Abstract
A juvenile male mixed breed dog was presented for lethargy, exercise intolerance, and aggression when touched on the head. Cyanosis, tachycardia, and tachypnea were observed and persisted during oxygen supplementation. Arterial blood gas analysis by co‐oximetry identified an increased methemoglobin concentration (27%; normal, <2%) with normal arterial oxygen tension. The methemoglobinemia and associated clinical signs resolved after administration of methylene blue (1 mg/kg) IV, and the dog was discharged. The affected dog's whole‐genome sequence contained 2 potentially causal heterozygous CYB5R3 missense mutations suggesting that cytochrome b5 reductase deficiency was responsible for the methemoglobinemia. This hypothesis was confirmed by enzyme analysis that identified cytochrome b5 reductase activity in the affected dog's erythrocytes to only approximately 6% of that in a control sample. Clinical signs recurred 11 days after discharge but normalized and the methemoglobin concentration decreased with methylene blue administration PO (1.5 mg/kg, initially daily and then every other day).
Keywords: Cyanosis, CYB5R3, Whole‐genome sequence, Blood gas
Abbreviations
- CBC
complete blood count
- FIO2
fraction of inspired oxygen
- MB
methylene blue
- methb%
methemoglobin percentage
- SpO2
pulse oximetry
Case Description
A 6‐month‐old male mixed breed dog was presented to the primary care veterinarian for castration and evaluation of a hacking cough. Preoperative laboratory screening identified increased serum activities of alanine aminotransferase (152 U/L; reference interval [RI], 10–125 U/L) and alkaline phosphatase (347 U/L; RI, 23–212 U/L). The dog was prescribed a liver supplement1 and orbifloxacin2 and was referred to the University of Missouri Veterinary Health Center for further evaluation.
Upon presentation, the owner reported that the dog had decreased appetite, an exertional hacking cough, and exercise intolerance for an approximately 2‐week period. Additionally, the dog had displayed aggressive behavior when its head or neck were touched. There had been no known access to onions, garlic, toxins, or drugs including acetaminophen. The dog had been fed a mixture of a dry commercial adult canine food and fried chicken livers. The dog was not current on vaccines and had not been treated with parasiticides.
Pertinent physical examination findings included heart and respiratory rates of approximately 200 beats and 35 breaths per minute, respectively, and cyanotic oral and preputial mucous membranes. Cardiothoracic auscultation disclosed a grade II/VI right apical systolic murmur. Palpation of the cervical trachea elicited a cough, but respiratory examination was otherwise normal. Furthermore, the dog was reluctant to comply with flexion and extension of the neck and attempted to bite and escape when any part of the head was palpated.
Because of the cyanosis, the dog was placed into an oxygen chamber (inspired oxygen concentration = 40%), but tachypnea and cyanosis persisted. Pulse oximetry (SpO2) yielded values of 76% and 79%, (RI, 95–100%) at fraction of inspired oxygen (FIO2) = 0.21 and approximately 0.40, respectively.
Thoracic radiographs disclosed a mild degree of peribronchial thickening and a slightly redundant dorsal tracheal membrane localized to the thoracic inlet. An echocardiogram identified mild tricuspid regurgitation. Abnormalities detected by routine serum biochemistry included increased activities of alanine aminotransferase (97 U/L; RI, 9–58 U/L) and alkaline phosphatase (489 U/L; RI, 5–129 U/L). Results of CBC, urinalysis and serum pre‐ and postprandial bile acid concentrations were normal. Arterial blood gas analysis3 with co‐oximetry identified methemoglobinemia concurrent with normal arterial oxygen tension at FIO2 = 0.21, which supported a nonrespiratory cause for cyanosis, tachypnea, and exercise intolerance (Table 1).
Table 1.
Serial blood gas analyses prior and after administration of methylene blue (1 mg/kg IV) in a dog with hereditary methemoglobinemia
| Day | 1 | 2 | 3 | 3 | 4 | 4 | 5 | |
|---|---|---|---|---|---|---|---|---|
| Time | 13:32 | 16.45a | 11:25 | 17:50 | 9:36 | 18:58 | 9:51 | |
| Sample | Arterial | Arterial | Arterial | Venous | Venous | Venous | Venous | Reference interval |
| pH | 7.401 | 7.425 | 7.457 | 7.44 | 7.425 | 7.412 | 7.397 | 7.36–7.44 (arterial) |
| 7.34–7.43 (venous) | ||||||||
| pCO2 | 35.3 | 32 | 32.4 | 38.2 | 33.4 | 35.1 | 35.9 | 27.1–38.7 mmHg (arterial) |
| 29.7–46 mmHg (venous) | ||||||||
| pO2 | 85.8 | 93.8 | 101 | 46.7 | 61.7 | 53.9 | 56.4 | 82.7–120 mmHg (arterial) |
| 31.6–63.9 mmHg (venous) | ||||||||
| HCO3 | 21.4 | 20.6 | 22.6 | 25.6 | 21.5 | 21.9 | 21.6 | 17.2–23 mEq/L (arterial) |
| 18.7–25.6 mEq/L (venous) | ||||||||
| HCT | 48.1 | 52.3 | 51.6 | 50.7 | 47.6 | 49.6 | 47.3 | |
| MetHb | 27.2 | 3.6 | 5.8 | 5.7 | 8.8 | 12 | 13.9 | 0.0–1.0% |
| FiO2(%) | 21 | 21 | 21 | 21 | 21 | 21 | 21 |
1 hour postintravenous MB administration.
On day 2, the dog was treated with a single dose of methylene blue4 (MB; 1 mg/kg IV over 20 minutes). An arterial blood gas analysis with co‐oximetry 1 hour after MB administration showed improvement (Table 1; Fig 1). Pulse oximetry before treatment with MB was 77% and improved to 97% after treatment (FIO2 = 0.21). In addition, the dog's heart and respiratory rates normalized (100 beats/min and 18 breaths/min, respectively), and cyanosis resolved completely. The dog was discharged on day 5.
Figure 1.
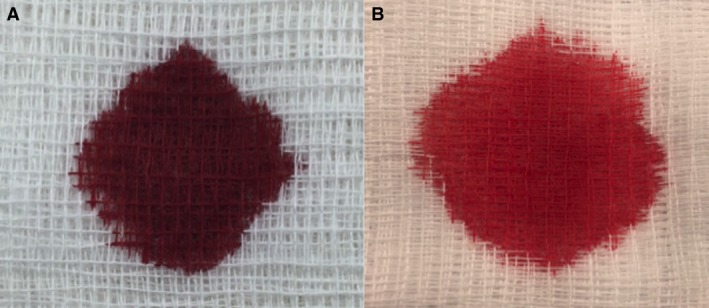
“Chocolate brown” blood from the dog (A) and resolution of discolored blood (B) 1 hour after IV administration of methylene blue.
Because of the patient's young age, the persistence of the methemoglobinemia, and lack of known toxin exposure, hereditary methemoglobinemia was thought to be the most likely differential diagnosis. To identify the molecular genetic cause, we used previously described methods to generate and analyze a 36‐fold average coverage whole‐genome sequence of the affected dog.1 The sequence data are available in the National Center for Biotechnology Information Sequence Read Archives (accession SRS1867904). The analysis focused on CYB5A and CYB5R3, 2 genes known to harbor methemoglobinemia‐causing sequence variants in humans. No potentially causal sequence variants were recognized within the CYB5A genic region but, we found 2 heterozygous CYB5R3 missense mutations: chr10:22,832,963G>A that predicted a CYB5R3:p.Gly72Ser amino acid substitution and chr10:22,836,951A>C that predicted a CYB5R3:p.Ile190Leu amino acid substitution. The chromosomal positions were based on the CanFam3.1 genome reference assembly, the cytochrome b5 reductase amino acids were numbered according to ENSCAFT00000046278, and the variants were reported according to nomenclature recommended by the Human Genome Variation Society (http://varnomen.hgvs.org/). Potential causal contributions to the dog's methemoglobinemia by either or both of the CYB5R3 missense mutations should be reflected by decreased cytochrome b5 reductase activity. Thus, fresh EDTA‐anticoagulated blood samples from the dog with methemoglobinemia and a healthy control dog were sent chilled to the PennGen laboratory of the School of Veterinary Medicine at the University of Pennsylvania, where established procedures2 were used to determine that the cytochrome b5 reductase activity in erythrocytes from the affected dog was only 0.27 IU/g Hb or 5.8% of the activity when compared to a simultaneously tested control sample (3.68 IU/g Hb; 100%).
When examined on day 16, the methemoglobin percentage (methb%) increased to a percentage similar to that determined before administration of IV MB on day 1 (Fig 2). Cyanosis persisted, as did exertional fatigue, and intermittent aggression. Additionally, a biochemical analysis showed that the dog's alanine aminotransferase activity was within the RI, whereas the alkaline phosphatase activity remained marginally increased above the RI (292 U/L; RI, 23–212 U/L). In an effort to decrease the clinical signs associated with methemoglobinemia, treatment with riboflavin5 (11 mg/kg PO q24h) was started. After 3 weeks of treatment with riboflavin, no appreciable change in the methb% was observed (Fig 2; day 38). Thus, riboflavin treatment was discontinued and ascorbic acid6 (12.5 mg/kg PO q24h) treatment was initiated. After 4.5 weeks of treatment with ascorbic acid, a venous blood gas with co‐oximetry showed no change in methb%. Therefore, administration of ascorbic acid was discontinued (Fig 2; day 69). On day 107, because of persistence of methemoglobinemia and clinical signs, PO treatment with MB7 (1.5 mg/kg PO q24h) was initiated. After a week of PO MB treatment (day 114), the dog's methb% decreased and clinical abnormalities (exertional fatigue and aggression) resolved (Fig 2). Oral MB treatment was continued. On day 142, the dog's CBC did not indicate any abnormalities, and methb% had decreased to approximately 10% (Fig 2). At that visit, the interval of MB administration was extended to every other day. This change in dosing maintained methb% near 10% and prevented the reappearance of the clinical abnormalities observed when the dog was evaluated on day 170 (Fig 2). Additionally, the dog's CBC did not show any abnormalities, and PO MB treatment was continued.
Figure 2.
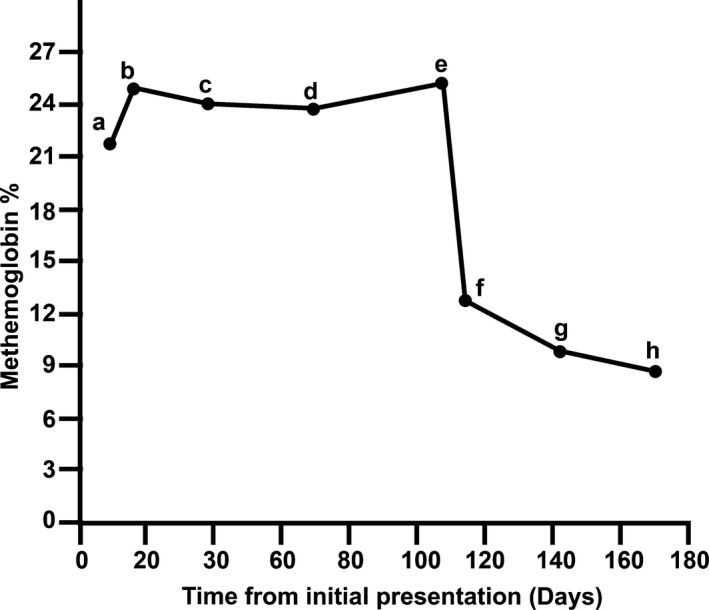
Methemoglobin concentrations (methb%) in a dog with hereditary methemoglobinemia without treatment, with intravenous methylene blue, oral riboflavin, oral ascorbic acid, or oral methylene blue (see Case Description for details). Methemoglobin concentrations were measured in venous blood with co‐oximetry. The dog received methylene blue 1.0 mg/kg IV on day 2 of hospitalization and the methb% gradually increased back to pretreatment levels (a; Day 9, b; Day 16). The dog was then treated with riboflavin 11 mg/kg PO q24h, for 21 days with a marginal effect on methb% (c). The dog was then treated with oral ascorbic acid 12.5 mg/kg PO q24h, for 31 days with an equivocal change in methb% (d). Ascorbic acid was discontinued (e), and oral MB 1.5 mg/kg PO q24h was started with reduction in methb% after 7 days (f). The methb% continued to decrease after an additional 28‐days of treatment with oral MB (g). The frequency of MB administration was decreased to 1.5 mg/kg PO every other day and after 28‐days no change in methb% (h).
Discussion
Methemoglobinemia forms when hemoglobin iron is oxidized from ferrous iron (Fe2+) to ferric iron (Fe3+).3, 4, 5, 6 Methemoglobin cannot bind oxygen and excess accumulation of methemoglobin can lead to cyanosis, impaired aerobic respiration, metabolic acidosis, and death in severe cases.3 Additionally, 1 ferriheme group in the hemoglobin tetramer causes allosteric interactions that left shift the oxygen‐hemoglobin dissociation curve and decrease release of oxygen at the tissue level. Under normal circumstances in dogs5 and people,4 3% of ferrous hemoglobin is oxidized to methemoglobin daily as a result of exposure to environmental or endogenous oxidants. The cytochrome b5/cytochrome b5 reductase enzyme system in erythrocytes functions to convert methemoglobin back to its reduced, ferrous form.5 The proteins cytochrome b5 reductase (encoded by CYB5R3) and cytochrome b5 (encoded by CYB5A) make up the cytochrome b5/cytochrome b5 reductase system, which accounts for 99% of daily methemoglobin reduction.3 This major physiologic pathway for reduction uses electrons donated from NADH to cytochrome b5 reductase that then are transferred to cytochrome b5, and finally to individual methemoglobin molecules resulting in its reduction to hemoglobin.3
A recent improvement in the instrumentation for massively parallel DNA sequencing has decreased the cost of generating mammalian whole‐genome sequences so that it is now a cost‐effective tool for diagnosing heritable diseases in veterinary patients.1, 7 Thus, once a diagnosis of inherited methemoglobinemia was suspected, we sequenced the dog's genome and identified 2 heterozygous CYB5R3 missense mutations that may be responsible for the cytochrome b5 reductase deficiency. There is strong indirect evidence for a causal contribution from 1 of them: CYB5R3:p.Gly72Ser. Indeed, 3 on‐line algorithms that estimate the functional consequences of sequence variants all predict impaired function for the Ser72 allele (Table 2). In addition, a heterozygous glycine‐to‐serine missense mutation at a homologous position in human cytochrome b5 reductase was reported to be partially responsible for the methemoglobinemia in a compound heterozygous human patient.8 Furthermore, in that study in vitro protein expression was used to produce cytochrome b5 reductase that had the homologous glycine‐to‐serine substitution and retained only 11% of the normal reductase activity.8 However, a single heterozygous mutation cannot by itself be responsible for an autosomal recessive disease such as cytochrome b5 reductase deficiency.
Table 2.
In silica predictors of variant effect on gene function
| Mutation | SIFT/prediction | PROVEAN/prediction | polyphen2/prediction |
|---|---|---|---|
| CYB5R3:p.Gly72Ser | 0.01/deleterious | −5.818/deleterious | 1/probably damaging |
| Cyb5R3:p.lle190Leu | 0/deleterious | 1.897/neutral | 0.035/benign |
SIFT score is considered deleterious at <0.05. The default score threshold for PROVEAN is considered deleterious at <−2.5. Polyphen2 is scored as “benign” to “probably damaging” on a scale of 0 to 1.
The only other potentially causal CYB5R3‐associated variant recognized in the affected dog's whole‐genome sequence was CYB5R3:p.Ile190Leu. Compared to the CYB5R3:p.Gly72Ser, there is less evidence that CYB5R3:p.Ile190Leu contributed to the methemoglobinemia. Only 1 of the 3 on‐line algorithms predicted that the Leu90 allele would impair function (Table 2), and we found no reports that mutations of this codon had caused methemoglobinemia in any species. Furthermore, we do not know if the 2 CYB5R3 missense mutations were both inherited from the same parent or if each was inherited from a different parent to produce compound heterozygosity with loss of reductase function. Until new information becomes available, we will consider the CYB5R3:p.Ile190Leu missense mutation to be of unknown consequence.
The whole‐genome sequence analysis suggested that the dog's methemoglobinemia was caused by a cytochrome b5 reductase deficiency. This was confirmed by enzymologic analysis, which indicated that the cytochrome b5 reductase activity in a sample from the affected dog was only about 6% of that in a control sample. The only other report of hereditary methemoglobinemia in a dog with genome sequencing identified a promoter deletion and a nonsynonymous coding variant in the gene encoding cytochrome b5 (CYB5A).9 A presumptive diagnosis of a cytochrome b5 defect was made based on the identified genetic variants in CYB5A and normal enzymologic evaluation of cytochrome b5 reductase.9
The severity of clinical signs associated with methemoglobinemia, independent of the cause, depends on how much of total hemoglobin has been oxidized to methemoglobin.3 Humans with normal hemoglobin concentrations and methemoglobin concentrations of 20–30% total hemoglobin can exhibit anxiety, lightheadedness, headache, and tachycardia. Concentrations that exceed 50–70% can result in coma, seizures, arrhythmias, acidosis, and death.3 The baseline methb% for the dog of our report was approximately 25%. Dogs with cytochrome b5 reductase deficiency have been reported to have intermittent exercise intolerance and lethargy.10 The intermittent bouts of aggression and reluctance to comply with palpation of the head and neck on physical examination ceased when PO treatment with MB was instituted, suggesting causality.
Currently, there are no long‐term treatment recommendations for dogs with hereditary methemoglobinemia. Treatment of methemoglobinemia in humans is suggested for infants (<6 months of age), and for individuals with concurrent anemia, lung disease, cardiac disease, sepsis, abnormal hemoglobins, sickle cell disease, and whenever methemoglobin concentrations exceed 20–30% in otherwise healthy individuals with no known comorbidities.4 Concurrent diseases that compromise oxygen‐carrying capacity, arterial oxygen tension, or perfusion will magnify the effects of methemoglobinemia.3, 4 In addition, inflammation or sepsis may further contribute to increased methemoglobin concentration by increased endogenous oxidant production.4 Moreover, some foods and drugs can act as oxidants and increase methemoglobin concentration.
The prophylactic use of MB in people with hereditary cytochrome b5 reductase deficiency and methemoglobin concentrations of >20% is recommended before induction of anesthesia.11 However, prophylactic use of MB in the aforementioned group of people with methemoglobin concentrations of <20% remains controversial.12, 13 The argument against its use is founded on the ability to carefully monitor people perioperatively and postoperatively using multiple wavelength co‐oximeters or co‐oximeter blood gas analyzers. These analyzers accurately detect oxyhemoglobin/methemoglobin saturation and methemoglobin concentration, respectively. Routine pulse oximetry in patients with methemoglobinemia is useless because methemoglobin absorbs both infrared and red light equally. The equal absorption interferes with the measured percentage of oxyhemoglobin and deoxyhemoglobin. Additionally, arterial blood gas analysis usually indicates normal oxygen tension because it only evaluates the partial pressure of dissolved oxygen in plasma. Because of the limited availability of multiple wavelength co‐oximeters and co‐oximeter blood gas analyzers in general practice, we recommend that dogs with hereditary methemoglobinemia as a result of cytochrome b5 reductase deficiency be treated prophylactically with MB IV. In dogs not treated prophylactically, clinicians should keep MB for IV use available in the hospital in the event the dog decompensates.
Anecdotal experience and various case reports show that many dogs with hereditary methemoglobinemia due to cytochrome b5 reductase deficiency do not routinely display relevant clinical signs. However, as was seen here, dogs can experience transient acute decompensation of their otherwise stable disease because of many factors (eg, any inflammatory response that increases endogenous oxidant production, oxidative toxins, drugs, foods, or anemia). The dog reported here had an inducible hacking cough on tracheal palpation that coincided with tachypnea and resolved with antibiotic treatment. We suspect that the dog acutely decompensated because of an upper respiratory infection. The acute worsening of clinical signs in people with recessive congenital methemoglobinemia has been associated with development of upper respiratory infections.14 Alternatively, the mild increase in serum liver enzyme activities with subsequent resolution of the increased serum alanine aminotransferase activity may indicate an acute hepatopathy caused by exposure to an exogenous oxidant. Our report highlights the potential need for clinicians to treat dogs with hereditary methemoglobinemia with IV or PO MB during periods of decompensation. Similarly, situations of oxidative stress (eg, toxicosis, drug exposure, infections) should be avoided.
In some human patients, methemoglobinemia can be controlled long‐term with PO administration of ascorbic acid, riboflavin, or MB.15, 16, 17, 18 The dog described here did not respond to treatment with either riboflavin or ascorbic acid at the doses given. However, treatment with MB PO resulted in a decrease in methb%, resolution of clinical signs (exercise intolerance and aggression) as well as improvement of cyanosis. No pharmacokinetic data exists for MB administered PO in dogs, and the dosages utilized were extrapolated from case reports in infants with hereditary methemoglobinemia.15, 16 It should be noted that MB has a narrow therapeutic index and thus must be dosed carefully.19 An NADPH‐driven reductase pathway converts MB to leukomethylene blue, the reducing agent responsible for reduction of methemoglobin to hemoglobin. Methylene blue actually is an oxidant. If the dose of MB saturates and overwhelms the NADPH‐reductase pathway, there will be a higher proportion of MB and thus magnified oxidative damage resulting in hemolysis and, paradoxically, worsening methemoglobinemia. Additional pharmacokinetic information is needed to determine optimal dosing and safety of PO MB in dogs. Many dogs with methemoglobinemia as a result of cytochrome b5 reductase deficiency have done well without treatment by avoiding oxidative injury and did not require long‐term treatment with PO MB.20, 21, 22 The application of the PO MB treatment regimen in client‐owned dogs with cytochrome b5 reductase deficiency should be made on a case‐by‐case basis as deemed necessary.
The dog described here represents a case of hereditary methemoglobinemia possibly caused by 2 heterozygous missense mutations in the CYB5R3 gene. The dog was effectively and safely treated with IV and then long‐term PO MB.
Acknowledgments
The authors thank the small animal internal medicine service for contributing partial funding toward diagnostic testing and treatment of the dog in this report. Additionally, the authors thank Kate Anderson for her editorial and literature search assistance. We also acknowledge Dr. John Harvey for his expert opinion related to canine methemoglobinemia. Lastly, the authors acknowledge the Respiratory Care Department at the University of Missouri Medical Hospital for their technical assistance and use of their blood gas analyzer.
Conflict of Interest Declaration
The University of Pennsylvania (PennGen) offers hematologic and biochemical testing for methemoglobinemia. The authors disclose no other conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Work performed at the University of Missouri and the University of Pennsylvania.
The work at the University of Pennsylvania was supported in part by grant from the NIH (OD 010939).
Footnotes
Denamarin, Nutramax Laboratories Inc, Edgewood, MD
Orbax, Intervet Inc, Summit, NJ
ABL800 FLEX blood gas analyzer, Radiometer Medical ApS, Denmark
Akorn Inc, Lake Forest, IL
Nature's Bounty, Bohemia, NY
Medisca Inc, Plattsburg, NY
Sigma‐Aldrich Co, St. Louis, MO
References
- 1. Kolicheski A, Johnson G, Villani N, et al. GM2 gangliosidosis in Shiba Inu dogs with an in‐frame deletion in HEXB . J Vet Intern Med 2017; https://doi.org/10.1111/jvim.14794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beutler E. Red Cell Metabolism: A Manual of Biochemical Methods, 3rd ed Orlando (FL): Grune & Stratton; 1984:10–83. [Google Scholar]
- 3. Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: Etiology, pharmacology, and clinical management. Ann Emerg Med 1999;34:646–656. [DOI] [PubMed] [Google Scholar]
- 4. Umbreit J. Methemoglobin – it's not just blue: A concise review. Am J Hematol 2007;82:134–144. [DOI] [PubMed] [Google Scholar]
- 5. Harvey JW. Evaluation of erythrocytes In: Harvey JW, ed. Veterinary Hematology: A Diagnostic Guide and Color Atlas. St Louis: Elsevier/Saunders; 2012:49–121. [Google Scholar]
- 6. Percy MJ, Lappin TR. Recessive congenital methaemoglobinaemia: Cytochrome b5 reductase deficiency. Brit J Haematol 2008;141:298–308. [DOI] [PubMed] [Google Scholar]
- 7. Mauler DA, Gandolfi B, Reinero CR, et al. Precision medicine in cats: Novel Niemann‐Pick type C1 diagnosed by whole‐genome sequencing. J Vet Intern Med 2017;32:539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Percy MJ, Crowley LJ, Roper D, et al. Identification and characterization of the novel FAD‐binding lobe G75S mutation in cytochrome b(5) reductase: An aid to determine recessive congenital methemoglobinemia status in an infant. Blood Cells Mol Dis 2006;36:81–90. [DOI] [PubMed] [Google Scholar]
- 9. McKenna JA, Sacco J, Son TT, et al. Congenital methemoglobinemia in a dog with a promoter deletion and a nonsynonymous coding variant in the gene encoding cytochrome b5. J Vet Intern Med 2014;28:1626–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harvey JW. Pathogenesis, laboratory diagnosis, and clinical implications of erythrocyte enzyme deficiencies in dogs, cats, and horses. Vet Clin Pathol 2006;35:144–156. [DOI] [PubMed] [Google Scholar]
- 11. Kus A, Berk D, Hosten T, et al. The role of preoperative evaluation for congenital methemoglobinemia. Turk J Anaesthesiol Reanim 2014;42:223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baraka AS, Ayoub CM, Yazbeck‐Karam V, et al. Prophylactic methylene blue in a patient with congenital methemoglobinemia. Can J Anaesth 2005;52:258–261. [DOI] [PubMed] [Google Scholar]
- 13. Sharma D, Pandia MP, Bithal PK. Methylene blue in congenital methemoglobinemia: Prophylactic or on demand? Can J Anaesth 2005;52:884–885. [DOI] [PubMed] [Google Scholar]
- 14. Huang YH, Tai CL, Lu YH, et al. Recessive congenital methemoglobinemia caused by a rare mechanism: Maternal uniparental heterodisomy with segmental isodisomy of a chromosome 22. Blood Cells Mol Dis 2012;49:114–117. [DOI] [PubMed] [Google Scholar]
- 15. Cooper M, Randal M, Rowell M, et al. Congenital methemoglobinemia type II – clinical improvement with short‐term methylene blue treatment. Pediatr Blood Cancer 2016;63:558–560. [DOI] [PubMed] [Google Scholar]
- 16. Da‐Silva SS, Sajan IS, Underwood JP 3rd. Congenital methemoglobinemia: A rare cause of cyanosis in the newborn – a case report. Pediatrics 2003;112:e158–e160. [DOI] [PubMed] [Google Scholar]
- 17. Fermo E, Bianchi P, Vercellati C, et al. Recessive hereditary methemoglobinemia: Two novel mutations in the NADH‐cytochrome b5 reductase gene. Blood Cells Mol Dis 2008;41:50–55. [DOI] [PubMed] [Google Scholar]
- 18. Hirano H, Matsuki T, Tanishima K, et al. Congenital methaemoglobinaemia due to NADH methaemoglobin reductase deficiency: Successful treatment with oral riboflavin. Brit J Haematol 1981;47:353–359. [DOI] [PubMed] [Google Scholar]
- 19. Papich MG. Methylene Blue 0.1%. In: Papich MG, ed. Saunders Handbook of Veterinary Drugs: Small and Large Animal, 4th ed St. Louis, MO: Elsevier Saunders; 2016: 514–516. [Google Scholar]
- 20. Harvey JW, Ling GV, Kaneko JJ. Methemoglobin reductase deficiency in a dog. J Am Vet Med Assoc 1974;164:1030–1033. [PubMed] [Google Scholar]
- 21. Letchworth GJ, Bentinck‐Smith J, Bolton GR, et al. Cyanosis and methemoglobinemia in two dogs due to NADH methemoglobin reductase deficiency. J Am Anim Hosp Assoc 1977;13:75–79. [Google Scholar]
- 22. Love L, Singer M. Anesthesia case of the month, Methemoglobinemia. J Am Vet Med Assoc 2013;242:753–756. [DOI] [PubMed] [Google Scholar]


