Abstract
Background
Oxidative stress plays a role in the pathophysiology of several diseases and has been documented as a contributor to disease in both the human and veterinary literature. One at‐risk cell is the erythrocyte, however, the role of oxidative stress in anemia in dogs has not been widely investigated.
Hypothesis/Objective
Anemic dogs will have an alteration in the activity of glutathione peroxidase (GPx), a decrease in of total antioxidant capacity (TAC), and an increased concentration of urinary 15‐F2‐isoprostanes (F2‐IsoP) when compared to healthy dogs.
Animals
40 client‐owned dogs with anemia (PCV <30%) age‐matched to 40 client‐owned healthy control dogs.
Methods
Prospective, cross‐sectional study. Whole blood GPx activity, plasma TAC, and urinary F2‐isoprostane concentrations were evaluated in each dog and compared between groups.
Results
Anemic dogs had significantly lower GPx activity (43.1 × 103 +/‐ 1.6 × 103 U/L) than did dogs in the control group (75.8 × 103 +/‐ 2.0 × 103 U/L; P < 0.0001). The GPx activity in dogs with hemolysis (103 +/‐ 0.8 × 103 U/L) was not significantly different (P = 0.57) than in dogs with nonhemolytic anemia (43.5 × 103 +/‐ 1.1 × 103 U/L). The TAC concentrations (P = 0.15) and urinary F2‐isoprostanes (P = 0.73) did not significantly differ between groups.
Conclusions and Clinical Importance
Glutathione peroxidase activity was significantly decreased in anemic dogs indicating oxidative stress. Additional studies are warranted to determine if antioxidant supplementation would improve survival and overall outcome as part of a therapeutic regimen for anemic dogs.
Keywords: Anemia, Antioxidants, Canine, Glutathione
Abbreviations
- ALT
alanine aminotransferase
- BUN
blood urea nitrogen
- F2‐IsoP
F2‐isoprostanes
- GPx
glutathione peroxidase
- GSH
glutathione
- GSSG
oxidized glutathione
- GSSG‐R
reduced glutathione
- HCT
hematocrit
- MDA
malondialdehyde
- NEAC
nonenzymatic antioxidant capacity
- NO
nitric oxide
- ROS
reactive oxygen species
- TAC
total antioxidant capacity
- TS
total solids
Oxidative stress results from a variety of inflamma tory and metabolic conditions and has been well documented as a contributor to disease in both the human and veterinary literature.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 Oxidative stress is characterized by the production of reactive oxygen species (ROS) that cause cellular injury through direct and indirect mechanisms. Erythrocytes especially are at risk, because of their ubiquity, fragile phospholipid membrane, oxygen and iron content, and unique redox metabolic processes.13, 14, 15 Clinically, oxidative damage to the erythrocyte is recognized through red blood cell morphologic changes (ie., Heinz bodies) noted on a blood smear and most commonly seen with certain toxin exposures or metabolic diseases.16 Other markers of red blood cell or systemic oxidative stress in anemic states have not been widely investigated, especially in dogs.
In anemia, increased oxygen demand occurs in peripheral tissues.13, 16 This increased demand can lead to increased production of pro‐oxidant compounds (ie., ROS) and depletion of antioxidants.13, 14, 15, 16 In people, oxidative stress has been documented in anemic states. Oxidative stress in humans with anemia is multifactorial, with comorbid diseases and the anemia itself contributing. The erythrocyte relies on antioxidant defenses, primarily the glutathione pathway and small molecule antioxidants (ie., selenium), to protect itself from injury.13, 16 Depletion of these antioxidants can lead to disruption of the glutathione pathway with generation of excessive ROS, which has been implicated in forms of hemolytic anemia in humans.17, 18, 19, 20, 21 Oxidative stress in anemic dogs evaluated by plasma malondialdehyde concentration (MDA, a marker of lipid peroxidation and oxidative stress) was found to be significantly higher in dogs with immune‐mediated hemolytic anemia compared with control dogs.22 However, it is unknown whether this finding can be attributed to the anemia, hemolysis, or both.
Oxidative stress is assessed primarily by the activity of antioxidant enzymes, the concentrations of endogenous antioxidants, and the presence of byproducts of oxidative damage. Measurement of specific antioxidants in blood or tissues can be inconsistent, and species variation is substantial. Therefore, markers of global antioxidant capacity (total antioxidant capacity, TAC), activity of antioxidant enzymes, and byproducts of oxidative damage often are used as more accurate assessments of oxidative stress in vivo.15
Common antioxidant enzymes include superoxide dismutase, catalase, and glutathione peroxidase. Glutathione peroxidase (GPx) plays an important role in protecting hemoglobin, red blood cell enzyme activity, and biological cell membranes against oxidative damage by increasing the concentration of reduced glutathione (GSSG‐R) in the process of aerobic glycolysis.14 Glutathione peroxidase is present in blood in 2 forms: erythrocyte‐derived and plasma. Few veterinary studies of GPx exist, and results have been variable. Erythrocyte‐derived GPx was found to be increased in dogs with dilated cardiomyopathy and decreased in dogs with leishmaniasis.6, 23 Anemic dogs with chronic kidney disease (CKD) were found to have normal erythrocyte‐derived GPx activity.10 Whole blood GPx activity is consistently decreased in people with anemia and increases the risk of early senescence, hemolysis, or both.14, 17, 18, 19, 20, 21 Therefore, investigation of whole blood GPx activity is warranted for anemic dogs.
Measurement of isoprostanes is a new and promising indicator of oxidative stress, specifically lipid peroxidation.24 Urine isoprostanes represent a unique series of prostaglandin‐like compounds that are produced by ROS‐catalyzed peroxidation of arachidonic acid.24 Increased concentrations of isoprostanes in tissue and urine correlate with disease severity in humans as well as in veterinary patients.7, 24, 25, 26, 27 Total antioxidant capacity measures overall antioxidant status, representing the action of endogenous antioxidants, proteins, and trace elements, but excluding antioxidant enzyme activity.28
The purpose of our study was to determine if oxidative stress is present in anemic dogs, as assessed by measuring markers of oxidative damage. We hypothesized that anemic dogs would have an alteration in the activity of whole blood GPx, a decrease in TAC, and an increased concentration of urinary F2‐isoprostanes when compared to healthy dogs. Documentation of oxidative stress in anemic states is appealing as it offers a novel therapeutic target and could justify antioxidant supplementation. However, further investigation of oxidative stress in anemic dogs is necessary before concluding that ROS play an important role in any specific disease process.
Materials and Methods
Study Design and Populations
This prospective, cross‐sectional study was performed at the Purdue University Veterinary Teaching Hospital. All skeletally mature dogs presented between October 1, 2015 and September 30, 2016 were eligible for enrollment into the study population. Dogs were included in the study if they were anemic defined as a packed cell volume (PCV) or hematocrit (HCT) <30%. Dogs were excluded from the study if they were <1 year of age or weighed <4 kg, because of concerns for blood collection in an anemic state. Dogs were excluded if they had received a transfusion of any blood product within 7 days of presentation or if they had received any corticosteroids systemically within 3 days of presentation. Other concurrent medications that prompted exclusion from the study included adjunctive immunosuppressive medications, chemotherapeutic agents, and supplemental antioxidants (including Denamarin,1 SAMe, milk thistle, omega 3 fatty acids, and vitamin E).
For dogs included in the study population, a detailed history was collected and physical examination performed. All dogs had a CBC including PCV and total solids (TS) and a serum biochemistry profile (based on clinician discretion) performed on the day of presentation. Study samples were collected 1 time, on the day anemia was identified. Based on clinical examination, laboratory findings, and the results of the diagnostic investigation performed at the clinicians' discretion, anemic dogs were subdivided into 2 groups: “hemolytic anemia” and “anemia due to nonhemolytic causes.” If no toxic cause for hemolysis was identified in the history or diagnostic investigation (eg., heavy metal, onion, and garlic), the anemia was defined as hemolytic if at least 1 of the following concurrent findings was identified: spherocytosis, auto‐agglutination, or a positive Coomb's test. The nonhemolytic anemia cases were further classified into those with neoplasia and then any non‐neoplastic, nonhemolytic causes were termed “other.”
A control population of skeletally mature, clinically healthy dogs was recruited from the employees, students, and staff of the veterinary teaching hospital and age‐matched to the study population +/‐ 1 year. Dogs were included if they met the age and weight criteria described above and if they were clinically healthy and not receiving any of the medications listed previously. Health status in these dogs was determined by a complete physical examination and assessment of a PCV and TS by refractometer, serum alanine aminotransferase activity (ALT), and assessment of blood urea nitrogen (BUN) using a drop of whole blood applied to a colorimetric reagent strip (Azostix).2
Client consent was obtained for clinically healthy and anemic dogs. In addition, the study protocol was reviewed, approved, and conducted in accordance with the Purdue University Animal Care and Use Committee.3
Sample Collection and Analysis
Blood samples were obtained by jugular venipuncture. Blood was collected into a lithium heparin tube.4 Once anticoagulated, 500 μL of heparinized whole blood was removed by micropipette and transferred into a cryotube and stored at ‐80°C until GPx analysis. The remaining heparinized blood was centrifuged (1,500 × g at 4°C for 15 minutes) and the plasma harvested and stored in cryotubes at ‐80°C until TAC analysis. Dogs in the control population also had blood collected into a serum tube.4 This blood was centrifuged (1,000 × g at 4°C for 15 minutes), and serum was harvested and submitted for determination of ALT activity on a commercial chemistry analyzer.5 A minimum of 0.5 mL of voided urine was collected from all dogs within 8 hours of blood collection and stored at ‐80°C until F2‐IsoP analysis. All samples were frozen within 1 hour of collection and shipped on dry ice. All samples were analyzed within 6 months of collection based on stability.
Determination of Whole Blood Glutathione Peroxidase Activity
Activity of GPx was measured using a commercial Ransel kit6 with a chemistry analyzer7 according to a previously described method.29 The GPx assay was performed by the Clinical and Translational Research Center Core Laboratory at the University of Colorado‐Denver, Anschutz Medical Campus (Denver, CO). This assay was previously validated in dogs.30 Glutathione peroxidase activity was determined indirectly by measuring the rate of formation of oxidized glutathione (GSSG). The rate of oxidation is measured spectrophotometrically as reduced absorbance at 340 nm and is proportional to the activity of GPx in the specimen. Activity of GPx is expressed as units/L (U/L).
Determination of Plasma Total Antioxidant Capacity
Total antioxidant capacity was determined spectrophotometrically (600 nm) using the commercially available TAS kit6 and a chemistry analyzer.7 The TAC assay was performed by the Clinical and Translational Research Center Core Laboratory at the University of Colorado‐Denver, Anschutz Medical Campus (Denver, CO). This assay was previously validated in dogs.31 The assay is based on the reduction in free radicals (ABTS, ‐2,2′‐azinobis‐(3‐ethylbenzothiazoline‐6‐sulfonate)) measured as a decrease in absorbance at 600 nm at 3 minutes by antioxidants. The results are expressed as mmol/L of Trolox equivalents. This reduction in free radical production is a representation of the TAC in the sample namely the activity of small molecule antioxidants and proteins.
Determination of Urinary 15‐F2‐Isoprostanes
Gas chromatography and negative ion chemical ionization‐mass spectrometry (GC/NICI‐MS) were utilized for measurement of urinary 15‐F2‐isoprostanes. The GC/NICI‐MS assay was performed by the Eicosanoid Core Laboratory in the Division of Clinical Pharmacology at Vanderbilt University Medical Center (Nashville, TN). Their methodology has been published previously and validated in dogs.32, 33 The GC/NICI‐MS approach is performed using stable isotope dilution. Urine creatinine concentration for normalization of isoprostane concentration was measured by the Jaffe reaction with a commercial chemistry analyzer8 at the Vanderbilt University Medical Center.
Statistical Analysis
Statistical power analysis was performed for sample size estimation, based on data from a previous study showing that the minimum clinically significant difference of GPx is 5.93 U/mL and that the pooled standard deviation is 6.28 U/mL.9 A sample size of 24 dogs would be needed in each of the control and anemia groups (α level = 0.05, power 90%) to detect a statistically significant difference in mean GPx activity between the 2 groups.
Records from the hematology analyzer9 at the Purdue University Veterinary Clinical Pathology Laboratory were searched for any sample from dogs with a HCT <30% identified in 1 year. An average of 39 anemic canine blood samples was analyzed per month. Therefore, a total of 40 anemic cases were recruited to represent a typical month, as well as to fulfill the needs of the power analysis.
Data were analyzed by commercial software.10 Normality was assessed by the Shapiro‐Wilk test. Values of selected variables (whole blood GPx activity, plasma TAC, and urinary 15‐F2‐isoprostanes) were compared between groups of dogs (anemic and healthy dogs; hemolytic anemia, nonhemolytic anemia [neoplasia and other], and healthy dogs). An unpaired t‐test was used for comparison of 2 groups, and 1‐way ANOVA for 3 groups, if the data were normally distributed (PCV and GPx). The Wilcoxon ranked sum test and Kruskal‐Wallis test by ranks were used for comparison of 2 and 3 groups, respectively, if the data were not normally distributed (TAC and urinary F2‐isoprostanes). Correlation was assessed by calculation of the Pearson product moment correlation coefficient (r). A value of P < 0.05 was considered significant.
Results
Forty dogs with anemia and 40 healthy control dogs were enrolled in the study. Sixty‐one dogs were evaluated for the control group, but 21 dogs were excluded because of laboratory results outside of the reference range (BUN, ALT, or both). The demographic information for anemic and control dogs is summarized in Table 1. Breeds that were represented more than once in the control group included mixed breed (18), Labrador retriever (4), Australian shepherd (3), Beagle (2), Boxer (2), Cocker spaniel (2), and Golden retriever (2). For the dogs with hemolytic and neoplastic causes of anemia, only the mixed breed dog was represented more than once (hemolysis, n = 2; neoplasia, n = 5). For the anemic dogs with other causes, breeds represented more than once included mixed breed (5), Pug (3), Australian Shepherd (2), Miniature schnauzer (2), and Shetland sheepdog (2). There was no significant difference in age (P = 0.89) or weight (P = 0.42) between the anemic dogs and healthy control dogs.
Table 1.
Demographic information for anemic dogs compared to healthy control dogs. Values represent the mean and standard deviation
| Age (years) | Sex | Body Weight | ||||
|---|---|---|---|---|---|---|
| F | FS | M | MC | (kg) | ||
| Control Dogs (N = 40) | 8.52 ± 3.51 | 3 | 21 | 1 | 15 | 22.44 ± 10.63 |
| Anemic Dogs (N = 40) | 8.62 ± 3.72 | 1 | 15 | 5 | 19 | 20.36 ± 12.08 |
| Hemolytic (N = 8) | 9.71 ± 3.04 | 0 | 4 | 1 | 3 | 19.54 ± 13.92 |
| Neoplastic (N = 9) | 9.28 ± 2.28 | 0 | 2 | 2 | 5 | 26.84 ± 13.05 |
| Other (N = 23) | 7.99 ± 4.33 | 1 | 9 | 2 | 11 | 18.10 ± 10.60 |
Eight anemic dogs met the criteria for hemolytic anemia (auto‐agglutination, n = 7; spherocytosis, n = 6; both auto‐agglutination and spherocytosis, n = 5). A total of 32 anemic dogs was classified as nonhemolytic. The classification of anemic dogs by cause is depicted in Figure 1.
Figure 1.
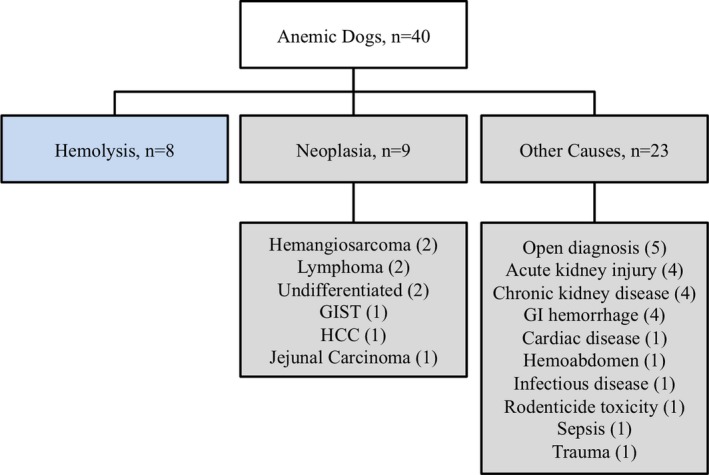
Flowchart depicting the categorization of 40 anemic dogs based on cause. GI, gastrointestinal; GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma.
The mean PCV of healthy control dogs was significantly higher than that of dogs in the anemia group (P < 0.001, Table 2). There was no significant difference in PCV for dogs with a hemolytic anemia compared to those with nonhemolytic causes (neoplasia and other; P = 0.084). The mean TS of healthy control dogs was significantly higher than that of dogs in the anemia group (P = 0.01, Table 2). The mean TS of the dogs with a hemolytic anemia was significantly higher than that of dogs with anemia due to any other cause (P = 0.01, Table 2). Of the anemic dogs, 16 of 40 (40%) had concurrent thrombocytopenia (reference interval ([RI]), 200‐500 K/μL), 15 of 40 (37.5%) dogs had concurrent neutrophilia (RI, 3.0‐12.0 K/μL), and 3 of 40 (7.5%) dogs had concurrent neutropenia. Two of 40 (5%) dogs had pancytopenia. Additional hematologic information is presented in Table 2. There was no significant association of other hematologic abnormalities with any cause for anemia.
Table 2.
Hematologic information for anemic dogs compared to healthy control dogs
| PCVa (%) | TS (g/dL) | Thrombocytopenia (N) (RI: 200‐500 K/μL)b | Neutrophilia (N)a (RI: 3.0‐12.0 K/μL) | Neutropenia (N) (RI: 3.0‐12.0 K/μL) | |
|---|---|---|---|---|---|
| Control Dogs (N = 40) | 47.70 ± 6.65 | 6.69 ± 0.49 | |||
|
Anemic Dogs (N = 40) |
22.78 ± 5.39 | 6.07 ± 1.30 |
N = 16 123 (84‐157) |
N = 15 19.4 (12.5‐62.2) |
N = 3 0.2 (0.0‐0.7) |
|
Hemolytic (N = 8) |
19.88 ± 4.09 | 6.99 ± 1.15 |
N = 4 123 (122‐124) |
N = 4 22.5 (13.1‐62.2) |
|
| Neoplastic (N = 9) | 24.00 ± 5.48 | 5.52 ± 0.98 |
N = 5 120.5 (84‐157) |
N = 4 16.1 (12.5‐20.9) |
N = 1 0.2 |
|
Other (N = 23) |
23.42 ± 5.64 | 6.11 ± 1.42 |
N = 7 118 (93‐143) |
N = 7 15.5 (12.9‐46.7) |
N = 2 0.4 (0.0‐0.7) |
PCV and TS values represent the mean and standard deviation.
If significant platelet clumping was noted on blood smear analysis, total platelet number was estimated as decreased, adequate, or increased.
Platelet and neutrophil values represent the median and range.
Platelet counts were not available for all anemic patients.
Biomarkers of Oxidative Stress
Anemic dogs had significantly lower GPx activity than did dogs in the control group (P < 0.001; Figure 2A). Glutathione peroxidase activity in dogs with hemolysis (41.1 × 103 +/‐ 0.8 × 103 U/L) was not significantly different (P = 0.57) than in dogs with nonhemolytic anemia (43.5 × 103 +/‐ 1.1 × 103 U/L). There was no significant difference when comparing the GPx activity of anemic dogs among the 3 major categories (hemolytic, neoplastic, or other; P = 0.80; Figure 3). A significant positive correlation was identified between PCV in anemic dogs and GPx activity (r = 0.878; P < 0.001; Figure 4).
Figure 2.
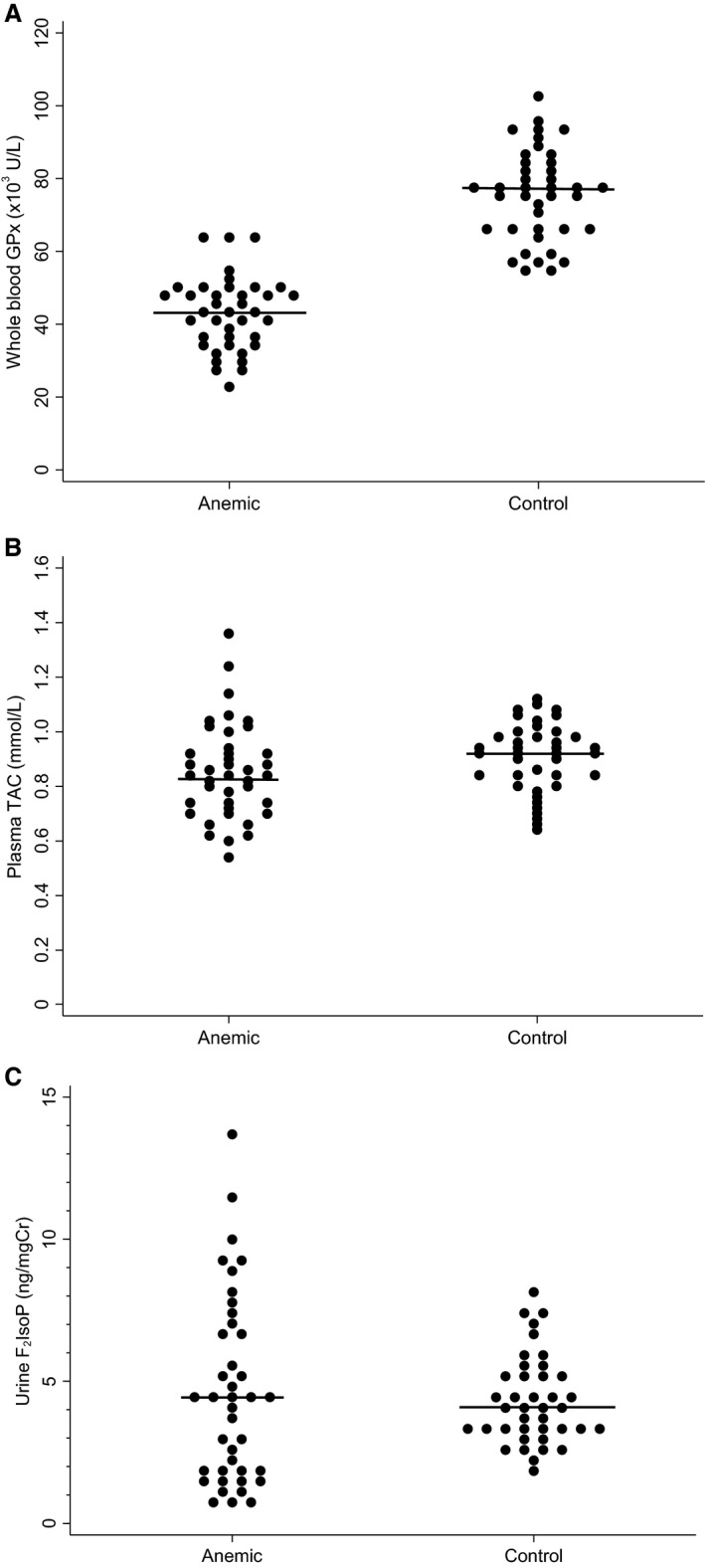
(A) Whole blood GPx activity in anemic dogs compared to healthy control dogs (P < 0.001). (B) Plasma TAC in anemic dogs compared to healthy control dogs (P = 0.15). (C) Urinary F2IsoP in anemic dogs compared to healthy control dogs (P = 0.73). Horizontal bars represent the median value. GPx, glutathione peroxidase; F2IsoP, 15‐F2‐Isoprostanes; TAC, total antioxidant capacity.
Figure 3.
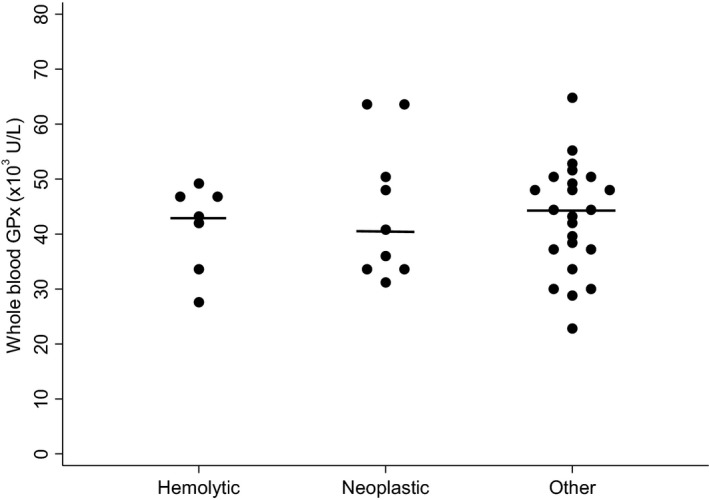
Whole blood GPx activity in “hemolytic” anemia (n = 8), “neoplastic” anemia (n = 9), and “other” anemia (n = 23). Horizontal lines represent the median value. No significant difference was identified between groups (P = 0.80). GPx, glutathione peroxidase.
Figure 4.
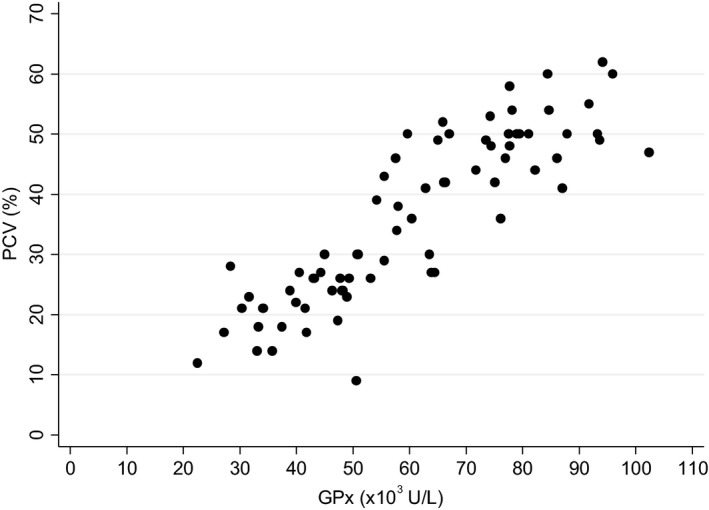
Correlation between mean PCV and whole blood GPx activity in anemic and nonanemic dogs (r = 0.878; P < 0.001). GPx, glutathione peroxidase; PCV, packed cell volume.
Plasma TAC was not significantly different between anemic dogs and healthy control dogs (P = 0.15; Figure 2B). There was no significant difference (P = 0.16) between dogs with hemolytic anemia (0.93 + /‐ 0.23 mmol/L) and dogs with nonhemolytic anemia (0.83 + /‐ 0.16 mmol/L) or control dogs (0.89 + /‐ 0.13 mmol/L; P = 0.56).
Concentrations of urinary F2‐isoprostanes were not significantly different between anemic dogs and healthy control dogs (P = 0.73; Figure 2C). There was no significant difference (P = 0.15) between dogs with a hemolytic anemia (5.99 + /‐ 3.36 ng/mg creatinine) and dogs with a nonhemolytic anemia (4.28 + /‐ 3.24 ng/mg creatinine) or control dogs (P = 0.17; 4.34 + /‐ 1.55 ng/mg creatinine).
Discussion
We identified that anemic dogs had significantly decreased whole blood activity of the antioxidant enzyme, glutathione peroxidase, when compared to healthy control dogs. This finding is similar to that in humans, in which a deficiency in GPx is associated with hemolytic anemia, iron deficiency anemia, and anemia of other causes.34, 35, 36 The mature erythrocyte depends upon nonoxidative metabolism for its major energy supply and is equipped with protective mechanisms against oxidant damage to its cell membrane, hemoglobin, and essential enzyme systems.36 One such mechanism is GPx, which permits reversible oxidation of glutathione (GSH) when the erythrocyte is exposed to oxidant compounds. Impaired activity might therefore be anticipated with anemia, and this decrease in GPx activity could render the red cell vulnerable to oxidative damage.
Glutathione peroxidase has been investigated in anemic dogs with CKD, and its activity was not significantly different when compared to the control population. In the CKD dogs, only erythrocyte‐derived GPx was analyzed, which excluded the plasma fraction of the enzyme activity.10 Four dogs in our study had anemia of CKD, and all had decreased whole blood GPx activity compared to the control population. This observation is in contrast to the erythrocyte‐derived GPx activity reported previously in dogs with CKD suggesting erythrocyte‐derived GPx is maintained whereas plasma GPx decreases in dogs with CKD.31 Systemic oxidative stress in anemic dogs could be more accurately be assessed by use of a whole blood rather than erythrocyte‐derived GPx assay.
We identified strong positive correlation between PCV and whole blood GPx activity, a correlation that has not been observed in humans. Diabetic people with anemia were investigated, and although oxidative stress was identified, characterized by decreased activity of whole blood GPx, no correlation with red blood cell concentration or HCT was identified.14 This finding suggests that erythrocyte defenses may differ among species. Furthermore, the results of our study did not determine whether the positive correlation between PCV and whole blood GPx activity reflects an increase in oxidative stress in anemic dogs or a reflection of the decrease in erythrocyte numbers and less erythrocyte‐derived GPx available to be quantified. A study evaluating these fractions separately would be required to investigate this possibility further.
There was no difference in GPx activity in dogs diagnosed with hemolytic anemia compared to dogs with anemia due to nonhemolytic causes. Our study aimed to investigate anemia of any cause, but the number of hemolytic cases was small and did not allow us to draw reliable conclusions about hemolysis and oxidative stress. Future studies with larger numbers of dogs with hemolytic anemia would be needed to determine the importance of oxidative stress in a hemolytic state.
Although a significant alteration in 1 antioxidant enzyme was identified, significant differences in plasma TAC and urinary F2‐IsoP were not found between dogs with anemia and healthy control dogs. Limitations of utilizing and interpreting TAC as a marker of oxidative stress have been described in people.37 The term TAC is not optimal because the assay measures only a portion of antioxidant capacity, often excluding enzymatic activities.37 It has been suggested that the term “Non‐enzymatic antioxidant capacity” (NEAC) would be more appropriate. This further supports our findings that oxidative stress in anemia likely occurs primarily through antioxidant enzymatic activity.
An increase in F2‐isoprostane production is expected in states of oxidative stress because it is a biomarker of lipid peroxidation.7, 15 Increased urinary F2‐IsoP concentrations are reported in dogs with intervertebral disk disease, dogs in congestive heart failure, and in sled dogs after exercise.5, 26, 27 Increased urinary F2‐IsoP concentrations also have been reported in a diverse group of hospitalized ill dogs.7 Urinary F2‐IsoP concentrations were not significantly different between the anemic and control dogs, and 1 possible explanation is that lipid peroxidation is not be a major mechanism contributing to oxidative damage to the red blood cell. A study evaluating F2‐isoprostane concentrations in people with anemia associated with renal disease and undergoing hemodialysis found no correlation between F2‐IsoP concentrations and the severity of anemia.38 Although the median F2‐isoprostane concentrations were similar between the anemic and control dogs, the highest data points were identified within the anemic dog population.
Glutathione precursors are readily available and could represent a practical therapeutic tool in anemic patients. Exogenous glutathione is not a viable supplement because it is unable to penetrate cell membranes.15 Therefore, clinicians often rely on treatment strategies that inhibit the formation of ROS. The most common include the glutathione precursors, SAMe, and N‐acetylcysteine, which increase production of glutathione by provision of cysteine to the hepatocytes and have been associated with improved outcomes in states of oxidative stress in dogs.7 Vitamin E and vitamin C also have been used for their antioxidant properties and are readily available.39, 40 Additional studies will be needed to evaluate the need for and impact of antioxidant supplementation in anemic dogs.
Our study had several limitations. Direct evaluation of ROS would have been ideal for detecting oxidative stress, but the volatility of ROS in blood and lack of assay availability were prohibitive. Therefore, we investigated markers of oxidative stress including the activity of 1 antioxidant enzyme, 1 byproduct of lipid peroxidation, and an overall assessment of plasma antioxidant capacity. A truly comprehensive evaluation of oxidative stress would include >50 different assays including endogenous antioxidants, antioxidant enzymes, trace elements, an iron profile, and byproducts of inflammation and oxidative damage.15 Such a study would be cost and time prohibitive and would not be practical in a clinical setting.
An additional limitation of our study was sample size. Our study had adequate power to identify a difference in GPx activity in anemic versus healthy dogs, but sample size was not large enough to identify meaningful differences in the other markers of oxidative stress (plasma TAC, urinary F2‐IsoP). Thus, a larger sample size could have found a significant difference in urinary F2‐IsoP between anemic dogs and healthy control dogs. Additionally, the study was underpowered to identify which categories of anemic dogs (hemolytic vs. nonhemolytic) had clinically relevant oxidative stress.
The addition of a third group consisting of sick, nonanemic dogs would have further supported that a deficiency in GPx activity was caused by the anemic state and not illness of other causes. The anemic dogs in our study had variable degrees of systemic illness and comorbidities, which could have influenced the severity of oxidative stress. However, a strong correlation between mean PCV and whole blood GPx activity was found, which suggests that alterations in whole blood GPx activity occur in anemic dogs and could be a supportive marker of oxidative stress (Figure 4).
Anemia of any cause was investigated in our study, but the anemic dogs were not evaluated based on evidence of regeneration, iron deficiency, or cause other than the presence of hemolysis. Oxidative stress has been identified in people with iron deficiency anemia, and iron status would have been useful to know in our study population.19 We did identify 2 dogs with microcytic hypochromic anemia. In those dogs, there was no significant difference in any of the markers of oxidative stress when compared to the other anemic dogs. These represented only a small number of dogs, without confirmation of iron deficiency, and further studies would be indicated to investigate the relationship between iron deficiency anemia in dogs and oxidative stress.
In conclusion, we demonstrated significantly decreased GPx activity and oxidative stress in anemic dogs. In addition, a strong correlation between decreased GPx activity and decreased mean PCV was found. Further studies are warranted to determine if antioxidant supplementation would improve survival and overall outcome as part of a therapeutic regimen for anemic dogs.
Acknowledgments
The authors thank Dr. Ginger Milne at the Eicosanoid Core Laboratory in the Division of Clinical Pharmacology at Vanderbilt University and Ms. Karen Morgenthaler at the Clinical and Translational Research Center Core Laboratory at the University of Colorado‐Denver, Anschutz Medical Campus for their assistance with sample analyses.
Conflict of Interest Declaration:
Dr. Moore serves as Consulting Editor for Experimental Design and Statistics for the Journal of Veterinary Internal Medicine. He was not involved in the review of this manuscript.
Off‐label Antimicrobial Declaration:
Authors declare no off‐label use of antimicrobials.
The research was conducted at the College of Veterinary Medicine, Purdue University, West Lafayette, Indiana.
This study was partially funded by the Purdue University, College of Veterinary Medicine, Veterinary Clinical Sciences, Graduate Student Competitive Research Grant.
Footnotes
Nutramax Laboratories, Lancaster, SC
Siemens Medical Solutions USA, Inc. Malvern, PA
PACUC Coeus Protocol Number 1509001296
BD Vacutainer; Bectin, Dickinson and Company, Frank
Ortho Clinical Diagnostic Vitros 5.1, Raritan, NJ
Randox Laboratories, Crumlin, UK
Beckman Coulter Inc., Brea, CA
Roche COBAS IntegraH 800, Basel, Switzerland
Abbott Cell Dyn 3700, Abbott Laboratories; Abbott Park, IL
Stata Corp LP, College Station, TX
References
- 1. Koren E, Kohen R, Ginsburg I. Polyphenols enhance total oxidant scavenging capacities of human blood by binding to red blood cells. Exp Biol Med 2010;235:689–699. [DOI] [PubMed] [Google Scholar]
- 2. Pasquini A, Gavazza A, Biagi G, et al. Oxidative stress in lymphoma: Similarities and differences between dog and human. Comp Clin Pathol 2015;24:69–73. [Google Scholar]
- 3. Sagols E, Priymenko N. Oxidative stress in dog with heart failure: The role of dietary fatty acids and antioxidants. Vet Med Int. 2011;2011:180206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Winter JL, Barber LG, Freeman L, et al. Antioxidant status and biomarkers of oxidative stress in dogs with lymphoma. J Vet Intern Med 2009;23:311–316. [DOI] [PubMed] [Google Scholar]
- 5. Freeman LM, Rush JE, Milbury PE, et al. Antioxidant status and biomarkers of oxidative stress in dogs with congestive heart failure. J Vet Intern Med 2005;19:537–541. [DOI] [PubMed] [Google Scholar]
- 6. Freeman LM, Brown DJ, Rush JE. Assessment of degree of oxidative stress and antioxidant concentrations in dogs with idiopathic dilated cardiomyopathy. J Am Vet Med Assoc 1999;215:644–646. [PubMed] [Google Scholar]
- 7. Viviano KR, VanderWielen B. Effect of N‐acetylcysteine supplementation on intracellular glutathione, urine isoprostanes, clinical score, and survival in hospitalized ill dogs. J Vet Intern Med 2013;27:250–258. [DOI] [PubMed] [Google Scholar]
- 8. Viviano KR, Lavergne SN, Goodman L, et al. Glutathione, cysteine, and ascorbate concentrations in clinically ill dogs and cats. J Vet Intern Med 2009;23:250–257. [DOI] [PubMed] [Google Scholar]
- 9. Krofic Zel M, Tozon N, Nemec Svete A. Plasma and erythrocyte glutathione peroxidase activity, serum selenium concentration, and plasma total antioxidant capacity in cats with IRIS stages I‐IV chronic kidney disease. J Vet Intern Med 2014;28:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kogika MM, Lustoza MD, Hagiwara MK, et al. Evaluation of oxidative stress in the anemia of dogs with chronic kidney disease. Vet Clin Pathol 2015;44:70–78. [DOI] [PubMed] [Google Scholar]
- 11. Chen T, Gionfriddo JR, Tai PY, et al. Oxidative stress increases in retinas of dogs in acute glaucoma but not in chronic glaucoma. Vet Ophthalmol 2015;18:261–270. [DOI] [PubMed] [Google Scholar]
- 12. Kapun AP, Salobir J, Levart A, et al. Oxidative stress markers in canine atopic dermatitis. Res Vet Sci 2012;92:469–470. [DOI] [PubMed] [Google Scholar]
- 13. Bhooshan Pandey K, Syed Rizvi I. Biomarkers of oxidative stress in red blood cells. Biomed Pap 2011;155:131–136. [DOI] [PubMed] [Google Scholar]
- 14. Waggiallah H, Alzohairy M. The effect of oxidative stress on human red cells glutathione peroxidase, glutathione reductase level, and prevalence of anemia among diabetics. North Am J Med Sci 2011;3:344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McMichael MA. Oxidative stress, antioxidants, and assessment of oxidative stress in dogs and cats. J Am Vet Med Assoc 2007;231:714–720. [DOI] [PubMed] [Google Scholar]
- 16. Edwards CJ, Fuller J. Oxidative stress in erythrocytes. Comp Haematol Int 1996;6:24–31. [Google Scholar]
- 17. Fibach E, Dana M. Oxidative stress in paroxysmal nocturnal hemoglobinuria and other conditions of complement‐mediated hemolysis. Free Radic Biol Med 2015;88:63–69. [DOI] [PubMed] [Google Scholar]
- 18. Fibach E, Rachmilewitz E. The role of oxidative stress in hemolytic anemia. Curr Mol Med 2008;8:609–619. [DOI] [PubMed] [Google Scholar]
- 19. Kurtoglu E, Ugur A, Baltaci AK, et al. Effect of iron supplementation on oxidative stress and antioxidant status in iron‐deficiency anemia. Biol Trace Elem Res 2003;96:117–123. [DOI] [PubMed] [Google Scholar]
- 20. Du W, Adam Z, Rani R, et al. Oxidative stress in fanconi anemia hematopoiesis and disease progression. Antioxid Redox Signal 2008;10:1909–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grune T, Sommerburg O, Siems WG. Oxidative stress in anemia. Clin Nephrol 2000;53:S18–S22. [PubMed] [Google Scholar]
- 22. Pesillo SA, Freeman LM, Rush JE. Assessment of lipid peroxidation and serum vitamin E concentration in dogs with immune‐mediated hemolytic anemia. Am J Vet Res 2004;65:1621–1624. [DOI] [PubMed] [Google Scholar]
- 23. Britti D, Sconza S, Morittu VM, et al. Superoxide dismutase and Glutathione peroxidase in the blood of dogs with Leishmaniasis. Vet Res Commun 2008;32:251–254. [DOI] [PubMed] [Google Scholar]
- 24. Morrow JD, Roberts LJ. The isoprostanes: Unique bioactive products of lipid peroxidation. Prog Lipid Res 1997;36:1–21. [DOI] [PubMed] [Google Scholar]
- 25. Kadiiska MB, Gladen BC, Baird DD, et al. Biomarkers of Oxidative Stress Study II: Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med 2005;38:698–710. [DOI] [PubMed] [Google Scholar]
- 26. McMichael MA, Ruaux CG, Baltzer WI, et al. Concentrations of 15F2t isoprostane in urine of dogs with intervertebral disk disease. Am J Vet Res 2006;67:1226–1231. [DOI] [PubMed] [Google Scholar]
- 27. Hinchcliff KW, Reinhart GA, DiSilvestro R, et al. Oxidant stress in sled dogs subjected to repetitive endurance exercise. Am J Vet Res 2000;61:512–517. [DOI] [PubMed] [Google Scholar]
- 28. Young I. Measurement of total antioxidant capacity. J Clin Pathol 2001;54:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967;70:158–169. [PubMed] [Google Scholar]
- 30. Plavec T, Nemec SA, Butinar J, et al. Antioxidant status in canine cancer patients. Acta Vet‐Beogr 2008;58:275–286. [Google Scholar]
- 31. Silva ACRA, de Almeida BFM, Soeiro CS, et al. Oxidative stress, superoxide production, and apoptosis of neutrophils in dogs with chronic kidney disease. Can J Vet Res 2013;77:136–141. [PMC free article] [PubMed] [Google Scholar]
- 32. Milne GL, Sanchez SC, Musiek ES, et al. Quantification of F2‐isoprostanes as a biomarker of oxidative stress. Nat Protoc 2007;2:221–226. [DOI] [PubMed] [Google Scholar]
- 33. Soffler C, Campbell VL, Hassel DM. Measurement of urinary F2‐isoprostanes as markers of in vivo lipid peroxidation: A comparison of enzyme immunoassays with gas chromatography‐mass spectrometry in domestic animal species. J Vet Diagn Investig 2010;22:200–209. [DOI] [PubMed] [Google Scholar]
- 34. Steinberg M, Brauer MJ, Necheles TF. Acute hemolytic anemia associated with erythrocyte glutathione‐peroxidase deficiency. Arch Intern Med 1970;125:302–303. [PubMed] [Google Scholar]
- 35. Hopkins J, Tudhope GR. Glutathione peroxidase in human red cells in health and disease. Br J Haematol 1973;25:563–575. [DOI] [PubMed] [Google Scholar]
- 36. Macdougall LG. Red cell metabolism in iron deficiency anemia. J Pediatr 1972;80:775–782. [DOI] [PubMed] [Google Scholar]
- 37. Bartosz G. Non‐enzymatic antioxidant capacity assays: Limitations of use in biomedicine. Free Radic Res 2010;44:711–720. [DOI] [PubMed] [Google Scholar]
- 38. Wiswedel I, Peter D, Gardemann A, et al. Serum concentrations of F2‐Isoprostanes and 4‐Hydroxynonenal in hemodialysis patients in relation to inflammation and renal anemia. Biomark Insights 2008;3:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Plevnik Kapun A, Salobir J, Levart A, et al. Vitamin E supplementation in canine atopic dermatitis: Improvement of clinical signs and effects on oxidative stress markers. Vet Rec 2014;175:560. [DOI] [PubMed] [Google Scholar]
- 40. Hesta M, Ottermans C, Krammer‐Lukas S, et al. The effect of vitamin C supplementation in healthy dogs on antioxidative capacity and immune parameters. J Anim Physiol Anim Nutr 2009;93:26–34. [DOI] [PubMed] [Google Scholar]


