulation of pronociceptive systems. Possible up-regulation of pronociceptive mechanisms, when paired with trigeminal hyperexcitability that probably occurs in the migraineur, may thus exacerbate and help to trigger migraine headache.
The following sections highlight the results of some recent studies that demonstrate a profound influence of prolonged exposure to migraine medications on neural events that have been hypothesized to play a role in triggering migraine headache. These events include cortical spreading depression, intracranial neurogenic inflammation, central sensitization and activation of brain pathways involved in the descending modulation of pain. The impact of prolonged exposure to migraine medications on each of these processes might thus exacerbate and help to trigger migraine headache, leading to the development of MOH.
Cortical spreading depression
While the pathophysiology of migraine is unknown, it is widely acknowledged that activation of trigeminal primary afferent neurons that innervate the intracranial blood vessels and dura is likely to be responsible for producing the headache pain. As evidence, stimulation of the large blood vessels and sinus in humans produces pain qualitatively similar to headache (21,22). How these neurons are activated during migraine or in response to headache triggers leading to migraine remains uncertain. Among the possible initiators of migraine headache pain are cortical spreading depression (CSD), vasodilation and neurogenic inflammation around the intracranial blood vessels.
Spreading depression is a transient depression of electrocorticographic waves that propagates at a rate of 3–6 mm/min following cortical depolarization (21). Recent studies suggest that CSD may have a causal relationship to migraine with aura, but not for migraine in the absence of aura (23). Tonabersat, a compound whose site and mechanism of action is still unknown, is a potent inhibitor of CSD events and was evaluated for the management of migraine (23). A placebo-controlled, double-blind crossover study revealed that tonabersat significantly reduced the incidence of migraine with aura but had no effect on development of headache without aura (23).
While CSD has been strongly linked to migraine aura, establishing a link between CSD and pain during migraine has remained a challenge. The expression of the c-fos protein in neurons is generally taken as an indicator of neuronal excitation. CSD appears to induce c-fos immunoreactivity in trigeminal nucleus caudalis in animal models, suggesting a link between spreading depression and activation of trigeminal afferents. Furthermore, sustained prophylactic treatments for migraine, such as topiramate and valproate, were shown to reduce the frequency of CSD events (24). In a potential connection with MOH, 30-day exposure to the analgesic paracetamol has recently been shown to increase the frequency of CSD events induced by application of potassium chloride to the cortex (25). Furthermore, chronic paracetamol administration increased CSD-evoked c-fos expression in superficial layers of the trigeminal nucleus caudalis, suggesting an increase in the activation of the nociceptive pathway involved in headache. CSDs initiated by KCl applied to the visual cortex enhanced neuronal responses of the trigeminal complex in response to meningeal stimulation (26). In other studies, CSDs elicited by KCl or pinprick applied to the visual cortex, or by electrical stimulation of the visual cortex, produced a doubling of meningeal nociceptor firing, indicating that CSDs can elicit a long-lasting (i.e. 30 to >68 min) activation of meningeal nociceptors (27).
Peripheral events
Vasodilation was once thought to play a significant role in migraine headache pain. It was believed that migraine occurred after an initial vasoconstriction and ischemia that was then followed by a rebound vasodilation that would activate trigeminal nerves innervating the dural and meningeal blood vessels (28). This theory was attractive because specific and effective anti-migraine medications such as triptans and ergotamine possess vasoconstrictive properties. However, intravenous infusion of vasoactive intestinal peptide (VIP), a potent vasodilator, does not cause headache in migraineurs (29). Moreover, brain imaging studies show no relationship between migraine attacks and cerebral blood flow, leading to the conclusion that “vasodilation is neither necessary nor sufficient to trigger migraine” (28). Although these recent findings call into question the necessity of vasodilation to the induction of migraine, evidence indicates a prominent role for two potent vasodilators, nitric oxide and calcitonin gene-related peptide (CGRP), in migraine.
A role for nitric oxide in the pathogenesis of migraine is suggested by observations that exposure to nitroglycerin produces an immediate headache in normal individuals as well as migraineurs and produces a secondary headache hours after exposure that is described as identical to a migraine attack in migraineurs (30). Furthermore, nitroglycerin-induced headache is more intense in individuals with migraine or tension-type headaches than in normal volunteers (30). Interestingly, although nitric oxide causes vasodilation of intracranial and extracranial arteries, the secondary migraine-like headache induced by sildenafil (31) or nitroglycerin (32) occurs in the absence of vasodilation. Recent clinical studies showed that the inhibitor of inducible nitric oxide synthase (iNOS) GW274150 failed in the prophylaxis (33) and acute (34) treatment of migraine. However, a recent preliminary study presented in abstract form suggested that selective inhibitors of neuronal NOS (nNOS) may show promise in the treatment of migraine (35). Based on these data showing a potential role for nNOS rather than iNOS, it is likely that the role of nitric oxide in the pathogenesis of migraine is related to its ability to promote nociceptive processing in the trigeminal system (30).
Although the mechanisms are not well established, CGRP is released from primary afferent neurons that innervate the intracranial blood vessels and plays a prominent role in the initiation of migraine headache. Administration of CGRP produces a migraine-like headache in humans (36). Increases in CGRP have been measured in the cranial circulation during migraine attacks (37). However, this remains controversial as others have also reported no release of CGRP during the onset of migraine with aura (38). Importantly, CGRP antagonists have been demonstrated to be clinically effective in treating migraine (39,40).
A potential connection between nitric oxide and CGRP has been found in both migraine and cluster headache. Nitroglycerin provoked headache in patients with cluster headaches only during the active period, and not during the headache-free remission period (41). In addition, basal blood levels of CGRP were elevated during the active period in patients with cluster headaches, and nitroglycerin further elevated blood CGRP levels coincident with the appearance of the evoked headache (42). These results suggest that nitric oxide activates an already sensitized trigeminal system to provoke headache and increase CGRP release. This is further demonstrated by the recent observation that intravenous infusion of nitroglycerin over a 20-minute period to healthy volunteers did not increase jugular levels of CGRP during the period of a mild immediate headache (43). Increased blood levels of CGRP have been observed during the late-phase migraine-like headache, but not during the immediate headache after nitroglycerin infusion (44).
Prolonged exposure to analgesics may lead to MOH through the up-regulation of neural regulators of vasodilation and neurogenic inflammation. It has been known for some time that sustained systemic delivery of morphine exposure increases CGRP content in dorsal root ganglion neurons (45–47). More recently, studies have shown that the number of dural afferent neurons that express CGRP and/or nNOS increased following morphine exposure (48). Prolonged morphine exposure also produced an increase in the expression of the transient receptor potential channel, TRPV1, in the dorsal root ganglia and trafficking to the periphery (49). This increase in TRPV1 expression was accompanied by an increase in capsaicin-induced plasma extravasation (49).
Recent studies have begun to examine the effect of prolonged exposure to triptans in initiating peripheral neuroplasticity that may promote headache. Continuous, persistent exposure of rats to triptans for a period of days resulted in a marked increase in the numbers of trigeminal ganglion cell bodies expressing CGRP and a modest increase in expression of substance P (50). The increase in CGRP was especially pronounced when only dural afferent nerves, identified by application of the retrograde tracer fluorogold to the dura, were considered. In addition, CGRP was increased in unmyelinated C-fibers that expressed binding for the isolectin IB4 (presumed to be ‘peptide-deficient’ nociceptors), as well as in myelinated afferents. Additionally, recent data demonstrate a marked increase in numbers of trigeminal ganglion neuronal profiles, and especially labeled dural afferents, that express nNOS after persistent exposure to sumatriptan (51). As triptans and opiates produce MOH in some patients, one possibility is that prolonged exposure to these medications produces long-lasting, apparently pronociceptive, neuroplastic changes in the peripheral nerves of the trigeminal system, that may lower the threshold to stimuli (i.e. ‘migraine triggers’) capable of precipitating migraine in the migraineur. For example, animals exposed to sumatriptan, but not saline, for 6 days and allowed to recover so that behavioral responses were returned to baseline levels showed sensitivity to subsequent exposure to environmental stress or to an NO donor (51). In addition to these potential peripheral mechanisms, several observations point to a potential contribution of centrally-mediated events in the onset of migraine and the development of MOH.
Trigeminal nucleus hyperexcitability
Increased excitability of the trigeminal nociceptive pathway in migraine sufferers has been demonstrated both during and in between migraine episodes (52–60). The most commonly used clinical correlate of this increased excitability has been the presence of cutaneous allodynia, which occurs in the head and face region during migraine. In the interictal period, increased temporal summation of pain produced by repeated mechanical stimulation (wind-up) suggests a reduced threshold for the induction of central sensitization in migraineurs (59).
Dural inflammation, produced in animal studies by a solution of proinflammatory mediators in high concentrations applied to the dura, has been used to examine the neural events leading to headache pain during migraine (61). In results that parallel the findings of allodynia in humans with migraine, intracranial mechanical hypersensitivity has been reported following dural inflammation in the rat (62–64). Following inflammation, primary afferent neurons become sensitized to mechanical stimulation of the dura, which could account for the throbbing pain associated with headache. In addition, dura sensitive neurons recorded in the trigeminal nucleus, which typically receive convergent input from the skin, become sensitized to both dural and cutaneous stimulation (65–67). Because these animal studies produce behavioral and neurophysiologic results that appear to correlate to some degree with clinical observations of sensitization and allodynia, such studies may lead to further insights into potential causes of MOH (50,52,61,65). Such sensitization is likely to be relevant to the cephalic allodynia observed during migraine headache.
Consistent with evidence for the development of central sensitization, exposure of rats to either morphine or triptans for a period of days results in behavioral responses to tactile stimuli suggestive of cutaneous allodynia, including allodynia of the periorbital region (48,50). In the case of triptans, these behavioral changes resolve over the 14 days following discontinuation of exposure to the drug. The elevation in expression of CGRP and of nNOS in identified dural afferents of the trigeminal ganglion is maintained even after behavioral responses to tactile stimuli have returned to baseline levels (50,51). Such pronociceptive adaptations may underlie a state of ‘latent sensitization’ in which animals previously exposed to triptans become hyper-responsive to presumed triggers of migraine such as environmental stress or challenge with a nitric oxide donor (51). Thus, migraine triggers elicit cutaneous allodynia only in animals previously exposed to triptans. Moreover, the cutaneous allodynia evoked in this animal model can be abolished by CGRP, but not NK-1, receptor antagonists (50), and by nNOS inhibitors (51). These observations mirror the clinical results showing that CGRP, but not NK-1, antagonists or iNOS inhibitors can effectively treat migraine headache in humans (68–70). Challenge of rats previously exposed to triptans with an NO donor also produced significant elevations in plasma levels of CGRP, similar to observations of elevated CGRP blood levels found during spontaneous and NO-precipitated migraine headaches (44,71,72).
Additional central mechanisms may promote increased excitability after chronic medications. An up-regulation of pronociceptive systems at the level of the spinal cord occurs after prolonged exposure to morphine, probably contributing to opiate-induced hyperalgesia and possibly antinociceptive tolerance (66,73–75). In spinal cord tissue, sustained morphine administration increased capsaicin-evoked release of CGRP and substance P, indicating an increase in excitatory neurotransmission (47,76). Additional studies have found a down-regulation of glutamate transporters after chronic morphine (77). Morphine-induced down-regulation of glutamate transporters may cause an increase in glutamatergic transmission and provide a mechanism for studies demonstrating increased activation of excitatory amino acid receptors following chronic administration of opiates. In particular, NMDA receptor activation, which contributes to central sensitization following inflammation, has been shown to also underlie, in part, opioid-induced tolerance and hyperalgesia (78–89). Whether similar changes occur in the trigeminal system is still unknown.
Studies indicate that many of the changes that occur in the spinal cord and trigeminal nucleus following inflammation and chronic drug exposure depend on brainstem neurons involved in the descending modulation of pain. The dysfunction of brainstem pain modulating neurons has also been implicated in migraine headache.
Descending control mechanisms
Throughout the course of a migraine, the region of hypersensitivity can spread from the head and face to encompass extracranial regions as well. The correlation of migraine headache with activation of the neuronal centers and pathways of the central nervous system that contribute to pain processing is not well established. However, an examination of descending pain modulatory pathways may provide some insights into the progression of migraine pain and allodynia to extracranial regions. The ventrolateral periaqueductal gray (PAG) and nucleus cuneiformis modulate pain through projections to the rostral ventromedial medulla (RVM). The RVM, in turn, can either inhibit or facilitate pain transmission through direct projections to the spinal and medullary dorsal horn (90). In a recent study in animals, dural inflammation produced extracranial allodynia that required the activation of pain facilitating ‘on’ cells in the RVM (62). Importantly, inactivation of the RVM prevented the cutaneous allodynia that resulted from dural inflammation (62). In humans, the PAG appears to be activated prominently in migraine and demonstrates significant structural abnormalities in migraine patients (91–95). The function of the nucleus cuneiformis also seems compromised in the interictal period (96), which may explain the reduced threshold required for induction of central sensitization that exists between migraine episodes (59).
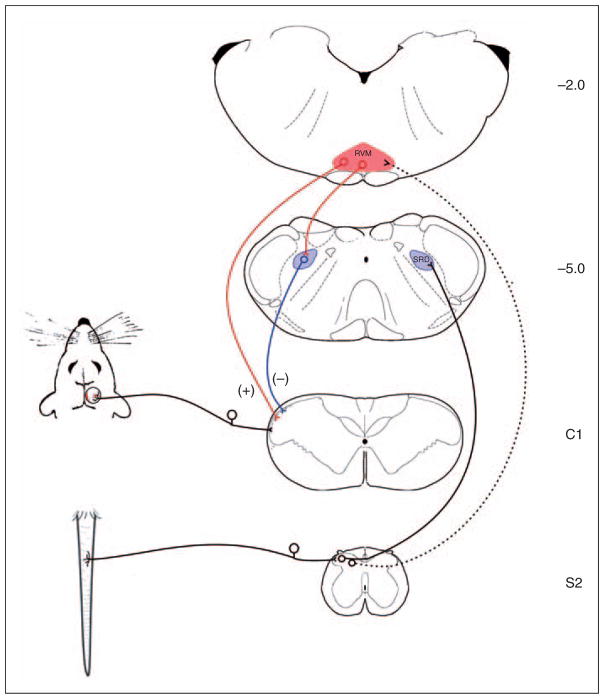
A separate pain modulatory pathway, one involved in producing diffuse noxious inhibitory controls (DNIC), has also been found to be dysfunctional in chronic daily headache patients (97–99). Sometimes referred to as counter-irritation, the effect of DNIC is observed as an inhibition of pain produced by a noxious stimulus applied to a remote part of the body. Chronic daily headache patients demonstrate a profound loss in DNIC, leading some to suggest that a reduction in descending inhibition may be responsible for migraine chronicity. Whether the loss of DNIC in chronic daily headache is related to changes in PAG and nucleus cuneiformis function remains unknown. Studies that have examined DNIC after chronic opiate exposure, however, have provided evidence for an interaction between these two descending modulatory systems (Figure 1).
Figure 1.
Schematic diagram illustrating the interaction of descending projections involved in the inhibition and facilitation of pain. Placement of the rat’s tail in hot water activates dorsal horn nociceptive neurons that project to the subnucleus reticularis dorsalis (SRD). Descending projections from the SRD inhibit nociceptive transmission produced by the activation of dural afferents. In addition, noxious tail stimulation activates pain-facilitating neurons in the rostral ventromedial medulla (RVM), mainly through indirect projections. Evidence indicates a shift in the balance between these two systems following chronic morphine exposure, such that descending facilitation dominates, masking DNIC.
Morphine-induced cutaneous allodynia and increased spinal cord neuronal excitability depends on descending input from the RVM (47). While acute administration of morphine inhibits pain through the activation of pain inhibitory ‘off’ cells in the RVM, it appears that more prolonged exposure to morphine can increase pain by enhancing the influence of pain facilitating ‘on’ cells (100). In addition to increasing descending facilitation from the RVM, chronic opiate exposure also inhibits DNIC. A study of chronic opiate users found that, although baseline pain thresholds remained unchanged, inhibition of pain produced by a remote noxious stimulus was greatly reduced when compared to control subjects (101). This result is consistent with a recent study that examined dura-sensitive neurons in morphine-treated animals (102). In rats exposed to chronic morphine, dura-sensitive neurons recorded from the trigeminal nucleus caudalis were not inhibited by noxious stimulation of the tail. The loss of DNIC in these animals could be re-established by inactivating the RVM, providing evidence that morphine exposure produces an increase in descending facilitation from the RVM that masks inhibition from DNIC. Results from these studies suggest that the transformation of periodic migraine to chronic daily headache, whether due to medication overuse or other factors, may be caused by an increase in descending facilitation from the RVM. The emergence of advanced imaging techniques has led to a growing interest in the examination of the role of these pain modulatory systems in the processing of nociception and pain perception in humans. It seems likely that as our understanding of these pain processing systems grows, the integration of these systems in the development of migraine headache or of MOH will become an important area of clinical research in the development of novel therapeutics and management protocols.
Conclusions
Evidence from observational, prospective clinic-based, case-control, and population-based studies indicates that the frequent use of acute medications to treat migraine headache, in a substantial subgroup of individuals, leads to the progression of migraine from an episodic disorder to a syndrome of daily or near daily migraine. The risk of and time to progression, the phenotype of the daily headache, and the duration of withdrawal symptoms appears to vary based on the acute medication and its frequency of use. These features, together with the unique and selective vulnerability of migraine patients to this phenomena, highlights the possibility that the mechanism(s) by which acute medications lead to this progression may differ depending on the particular drug, and may be similar to the underlying biology of acute attacks of migraine.
Preclinical studies have now provided evidence for several potential mechanisms for the development of MOH, including increases in evoked CSD, a possible role for neurogenic inflammation, peripheral and central sensitization, and descending facilitation. A more thorough examination comparing the effect of different classes of medication on each of these factors should provide further insight into the pathophysiological mechanisms of both MOH and migraine.
Acknowledgments
Funding
This work was supported by the National Institute on Drug Abuse [grant number DA029112].
References
- 1.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. 2nd ed. Cephalalgia. 2004;24(Suppl 1):1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 2.Colas R, Munoz P, Temprano R, et al. Chronic daily headache with analgesic overuse: epidemiology and impact on quality of life. Neurology. 2004;62:1338–1342. doi: 10.1212/01.wnl.0000120545.45443.93. [DOI] [PubMed] [Google Scholar]
- 3.Dyb G, Holmen TL, Zwart JA. Analgesic overuse among adolescents with headache: the Head-HUNT-Youth Study. Neurology. 2006;66:198–201. doi: 10.1212/01.wnl.0000193630.03650.19. [DOI] [PubMed] [Google Scholar]
- 4.Zwart JA, Dyb G, Hagen K, et al. Analgesic overuse among subjects with headache, neck, and low-back pain. Neurology. 2004;62:1540–1544. doi: 10.1212/01.wnl.0000123262.96132.fc. [DOI] [PubMed] [Google Scholar]
- 5.Castillo J, Munoz P, Guitera V, et al. Kaplan Award 1998. Epidemiology of chronic daily headache in the general population. Headache. 1999;39:190–196. doi: 10.1046/j.1526-4610.1999.3903190.x. [DOI] [PubMed] [Google Scholar]
- 6.Chakravarty A. Chronic daily headaches: clinical profile in Indian patients. Cephalalgia. 2003;23:348–353. doi: 10.1046/j.1468-2982.2003.00514.x. [DOI] [PubMed] [Google Scholar]
- 7.Lu SR, Fuh JL, Chen WT, et al. Chronic daily headache in Taipei, Taiwan: prevalence, follow-up and outcome predictors. Cephalalgia. 2001;21:980–986. doi: 10.1046/j.1468-2982.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- 8.Mathew NT, Reuveni U, Perez F. Transformed or evolutive migraine. Headache. 1987;27:102–106. doi: 10.1111/j.1526-4610.1987.hed2702102.x. [DOI] [PubMed] [Google Scholar]
- 9.Meskunas CA, Tepper SJ, Rapoport AM, et al. Medications associated with probable medication overuse headache reported in a tertiary care headache center over a 15-year period. Headache. 2006;46:766–772. doi: 10.1111/j.1526-4610.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 10.Rapoport A, Stang P, Gutterman DL, et al. Analgesic rebound headache in clinical practice: data from a physician survey. Headache. 1996;36:14–19. doi: 10.1046/j.1526-4610.1996.3601014.x. [DOI] [PubMed] [Google Scholar]
- 11.Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71:1821–1828. doi: 10.1212/01.wnl.0000335946.53860.1d. [DOI] [PubMed] [Google Scholar]
- 12.Diamond S, Bigal ME, Silberstein S, et al. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache. 2007;47:355–363. doi: 10.1111/j.1526-4610.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- 13.Bahra A, Walsh M, Menon S, et al. Does chronic daily headache arise de novo in association with regular use of analgesics? Headache. 2003;43:179–190. doi: 10.1046/j.1526-4610.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 14.Bigal ME, Lipton RB. Clinical course in migraine: conceptualizing migraine transformation. Neurology. 2008;71:848–855. doi: 10.1212/01.wnl.0000325565.63526.d2. [DOI] [PubMed] [Google Scholar]
- 15.Paemeleire K, Bahra A, Evers S, et al. Medication-overuse headache in patients with cluster headache. Neurology. 2006;67:109–113. doi: 10.1212/01.wnl.0000223332.35936.6e. [DOI] [PubMed] [Google Scholar]
- 16.Scher AI, Stewart WF, Liberman J, et al. Prevalence of frequent headache in a population sample. Headache. 1998;38:497–506. doi: 10.1046/j.1526-4610.1998.3807497.x. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson SM, Becker WJ, Heine JA. Opiate use to control bowel motility may induce chronic daily headache in patients with migraine. Headache. 2001;41:303–309. doi: 10.1046/j.1526-4610.2001.111006303.x. [DOI] [PubMed] [Google Scholar]
- 18.Williams L, O’Connell K, Tubridy N. Headaches in a rheumatology clinic: when one pain leads to another. Eur J Neurol. 2008;15:274–277. doi: 10.1111/j.1468-1331.2008.02050.x. [DOI] [PubMed] [Google Scholar]
- 19.Limmroth V, Katsarava Z, Fritsche G, et al. Features of medication overuse headache following overuse of different acute headache drugs. Neurology. 2002;59:1011–1014. doi: 10.1212/wnl.59.7.1011. [DOI] [PubMed] [Google Scholar]
- 20.Katsarava Z, Fritsche G, Muessig M, et al. Clinical features of withdrawal headache following overuse of triptans and other headache drugs. Neurology. 2001;57:1694–1698. doi: 10.1212/wnl.57.9.1694. [DOI] [PubMed] [Google Scholar]
- 21.Ayata C. Spreading depression: from serendipity to targeted therapy in migraine prophylaxis. Cephalalgia. 2009;29:1095–1114. doi: 10.1111/j.1468-2982.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 22.Ray BS, Wolff HG. Experimental studies on headache pain sensitive structures of the head and their significance in headache. Arch Surg. 1940;41:813–856. [Google Scholar]
- 23.Hauge AW, Asghar MS, Schytz HW, et al. Effects of tonabersat on migraine with aura: a randomised, double-blind, placebo-controlled crossover study. Lancet Neurol. 2009;8:718–723. doi: 10.1016/S1474-4422(09)70135-8. [DOI] [PubMed] [Google Scholar]
- 24.Ayata C, Moskowitz MA. Cortical spreading depression confounds concentration-dependent pial arteriolar dilation during N-methyl-D-aspartate superfusion. Am J Physiol Heart Circ Physiol. 2006;290:H1837–H1841. doi: 10.1152/ajpheart.01102.2005. [DOI] [PubMed] [Google Scholar]
- 25.Supornsilpchai W, le Grand SM, Srikiatkhachorn A. Cortical hyperexcitability and mechanism of medication-overuse headache. Cephalalgia. 2010;30:1101–1109. doi: 10.1177/0333102409355600. [DOI] [PubMed] [Google Scholar]
- 26.Noseda R, Constandil L, Bourgeais L, et al. Changes of meningeal excitability mediated by corticotrigeminal networks: a link for the endogenous modulation of migraine pain. J Neurosci. 2010;30:14420–14429. doi: 10.1523/JNEUROSCI.3025-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Levy D, Noseda R, et al. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci. 2010;30:8807–8814. doi: 10.1523/JNEUROSCI.0511-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol. 2010;6:573–582. doi: 10.1038/nrneurol.2010.127. [DOI] [PubMed] [Google Scholar]
- 29.Rahmann A, Wienecke T, Hansen JM, et al. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28:226–236. doi: 10.1111/j.1468-2982.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- 30.Olesen J. Nitric oxide-related drug targets in headache. Neurotherapeutics. 2010;7:183–190. doi: 10.1016/j.nurt.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruuse C, Thomsen LL, Birk S, et al. Migraine can be induced by sildenafil without changes in middle cerebral artery diameter. Brain. 2003;126:241–247. doi: 10.1093/brain/awg009. [DOI] [PubMed] [Google Scholar]
- 32.Schoonman GG, van der Grond J, Kortmann C, et al. Migraine headache is not associated with cerebral or meningeal vasodilatation – a 3T magnetic resonance angiography study. Brain. 2008;131:2192–2200. doi: 10.1093/brain/awn094. [DOI] [PubMed] [Google Scholar]
- 33.Hoivik HO, Laurijssens BE, Harnisch LO, et al. Lack of efficacy of the selective iNOS inhibitor GW274150 in prophylaxis of migraine headache. Cephalalgia. 2010;30:1458–1467. doi: 10.1177/0333102410370875. [DOI] [PubMed] [Google Scholar]
- 34.Van der Schueren BJ, Lunnon MW, Laurijssens BE, et al. Does the unfavorable pharmacokinetic and pharmacodynamic profile of the iNOS inhibitor GW273629 lead to inefficacy in acute migraine? J Clin Pharmacol. 2009;49:281–290. doi: 10.1177/0091270008329548. [DOI] [PubMed] [Google Scholar]
- 35.Medve RA, Andrews JS. Effects of fixed dose combination of nNOS inhibition and 5HT agonism on progression of migraine with and without aura. Cephalalgia. 2009;29:126. [Google Scholar]
- 36.Lassen LH, Haderslev PA, Jacobsen VB, et al. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 37.Gallai V, Sarchielli P, Floridi A, et al. Vasoactive peptide levels in the plasma of young migraine patients with and without aura assessed both interictally and ictally. Cephalalgia. 1995;15:384–390. doi: 10.1046/j.1468-2982.1995.1505384.x. [DOI] [PubMed] [Google Scholar]
- 38.Friberg L, Olesen J, Olsen TS, et al. Absence of vasoactive peptide release from brain to cerebral circulation during onset of migraine with aura. Cephalalgia. 1994;14:47–54. doi: 10.1046/j.1468-2982.1994.1401047.x. [DOI] [PubMed] [Google Scholar]
- 39.Doods H, Arndt K, Rudolf K, et al. CGRP antagonists: unravelling the role of CGRP in migraine. Trends Pharmacol Sci. 2007;28:580–587. doi: 10.1016/j.tips.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Olesen J, Diener HC, Husstedt IW, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 41.Olesen J, Thomsen LL, Iversen H. Nitric oxide is a key molecule in migraine and other vascular headaches. Trends Pharmacol Sci. 1994;15:149–153. doi: 10.1016/0165-6147(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 42.Fanciullacci M, Alessandri M, Figini M, et al. Increase in plasma calcitonin gene-related peptide from the extracerebral circulation during nitroglycerin-induced cluster headache attack. Pain. 1995;60:119–123. doi: 10.1016/0304-3959(94)00097-X. [DOI] [PubMed] [Google Scholar]
- 43.Kruuse C, Iversen HK, Jansen-Olesen I, et al. Calcitonin gene-related peptide (CGRP) levels during glyceryl trinitrate (GTN)-induced headache in healthy volunteers. Cephalalgia. 2010;30:467–474. doi: 10.1111/j.1468-2982.2009.01963.x. [DOI] [PubMed] [Google Scholar]
- 44.Juhasz G, Zsombok T, Modos EA, et al. NO-induced migraine attack: strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain. 2003;106:461–470. doi: 10.1016/j.pain.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Belanger S, Ma W, Chabot JG, et al. Expression of calcitonin gene-related peptide, substance P and protein kinase C in cultured dorsal root ganglion neurons following chronic exposure to mu, delta and kappa opiates. Neuroscience. 2002;115:441–453. doi: 10.1016/s0306-4522(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 46.Menard DP, van Rossum D, Kar S, et al. A calcitonin gene-related peptide receptor antagonist prevents the development of tolerance to spinal morphine analgesia. J Neurosci. 1996;16:2342–2351. doi: 10.1523/JNEUROSCI.16-07-02342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardell LR, Wang R, Burgess SE, et al. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci. 2002;22:6747–6755. doi: 10.1523/JNEUROSCI.22-15-06747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Felice M, Porreca F. Opiate-induced persistent pronociceptive trigeminal neural adaptations: potential relevance to opiate-induced medication overuse headache. Cephalalgia. 2009;29:1277–1284. doi: 10.1111/j.1468-2982.2009.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vardanyan A, Wang R, Vanderah TW, et al. TRPV1 receptor in expression of opioid-induced hyperalgesia. J Pain. 2009;10:243–252. doi: 10.1016/j.jpain.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Felice M, Ossipov MH, Wang R, et al. Triptan-induced latent sensitization: a possible basis for medication overuse headache. Ann Neurol. 2010;67:325–337. doi: 10.1002/ana.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Felice M, Ossipov MH, Wang R, et al. Triptan-induced enhancement of neuronal nitric oxide synthase in trigeminal ganglion dural afferents underlies increased responsiveness to potential migraine triggers. Brain. 2010;133:2475–2488. doi: 10.1093/brain/awq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack: clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123(Pt 8):1703–1709. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- 53.de Tommaso M, Valeriani M, Guido M, et al. Abnormal brain processing of cutaneous pain in patients with chronic migraine. Pain. 2003;101:25–32. doi: 10.1016/s0304-3959(02)00299-3. [DOI] [PubMed] [Google Scholar]
- 54.Drummond PD. Scalp tenderness and sensitivity to pain in migraine and tension headache. Headache. 1987;27:45–50. doi: 10.1111/j.1526-4610.1987.hed2701045.x. [DOI] [PubMed] [Google Scholar]
- 55.Katsarava Z, Giffin N, Diener HC, et al. Abnormal habituation of ‘nociceptive’ blink reflex in migraine – evidence for increased excitability of trigeminal nociception. Cephalalgia. 2003;23:814–819. doi: 10.1046/j.1468-2982.2003.00591.x. [DOI] [PubMed] [Google Scholar]
- 56.Katsarava Z, Lehnerdt G, Duda B, et al. Sensitization of trigeminal nociception specific for migraine but not pain of sinusitis. Neurology. 2002;59:1450–1453. doi: 10.1212/wnl.59.9.1450. [DOI] [PubMed] [Google Scholar]
- 57.Kaube H, Katsarava Z, Przywara S, et al. Acute migraine headache: possible sensitization of neurons in the spinal trigeminal nucleus? Neurology. 2002;58:1234–1238. doi: 10.1212/wnl.58.8.1234. [DOI] [PubMed] [Google Scholar]
- 58.Valeriani M, de Tommaso M, Restuccia D, et al. Reduced habituation to experimental pain in migraine patients: a CO(2) laser evoked potential study. Pain. 2003;105:57–64. doi: 10.1016/s0304-3959(03)00137-4. [DOI] [PubMed] [Google Scholar]
- 59.Weissman-Fogel I, Sprecher E, Granovsky Y, et al. Repeated noxious stimulation of the skin enhances cutaneous pain perception of migraine patients in-between attacks: clinical evidence for continuous sub-threshold increase in membrane excitability of central trigeminovascular neurons. Pain. 2003;104:693–700. doi: 10.1016/S0304-3959(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 60.Moulton EA, Becerra L, Maleki N, et al. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine states. Cereb Cortex. 2011;21:435–448. doi: 10.1093/cercor/bhq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain. 2001;89:107–110. doi: 10.1016/s0304-3959(00)00478-4. [DOI] [PubMed] [Google Scholar]
- 62.Edelmayer RM, Vanderah TW, Majuta L, et al. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol. 2009;65:184–193. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007;47:1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wieseler J, Ellis A, Sprunger D, et al. A novel method for modeling facial allodynia associated with migraine in awake and freely moving rats. J Neurosci Methods. 2010;185:236–245. doi: 10.1016/j.jneumeth.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamura H, Malick A, Chamberlin NL, et al. Cardiovascular and neuronal responses to head stimulation reflect central sensitization and cutaneous allodynia in a rat model of migraine. J Neurophysiol. 1999;81:479–493. doi: 10.1152/jn.1999.81.2.479. [DOI] [PubMed] [Google Scholar]
- 66.Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004;55:27–36. doi: 10.1002/ana.10785. [DOI] [PubMed] [Google Scholar]
- 67.Burstein R, Yamamura H, Malick A, et al. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 68.Olesen J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol Ther. 2008;120:157–171. doi: 10.1016/j.pharmthera.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 69.Goldstein DJ, Wang O, Saper JR, et al. Ineffectiveness of neurokinin-1 antagonist in acute migraine: a crossover study. Cephalalgia. 1997;17:785–790. doi: 10.1046/j.1468-2982.1997.1707785.x. [DOI] [PubMed] [Google Scholar]
- 70.Ho TW, Ferrari MD, Dodick DW, et al. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet. 2008;372:2115–2123. doi: 10.1016/S0140-6736(08)61626-8. [DOI] [PubMed] [Google Scholar]
- 71.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 72.Juhasz G, Zsombok T, Jakab B, et al. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia. 2005;25:179–183. doi: 10.1111/j.1468-2982.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- 73.Laulin JP, Celerier E, Larcher A, et al. Opiate tolerance to daily heroin administration: an apparent phenomenon associated with enhanced pain sensitivity. Neuroscience. 1999;89:631–636. doi: 10.1016/s0306-4522(98)00652-6. [DOI] [PubMed] [Google Scholar]
- 74.Laulin JP, Larcher A, Celerier E, et al. Long-lasting increased pain sensitivity in rat following exposure to heroin for the first time. Eur J Neurosci. 1998;10:782–785. doi: 10.1046/j.1460-9568.1998.00083.x. [DOI] [PubMed] [Google Scholar]
- 75.Ossipov MH, Lai J, Vanderah TW, et al. Induction of pain facilitation by sustained opioid exposure: relationship to opioid antinociceptive tolerance. Life Sci. 2003;73:783–800. doi: 10.1016/s0024-3205(03)00410-7. [DOI] [PubMed] [Google Scholar]
- 76.King T, Gardell LR, Wang R, et al. Role of NK-1 neurotransmission in opioid-induced hyperalgesia. Pain. 2005;116:276–288. doi: 10.1016/j.pain.2005.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mao J, Sung B, Ji RR, et al. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ben-Eliyahu S, Marek P, Vaccarino AL, et al. The NMDA receptor antagonist MK-801 prevents long-lasting non-associative morphine tolerance in the rat. Brain Res. 1992;575:304–308. doi: 10.1016/0006-8993(92)90094-p. [DOI] [PubMed] [Google Scholar]
- 79.Bilsky EJ, Inturrisi CE, Sadee W, et al. Competitive and non-competitive NMDA antagonists block the development of antinociceptive tolerance to morphine, but not to selective mu or delta opioid agonists in mice. Pain. 1996;68:229–237. doi: 10.1016/s0304-3959(96)03185-5. [DOI] [PubMed] [Google Scholar]
- 80.Celerier E, Laulin J, Larcher A, et al. Evidence for opiate-activated NMDA processes masking opiate analgesia in rats. Brain Res. 1999;847:18–25. doi: 10.1016/s0006-8993(99)01998-8. [DOI] [PubMed] [Google Scholar]
- 81.Celerier E, Laulin JP, Corcuff JB, et al. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci. 2001;21:4074–4080. doi: 10.1523/JNEUROSCI.21-11-04074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Celerier E, Rivat C, Jun Y, et al. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92:465–472. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- 83.Gutstein HB, Trujillo KA. MK-801 inhibits the development of morphine tolerance at spinal sites. Brain Res. 1993;626:332–334. doi: 10.1016/0006-8993(93)90597-g. [DOI] [PubMed] [Google Scholar]
- 84.Kest B, Mogil JS, Shamgar BE, et al. The NMDA receptor antagonist MK-801 protects against the development of morphine tolerance after intrathecal administration. Proc West Pharmacol Soc. 1993;36:307–310. [PubMed] [Google Scholar]
- 85.Larcher A, Laulin JP, Celerier E, et al. Acute tolerance associated with a single opiate administration: involvement of N-methyl-D-aspartate-dependent pain facilitatory systems. Neuroscience. 1998;84:583–589. doi: 10.1016/s0306-4522(97)00556-3. [DOI] [PubMed] [Google Scholar]
- 86.Manning BH, Mao J, Frenk H, et al. Continuous co-administration of dextromethorphan or MK-801 with morphine: attenuation of morphine dependence and naloxone-reversible attenuation of morphine tolerance. Pain. 1996;67:79–88. doi: 10.1016/0304-3959(96)81972-5. [DOI] [PubMed] [Google Scholar]
- 87.Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci. 1994;14:2301–2312. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marek P, Ben-Eliyahu S, Gold M, et al. Excitatory amino acid antagonists (kynurenic acid and MK-801) attenuate the development of morphine tolerance in the rat. Brain Res. 1991;547:77–81. doi: 10.1016/0006-8993(91)90576-h. [DOI] [PubMed] [Google Scholar]
- 89.Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- 90.Heinricher MM, Tavares I, Leith JL, et al. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Afridi SK, Giffin NJ, Kaube H, et al. A positron emission tomographic study in spontaneous migraine. Arch Neurol. 2005;62:1270–1275. doi: 10.1001/archneur.62.8.1270. [DOI] [PubMed] [Google Scholar]
- 92.Haas DC, Kent PF, Friedman DI. Headache caused by a single lesion of multiple sclerosis in the periaqueductal gray area. Headache. 1993;33:452–455. doi: 10.1111/j.1526-4610.1993.hed3308452.x. [DOI] [PubMed] [Google Scholar]
- 93.Weiller C, May A, Limmroth V, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med. 1995;1:658–660. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- 94.Goadsby PJ. Neurovascular headache and a midbrain vascular malformation: evidence for a role of the brainstem in chronic migraine. Cephalalgia. 2002;22:107–111. doi: 10.1046/j.1468-2982.2002.00323.x. [DOI] [PubMed] [Google Scholar]
- 95.Welch KM, Nagesh V, Aurora SK, et al. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001;41:629–637. doi: 10.1046/j.1526-4610.2001.041007629.x. [DOI] [PubMed] [Google Scholar]
- 96.Moulton EA, Burstein R, Tully S, et al. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One. 2008;3:e3799. doi: 10.1371/journal.pone.0003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cathcart S, Winefield AH, Lushington K, et al. Noxious inhibition of temporal summation is impaired in chronic tension-type headache. Headache. 2010;50:403–412. doi: 10.1111/j.1526-4610.2009.01545.x. [DOI] [PubMed] [Google Scholar]
- 98.de Tommaso M, Sardaro M, Pecoraro C, et al. Effects of the remote C fibres stimulation induced by capsaicin on the blink reflex in chronic migraine. Cephalalgia. 2007;27:881–890. doi: 10.1111/j.1468-2982.2007.01357.x. [DOI] [PubMed] [Google Scholar]
- 99.Pielsticker A, Haag G, Zaudig M, et al. Impairment of pain inhibition in chronic tension-type headache. Pain. 2005;118:215–223. doi: 10.1016/j.pain.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 100.Meng ID, Harasawa I. Chronic morphine exposure increases the proportion of on-cells in the rostral ventromedial medulla in rats. Life Sci. 2007;80:1915–1920. doi: 10.1016/j.lfs.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ram KC, Eisenberg E, Haddad M, et al. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain – new perspective of opioid-induced hyperalgesia. Pain. 2008;139:431–438. doi: 10.1016/j.pain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 102.Okada-Ogawa A, Porreca F, Meng ID. Sustained morphine-induced sensitization and loss of diffuse noxious inhibitory controls in dura-sensitive medullary dorsal horn neurons. J Neurosci. 2009;29:15828–15835. doi: 10.1523/JNEUROSCI.3623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]