Abstract
Background
Inflammatory bowel disease (IBD) is common in dogs. Despite the known importance of intestinal lymphocytes in its pathogenesis, little is known about the role of peripheral blood lymphocytes (PBLs) in IBD.
Objectives
The aims of this study were (1) comparison of PBLs analyzed by flow cytometry (FCM) in IBD dogs and healthy controls and (2) comparison of PBLs in IBD dogs at the time of diagnosis and in dogs in clinical remission.
Animals
Whole blood samples of 19 IBD dogs at the time of diagnosis and blood samples of 6 dogs in clinical remission were collected. Ten healthy dogs served as controls.
Methods
In this prospective observational study, PBLs were analyzed with multicolor FCM by staining with a panel of anticanine and cross‐reactive monoclonal antibodies against T‐ and B‐cell differentiation antigens, including CD45, CD3, CD4, CD8α, CD8β, TCRαβ, TCRγδ, CD79αcy, and CD21.
Results
The IBD patients’ PBLs had significantly decreased percentages of TCRγδ+ T lymphocytes (median: healthy dogs, 3.32; IBD dogs, 0.97; P = 0.03) and CD21+ B cells (median: healthy dogs, 27.61; IBD dogs, 17.26; P = 0.04). There were no significant differences in PBLs between pretreatment and follow‐up samples.
Conclusions and Clinical Importance
The differences between PBLs in healthy and IBD dogs analyzed by FCM indicate an imbalance of lymphocytes with different immunologic functions and emphasize the potential value of this technique in a larger cohort of dogs. The PBLs did not differ between IBD dogs before treatment and clinically well‐controlled dogs after treatment.
Keywords: CD21 B cells, Flow cytometry, IBD, TCRγδ‐cells
Abbreviations
- CBC
complete blood count
- CD
cluster of differentiation
- CIBDAI
canine Inflammatory Bowel Disease Activity Index
- CrD
Crohn's disease
- EDTA
ethylenediaminetetraacetic acid disodium salt solution
- FCM
flow cytometry
- IBD
inflammatory bowel disease
- IEL
intraepithelial lymphocytes
- LPE
lymphocytic‐plasmacytic enteritis
- mAb
monoclonal antibody
- PBL
peripheral blood lymphocyte
- PBS
phosphate‐buffered saline
- RBC
red blood cell
- TCR
T‐cell receptor
- UC
ulcerative colitis
- WSAVA
World Small Animal Veterinary Association
Canine inflammatory bowel disease (IBD) is a common cause for chronic or relapsing gastrointestinal signs such as diarrhea, vomiting, and weight loss.1 Canine IBD is diagnosed by exclusion of infectious, endocrine, and neoplastic causes for gastrointestinal signs and by histologic evidence of intestinal mucosal inflammation.2 Canine IBD can be classified histopathologically, based on the predominant type of inflammatory cells in the intestinal mucosa.3 Lymphocytic‐plasmacytic enteritis (LPE) is the most commonly described form of canine IBD.1, 2, 3 Using immunohistochemistry, increased numbers of intestinal mucosal CD3+ T cells,4, 5, 6 plasma cells,4, 5 and B cells6 have been documented in dogs with IBD. One recent study using flow cytometry (FCM) for characterization of canine intestinal intraepithelial lymphocytes (IEL) reported increased numbers of TCRγδ+ T cells in dogs with IBD.7 In contrast, in a previous study using immunohistochemistry, decreased numbers of CD3+ and immunoglobulin G‐secreting plasma cells were reported in mucosal samples of IBD dogs.8
These studies point toward an important role of intestinal lymphocytes in canine IBD. Flow cytometry analysis of immune cells could improve this understanding of the canine intestinal immune system and its dysregulation in disease states. The intestinal inflammatory processes may be accompanied by changes in the peripheral lymphocyte populations of affected dogs, which may provide a noninvasive opportunity for the identification of IBD dogs and the monitoring of therapeutic responses. A previous study, comparing selected lymphocyte subsets in the peripheral blood of healthy dogs and dogs with chronic enteropathies using FCM, showed no significant differences between the investigated groups after labeling with antibodies against CD3, CD4, CD8, CD21, and MHC‐II.9
The purpose of our study was the phenotypic analysis and comparison of PBLs in healthy dogs and dogs with IBD by FCM with an extensive multicolor antibody panel. The hypothesis was that dogs with IBD would have altered distributions of their PBL subpopulations. This could reflect a dysregulated immune function contributing to the development of the disease, or occur as consequence of the inflammatory character of canine IBD. Furthermore, we aimed to compare PBL subpopulations in IBD dogs before treatment with dogs in clinical remission after treatment, to investigate FCM as an objective diagnostic tool in the follow‐up for IBD dogs.
Materials and Methods
Ethical Approvals
The study was approved by the Institutional Ethics Committee and the Advisory Committee for Animal Experiments (§12 of Law for Animal Experiments, Tierversuchsgesetz [TVG]) and the Federal Ministry of Research and Science (bmwf: GZ 68.205/201‐II/3b/2010).
Owners signed a written consent form to take part in the study.
Animals
Client‐owned dogs (n = 19) with IBD, presented to the Clinic for Internal Medicine and Infectious Diseases of the University of Veterinary Medicine Vienna, Austria, between September 2011 and November 2013 were prospectively enrolled. Inclusion criteria were a history of ≥2 gastrointestinal signs comprising vomiting, diarrhea, anorexia, or weight loss for at least a 4‐week duration, thorough diagnostic evaluation to eliminate other possible causes for gastrointestinal signs and gastroduodenoscopy (including histopathologic examination of duodenal biopsy samples) to confirm intestinal lymphocytic‐plasmacytic infiltration. Diagnostic tests performed to exclude infectious, endocrine, or neoplastic diseases included a CBC, serum biochemical profile, assessment of serum concentration of trypsin‐like immunoreactivity and cortisol concentrations, analysis of fecal samples by flotation, fecal Giardia antigen test, and abdominal ultrasonography. Dogs with other possible causes for inflammatory intestinal changes and dogs with a history of administration of immunosuppressive drugs, antibiotics, or both 10 days before blood sampling were excluded. The diagnosis of IBD was based on the chronicity of gastrointestinal signs, the exclusion of underlying infectious, endocrine or neoplastic diseases, and the intestinal histopathologic inflammatory findings and immunophenotypic analysis of PBLs by FCM were performed. In clinically well‐controlled dogs, defined as having normal activity level and no ongoing gastrointestinal signs as reported by the owners, immunophenotypic FCM analysis of PBLs was repeated after treatment.
The control group (n = 10) consisted of staff‐owned dogs, without any history of gastrointestinal signs. They were not receiving any medication and were clinically healthy as verified by physical and hematologic examination and blood biochemistry.
For FCM analysis, blood samples were taken from the jugular vein of each dog. Blood collected in EDTA was used for white blood cell countsa and immunophenotypic analysis of PBLs using 3‐color FCM.
Clinical and Histopathologic Scoring
The IBD dogs were clinically scored according to the canine IBD activity index (CIBDAI).10 Intestinal biopsies of the IBD dogs were graded by 1 independent board‐certified pathologist according to the World Small Animal Veterinary Association (WSAVA) International Gastrointestinal Standardization Group guidelines.2 As a result of suboptimal orientation of the mucosa in the tissue blocks in a number of samples, the morphologic criterion of villus stunting was not taken into account. Therefore #bib4 morphologic variables (epithelial injury, crypt distension, lacteal dilatation, and mucosal fibrosis) and 4 inflammatory histologic variables (intraepithelial lymphocytes, lamina propria lymphocytes and plasma cells, lamina propria eosinophils, and lamina propria neutrophils) were scored as normal (0) or showing mild (1), moderate (2), or marked (3) changes. Based on the sum of the scores, dogs were subdivided into histologic severity groups: WSAVA score of 0 = normal #bib13 #bib14 #bib15 = mild #bib13 #bib14 #bib15 = moderate #bib13 #bib14 #bib15 = severe, and >18 = very severe IBD.11
Flow Cytometry
For phenotypic characterization, multicolor analyses of PBLs were performed with anticanine‐specific and anti‐human cross‐reactive monoclonal antibodies (mAb) against CD45,b CD21,c CD79αcy,d CD3,b TCRαβ,e TCRγδ,e CD4,b CD8α,b and CD8β,b listed in Table 1. Blood samples were diluted 1:1 with phosphate‐buffered saline (PBS),f without Ca2+ an Mg2+, incubated with primary mAbs for 20 minutes at room temperature either for double or triple labeling of the PBLs (combinations of mAbs against CD21/CD45; CD79αcy/CD3; TCRαβ/CD8α/CD4; TCRαβ/CD8α/CD8β; CD8β/TCRγδ/CD8α) and washed with PBS. Samples containing mAb without directly conjugated fluorochromes were labeled with anti‐mouse secondary antibodies and incubated for an additional 20 minutes at room temperature (Table 1). To lyse red blood cells, a commercially available red blood cell (RBC) lysing solution (ADG‐lyse)g was used according to the manufacturer's instructions. The mAbs used for identification of CD3 and CD79αcy recognize intracellular epitopes. Thus, to enable intracellular staining, the IntraStain‐Kitd was used according to manufacturer's instructions in samples stained with these antibodies. Appropriate isotype controls were used for each sample (Table 1). After the last incubation step, cells were washed again and subsequently analyzed with a FACS Canto II flow cytometer.c
Table 1.
List of canine‐specific and anti‐human cross‐reactive mAbs used for FCM analysis of canine PBLs
| mAb | Clone | Isotype | Fluorescence Labeling | Target Species/Species Cross‐Reactivitya |
|---|---|---|---|---|
| CD45 | YKIX716.13 | rIgG2b | APC | Anti‐canine or Antihuman |
| CD3 | CD3‐12 | rIgG1 | FITC | Anti‐humana |
| CD4 | YKIX302.9 | rIgG2a | APC | Anticanine |
| CD8α | YCATE 55.9 | rIgG1 | PE | Anticanine |
| CD8β | CA15.4G2 | mIgG1 | α‐mIgG1‐AlexaFluor647b , c | Anticanine |
| CD21 | B‐ly‐4 | mIgG1 | APC | Anti‐human37 |
| CD79αcy | HM57 | mIgG1 | PE | Anti‐human38 |
| TCRαβ | CA15.8G7 | mIgG1 | α‐mIgG1‐AlexaFluor488b , d | Anticanine |
| TCRγδ | CA20.8H1 | mIgG2a | α‐mIgG2a‐FITCb , e | Anticanine |
mAb, monoclonal antibodies; CD, cluster of differentiation; IgG, immunoglobulin G; TCR, T‐cell receptor; m, mouse; α‐m, anti‐mouse; r, rat; FITC, fluorescein isothiocyanate; APC, allophycocyanin; PE, phycoerythrin; a, cross‐reactivity established by: CD3‐12, Serotec, technical datasheet MCA1477, CD21, see reference 37, CD79, see reference 38; b, fluorescence labeling was achieved by use of a secondary antibody; c, goat anti‐mouse IgG1‐Alexa647; Life Technologies, Carlsbad, CA; d, goat anti‐mouse IgG1‐Alexa488; Life Technologies, Carlsbad, CA; e, goat F(ab)2 anti‐mouse IgG2a‐FITC; SouthernBiotech, Birmingham, AL.
The lymphocyte population was gated based on relative size and granularity using forward and sideward scatter properties to exclude other cell populations and cell debris (Figs 1 and 2). For each sample, data of at least 10.000 events in the lymphocyte gate were recorded. Data analysis was performed by the FACS Diva software, version 6.1.3.c The results of the CD45+ cell populations were expressed as percentage of positive cells within the lymphocyte gate. The results of the remaining populations finally were normalized by calculating the percentages of the subpopulations positive for the cell surface markers within the CD45+ cell population.12
Figure 1.
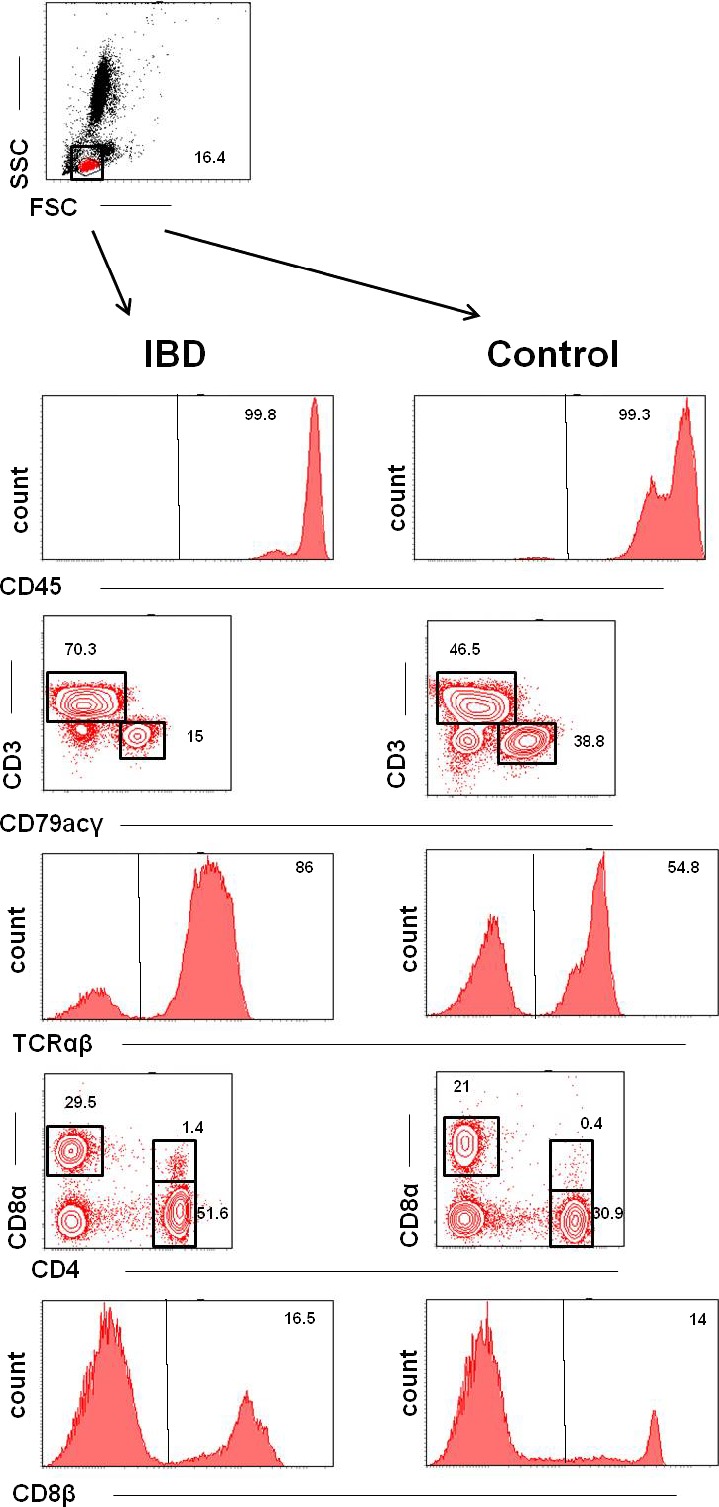
Phenotypes of PBLs in dogs with IBD (left) and healthy control dogs (right). Both columns are representative examples of the respective dog groups. Data are presented by histograms showing the expressions of 1 parameter per histogram and by contour plots showing percentages of cells with various properties. Top row: Representative dot blot showing the forward/sideward scatter (FSC/SSC) properties of the analyzed PBLs gating the lymphocyte population (red). Rows 2 #bib4, and 6 show representative histograms of CD45, TCRαβ, and CD8β expressions with negative cells on the left and positive cells on the right side of the graphs. The percentages of the respective positive populations are indicated by the numbers in the right upper corners of the graphs. Row 3 shows representative contour plots of gated cells labeled with mAb against CD3 and CD79. CD3+ cells are displayed in the upper left corner; CD79αcγ+ cells are displayed in the lower right corner of the graphs. The percentages of the respective positive populations are indicated by the adjacent numbers. Row 5 shows representative contour plots of gated cells labeled with mAb against CD8α and CD4. CD8α+ cells are displayed in the upper left corner, and CD4+ cells are displayed in the lower right corner of the contour plot. There is a small population of double‐positive cells (CD4+ CD8+) displayed in the right upper corner of the graphs. The percentages of the respective positive populations are indicated by the adjacent numbers.
Figure 2.
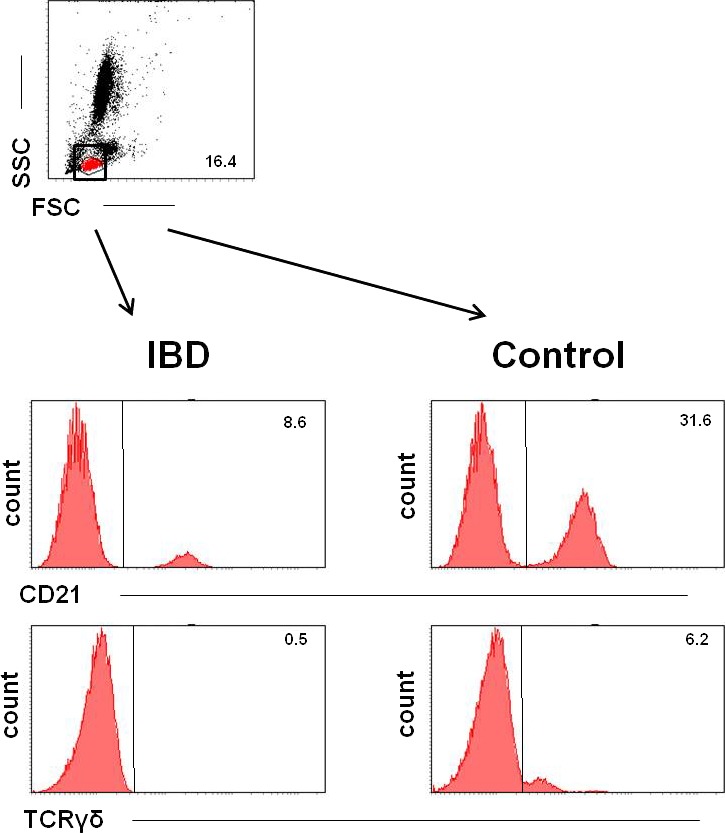
Representative FCM analyses of PBLs in dogs with IBD (left) and healthy control dogs (right). Data are presented by histograms showing the expressions of 1 parameter per histogram. Top row: Representative dot blot showing the forward/sideward scatter (FSC/SSC) properties of the analyzed PBLs gating the lymphocyte population (red). Rows 2 and 3 show representative histograms of TCRγδ and CD21 expressions with negative cells on the left and positive cells on the right side of the graphs. The percentages of the respective positive populations are indicated by the number in the right upper corner of the graphs, IBD dogs, and healthy control dogs showing significant differences in these subpopulations between groups.
Statistical Analysis
Sex of healthy dogs and dogs with IBD were compared by a chi‐square test. Age and body weight of diseased dogs and healthy control dogs were tested for normal distribution by the Shapiro‐Wilk test and were consecutively compared by Student's t‐test. After testing percentages of PBL subpopulations for normal distribution by the Shapiro‐Wilk test, data obtained from dogs with IBD were compared to data of healthy dogs by use of Student's t‐test in the case of normal distribution or by Mann–Whitney U‐test in case of non‐normally distributed data. The FCM data of dogs before therapy were compared to data of dogs receiving treatment after testing for normal distribution by use of the Shapiro‐Wilk test. Normally distributed data were analyzed with a paired Student's t‐test and non‐normally distributed data were compared by the Wilcoxon‐signed rank test. P‐values <0.05 were considered significant. All statistical analyses were performed by SPSS version 15.h
Results
Animal Characteristics
IBD Group
The IBD group (n = 19) included 10 female (8 spayed) and 9 male neutered dogs of different breeds. Crossbreeds (n = 4) and Boxers (n = 2) were most common. The mean age was 5.1 years (±2.8 years), and mean body weight was 21.9 kg (±12.9 kg). The major reason for presentation was diarrhea (n = 19) followed by vomiting (n = 7) and weight loss (n = 5). Five dogs were pretreated for their gastrointestinal signs until 10 days before sampling. Of these 5 dogs #bib3 were treated with antibiotics (1 with amoxicillin/clavulanic acid #bib1 with metronidazole, and 1 with tylosin) and 2 dogs were treated with prednisolone. The diseased dogs were suffering from moderate‐to‐severe IBD with a mean CIBDAI score of 6.6 (±3.1). They were classified as having lymphoplasmacytic IBD with WSAVA scores of intestinal tissue samples ranging from 1 to 6 (median #bib2) corresponding to mild histopathologic changes.
Follow‐up IBD Group
Six clinically well‐controlled IBD dogs were available for re‐evaluation of PBLs after treatment. Five dogs were not controlled satisfactorily. In 3 dogs, follow‐up was lost, and the owners of 5 dogs in clinical remission did not want to participate further in the study. Five dogs were resampled after 2 months, and in 1 dog, resampling was done 11 months postdiagnosis. The group of re‐evaluated dogs included 3 female spayed and 3 male neutered dogs of different breeds. Five dogs were resampled after 2 months, and in 1 dog, resampling was done 11 months postdiagnosis. The dogs were between 3 and 11 years old (median #bib5.5 years) and had body weights between 14.1 and 58 kg (median, 26 kg). At the time of presentation, CIBDAI scores of the affected dogs ranged from 4 to 10 (median, 5.5) and WSAVA scores from 1 to 5 (median, 2.5). Two dogs were clinically well controlled receiving a hydrolyzed (n = 1) or single protein (n = 1) hypoallergenic diet and 4 dogs by administration of corticosteroids. The CIBDAI scores had improved in all 6 dogs and ranged from 0 to 2 (median, 1) after treatment.
Healthy Control Group
The control group (n = 10) consisted of 7 female (4 spayed) and 3 male dogs of various breeds. Flat‐coated retrievers (n = 2) and Crossbreeds (n = 3) were most common. The mean age of the dogs was 4.2 (±2.6 years). The mean body weight was 25.1 kg (±11.2 kg). Sex, age, and body weight did not differ significantly between healthy and IBD dogs.
Immunophenotyping by Flow Cytometry
Representative examples of lymphocyte subpopulations in IBD dogs and healthy control dogs are shown in Figures 1 and 2. Summarized data including median, mean, standard deviation, as well as minimum and maximum values of all dogs, are shown in Tables 2 and 3.
Table 2.
FCM analyses of PBL subpopulations presented in percentages of CD45+ lymphocytes of healthy dogs and IBD dogs at initial diagnosis
| Median | Mean | ±SD | Min. | Max. | ||
|---|---|---|---|---|---|---|
| CD45+ | Healthy dogs | 98.65 | 98.12 | ±1.55 | 94.90 | 99.70 |
| IBD dogs | 98.00 | 96.17 | ±6.07 | 73.20 | 99.60 | |
| CD3+ | Healthy dogs | 65.69 | 66.46 | ±9.24 | 51.46 | 79.31 |
| IBD dogs | 68.95 | 69.69 | ±13.33 | 39.10 | 95.86 | |
| CD4+ | Healthy dogs | 42.42 | 42.69 | ±8.58 | 30.51 | 56.22 |
| IBD dogs | 42.02 | 42.30 | ±10.88 | 34.31 | 78.70 | |
| CD21+ a | Healthy dogs | 27.61 | 24.40 | ±11.46 | 8.17 | 40.89 |
| IBD dogs | 17.26 | 17.33 | ±7.59 | 3.28 | 27.31 | |
| CD8α+ | Healthy dogs | 19.88 | 20.68 | ±9.89 | 11.74 | 45.40 |
| IBD dogs | 20.24 | 19.47 | ±6.38 | 8.66 | 29.08 | |
| CD8β+ | Healthy dogs | 16.88 | 15.33 | ±6.35 | 8.64 | 32.70 |
| IBD dogs | 15.37 | 17.60 | ±13.45 | 3.08 | 65.58 | |
| CD79αcγ+ | Healthy dogs | 30.28 | 29.19 | ±10.96 | 14.24 | 46.15 |
| IBD dogs | 22.93 | 21.67 | ±9.82 | 0.93 | 38.71 | |
| TCRαβ+ | Healthy dogs | 66.72 | 66.01 | ±11.63 | 29.89 | 86.61 |
| IBD dogs | 68.69 | 70.56 | ±11.15 | 52.97 | 88.23 | |
| TCRγδ+ a | Healthy dogs | 3.32 | 4.25 | ±3.64 | 0.10 | 9.88 |
| IBD dogs | 0.97 | 1.17 | ±0.82 | 0.20 | 3.12 | |
| CD4+CD8+ | Healthy dogs | 0.52 | 0.53 | ±0.28 | 0.11 | 0.92 |
| IBD dogs | 0.71 | 1.04 | ±1.39 | 0.14 | 6.25 |
PBLs, peripheral blood lymphocytes; FCM, flow cytometry; IBD, inflammatory bowel disease; CD, Cluster of differentiation; TCR, T‐cell receptor; SD, standard deviation; min., minimum; max., maximum.
Significant differences compared to healthy dogs.
Table 3.
FCM analyses of PBL subpopulations presented in percentages of CD45+ lymphocytes of 6 IBD dogs at the time of diagnosis and after treatment
| Median | Mean | ±SD | Min. | Max. | ||
|---|---|---|---|---|---|---|
| CD45+ | Pretreatment | 98.45 | 97.87 | ±1.71 | 95.40 | 99.60 |
| Post‐treatment | 95.50 | 93.90 | ±5.86 | 84.20 | 99.30 | |
| CD3+ | Pretreatment | 75.80 | 68.45 | ±21.36 | 37.30 | 84.90 |
| Post‐treatment | 70.72 | 68.12 | ±8.56 | 54.16 | 75.77 | |
| CD4+ | Pretreatment | 50.35 | 47.98 | ±6.55 | 37.60 | 54.70 |
| Post‐treatment | 45.46 | 47.01 | ±8.23 | 38.23 | 59.55 | |
| CD21+ | Pretreatment | 15.90 | 16.00 | ±6.98 | 8.30 | 23.90 |
| Post‐treatment | 16.18 | 16.73 | ±10.43 | 3.66 | 33.25 | |
| CD8α+ | Pretreatment | 18.43 | 19.06 | ±7.72 | 10.67 | 27.83 |
| Post‐treatment | 21.50 | 20.04 | ±11.29 | 4.67 | 32.43 | |
| CD8β+ | Pretreatment | 16.10 | 15.67 | ±8.01 | 4.40 | 26.60 |
| Post‐treatment | 13.22 | 13.69 | ±8.80 | 4.21 | 23.80 | |
| CD79αcγ+ | Pretreatment | 23.30 | 21.02 | ±7.22 | 11.70 | 28.50 |
| Post‐treatment | 20.97 | 24.18 | ±12.29 | 12.81 | 41.92 | |
| TCRαβ+ | Pretreatment | 79.30 | 74.93 | ±13.07 | 56.20 | 87.70 |
| Post‐treatment | 77.80 | 74.58 | ±15.64 | 52.56 | 91.68 | |
| TCRγδ+ | Pretreatment | 0.50 | 0.70 | ±0.63 | 0.20 | 1.80 |
| Post‐treatment | 1.94 | 1.61 | ±0.81 | 0.59 | 2.33 | |
| CD4+CD8+ | Pretreatment | 0.80 | 0.85 | ±0.61 | 0.20 | 1.70 |
| Post‐treatment | 0.41 | 0.47 | ±0.33 | 0.12 | 0.92 |
PBLs, peripheral blood lymphocytes; FCM, flow cytometry; IBD, inflammatory bowel disease; CD, cluster of differentiation; TCR, T‐cell receptor; SD, standard deviation; min., minimum; max., maximum.
In both healthy and IBD dogs, almost 100% of the cells in the lymphocyte gate were CD45+. In all groups, the proportion of T cells (CD3+) was higher than the B cells (CD21+, CD79αcy+) proportion. The majority of T cells (CD3+) were T helper cells (CD4+), whereas cytotoxic T cells (CD8+) were detected in lower numbers. Most T cells expressed TCRαβ, whereas only a small percentage expressed TCRγδ. A very small percentage of cells were CD4+CD8+ double‐positive (Fig 1, Tables 2 and 3).
The percentages of TCRγδ+ cells (median: healthy dogs, 3.32; IBD dogs, 0.97; P = 0.03) and CD21+ B cells (median: healthy dogs, 27.61; IBD dogs, 17.26; P = 0.04) were significantly lower in dogs with IBD compared to healthy dogs (Figs 2, 3, 4, Table 2). None of the other subpopulations positive for CD45 (P = 0.33), CD3 (P = 0.63), CD4 (P = 0.44), CD79αcy+ (P = 0.07), CD8α (P = 0.91), CD8β (P = 0.86), TCRαβ (P = 0.31), CD4, and CD8 (P = 0.86) showed significant differences between dogs with IBD and healthy controls (Table 2).
Figure 3.
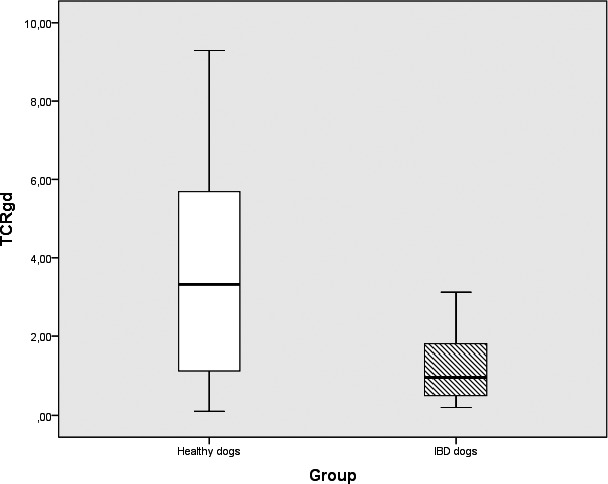
Box plots comparing percentages of TCRγδ expression in IBD (n = 19) and healthy control dogs (n = 10). PBLs in IBD dogs and healthy control dogs showing significant differences in the subpopulations between groups (P = 0.026). Box plots represent the 25–75th percentile; the median is shown as the heavy dark horizontal line. Vertical lines extend to the minimum and maximum values.
Figure 4.
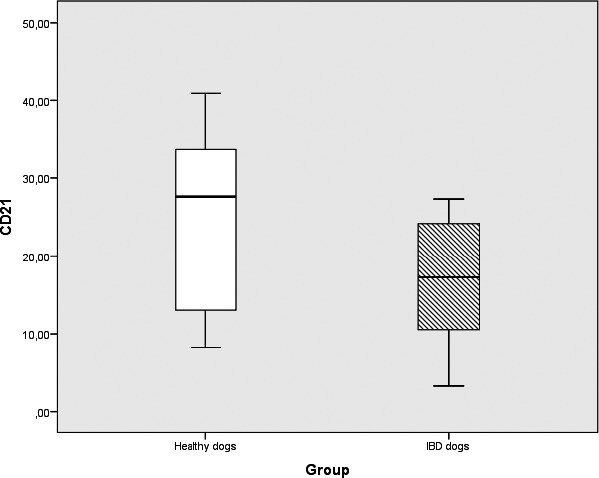
Box plots comparing percentages of CD21+ PBLs in IBD dogs (n = 19) and healthy control dogs (n = 10) showing significant differences in the subpopulations between groups (P = 0.04). Box plots represent the 25–75th percentile; the median is shown as the heavy dark horizontal line. Vertical lines extend to the minimum and maximum values.
Furthermore, there was no statistical difference in the TCRγδ+ cell (P = 0.09) and CD21+ cell (P = 0.88) proportions or in any other PBL subpopulations between IBD dogs before and after treatment (Table 3, other P‐values not shown).
Discussion
Our study was performed to compare lymphocyte subpopulations of canine PBLs in healthy dogs and dogs with IBD and indicated that the TCRγδ+ and CD21+ subpopulations differed significantly between these 2 groups. Treatment did not change the lymphocyte proportions significantly.
Different PBL subsets have been phenotypically analyzed before in healthy dogs9, 13, 14, 15, 16 and in dogs with chronic enteropathies.9, 17 We aimed to recruit control dogs of similar age and sex for our study because the proportion of CD21+ B cells decreases whereas the percentage of CD8+ cells increases with advancing age, and sex may have an influence on lymphocyte subpopulations.13 Furthermore, both groups included dogs of a range of breeds in an attempt to avoid potential breed‐associated differences in lymphocyte distribution.13 The results of our study, showing CD3+ T cells as the major population and a lower number of CD21+, respectively, CD79αcy+ B cells in canine peripheral blood, as well as the higher expression of CD4 rather than CD8, mirrored previous results in dogs.9, 13, 14, 15, 17 However, in the dogs of the healthy control group, percentages of CD21+ B cells were higher and those of CD3+ T cells were slightly lower, compared to previous studies.13, 14, 15, 17 We found a small subpopulation of double‐positive CD4+CD8+ PBLs confirming results of an earlier study,16 in which canine CD4+CD8+ T cells were identified to be activated effector/memory cells. T cells expressing TCRαβ clearly outnumbered cells expressing TCRγδ, mirroring results of previous studies.13, 14, 15, 17 The γδ T cells are preferentially localized in epithelial tissues, and a substantial proportion of unconventional TCRγδ+ T cells have been observed in the canine IEL pool,7, 12 although they normally comprise only a small fraction of circulating T lymphocytes.13, 14, 15, 17
In our study, IBD dogs had lower percentages of CD21+ and TCRγδ+ PBLs compared with the healthy control group. These results are in contrast with a study that indicated no differences in the peripheral CD21+ subset of dogs with chronic gastrointestinal disease compared to healthy dogs.9 Data comparing circulating γδ T cells in IBD dogs and healthy dogs are lacking. In humans with IBD, results regarding peripheral γδ T cells are contradictory. A previous study identified increased numbers of peripheral γδ T cells in patients with active Crohn′s disease (CrD) and ulcerative colitis (UC), but not in patients in remission.18 Two further studies identified increased circulating γδ T cells in patients with CrD, but not in patients with UC.19, 20 However, in a more recent study, a peripheral γδ T cell deficiency in CrD patients with active and quiescent disease was observed.21 The reason for the partly discordant results compared to our study is not clear. It may lie in the heterogeneity of the syndromes classified under the umbrella term IBD.1, 22 Differences in genetic background, environmental factors, intestinal inflammatory profiles as well as their location and severity, the influence of treatment or the phase of disease may have had an impact on the observed results.
Although canine IBD shares many features with human IBD, there are several species‐specific differences,1, 22 which additionally could account for the discordant results regarding the γδ T cells of our study, compared to studies of humans.
In addition, the lower percentages of CD21+ B cells in our IBD dogs of this study mirrored the results of previous studies in healthy dogs13, 14, 15, 17 more closely than the higher percentages obtained in the healthy control group. Similarly, the very low proportion of PBLs expressing TCRγδ in the IBD group was comparable to previous results in healthy dogs, whereas the obtained values in the healthy control group were slightly higher.13, 14, 15 Furthermore, we observed a large range in the γδ T cell proportions, especially in the healthy control group, as shown by the minimum and maximum values as well as the standard deviation of this variable (Table 2). Individual biologic variation caused by regional differences and variables such as breed, nutrition, and environmental factors may have contributed to these results, especially considering the small sample size.
However, the differences in PBL subsets detected in IBD dogs in our study may have occurred as a consequence of disease or the lower proportions of TCRγδ+ and CD21+ PBLs in IBD dogs could represent the cause rather than effect of the disease. Decreased proportions of CD21+ cells and γδ T cells may reflect dysregulation of immune function, particularly considering the proposed regulatory and protective function of γδ T cells, and may therefore play a role in the pathogenesis of canine IBD.
As already mentioned, γδ T cells are a substantial proportion of the canine IEL pool.7, 12 In an animal colitis model, depletion of γδ T cells resulted in significantly increased mortality and histologically more severe colitis compared to controls.23 Similarly, in murine IBD models, depletion or deficiency of γδ T cells aggravated intestinal inflammation, suggesting an important regulatory and protective function of γδ T cells, especially in the early phase of intestinal inflammation.24 Furthermore, transferred γδ T cells acted protectively on experimentally induced colitis in mice.25 Considering the proposed regulatory and protective function of γδ T cells, the observed lower proportions in IBD dogs in our study may reflect dysregulation of immune function, predisposing these dogs to the development of IBD.
Alternatively, γδ T cells also seem to have proinflammatory properties, because IL‐17‐producing TCRγδ+ cells were able to induce colitis in murine IBD models.26 Furthermore, in human IBD patients, a direct correlation between numbers of TCRγδ+ cells in the intestinal mucosa and disease severity has been demonstrated,27, 28 and a previous study identified increased numbers of TCRγδ+ IELs in dogs with IBD compared to healthy controls.7 It is still not clear whether lymphocyte precursors migrate to the epithelium and become γδ T cells in situ or whether activated γδ T cells migrate to the gut epithelium as a result of a specific tropism, suggesting an extrathymic or thymic differentiation of these cells, respectively.29 In the latter case, homing of activated γδ T cells to the IEL pool could cause alterations in the PBL pool. With activation and clonal expression, an increase of peripheral γδ T cells could be expected, but with ongoing migration to the gut epithelium, decreased γδ T cells could be encountered in the peripheral blood. Likewise, decreased percentages in the CD21+ PBL subpopulation could be caused by increased homing of CD21+ cells to the inflamed intestinal lamina propria in IBD dogs. The CD21 molecule is expressed on relatively mature B cells,30 whereas CD79αcγ is expressed from before the pre‐B‐cell stage to the plasma cell differentiation stage;31 thus, different stages of maturation may explain the significant difference encountered in the CD21+ subpopulation but not in CD79αcγ+ cells between dogs with IBD and healthy controls in our study.
Alterations in PBL subsets reflecting disease activity would be useful to monitor therapeutic responses in dogs with IBD. However, although TCRγδ+ cells increased in treated IBD dogs in our study, no significant changes were observed in the proportions of TCRγδ+ cells, CD21+ cells, or any other PBL subsets during treatment of IBD despite clinical improvement of affected dogs. Previous studies have shown that the histopathologic lesions in intestinal biopsy specimens of IBD dogs did not change despite clinical improvement with treatment.32, 33, 34, 35 Furthermore, no difference was observed in the numbers of intestinal mucosal CD3+ cells35, 36 and immunoglobulin A+ cells36 in intestinal biopsy specimens of dogs with chronic enteropathies taken before treatment and in clinically well‐controlled dogs. These results suggest an ongoing immune cell dysregulation despite an improved clinical state in affected dogs. However, we did not reassess small intestinal biopsy specimens in the treated IBD dogs in our study, and we can only speculate on the intestinal histopathologic appearance. Other explanations for the lack of differences between pre‐ and post‐treatment FCM results in our study may lie in the small sample size of reassessed dogs and the time frame chosen for reassessment, which may have been insufficient to revert immune system dysfunction.
Although examination of PBLs may help identify alterations in the immune status and may give rise to a better understanding of the immunopathogenesis of canine IBD, caution must be taken in the interpretation of these results because the behavior of PBLs does not necessarily represent the ongoing processes in the inflamed mucosa, and the composition of PBL subpopulations can be influenced by many factors. The possibility that the observed decrease in CD21+ B cells and TCR γδ+ T cells in our study is associated with rapid migration of peripheral lymphocytes to the gut raises the question of whether or not these results are specific to IBD. Other acute or chronic conditions causing intestinal inflammation or epithelial injury may share the observed alterations in PBLs. Corresponding data from dogs with different gastrointestinal diseases are lacking. In addition, pretreatment in 5 IBD dogs in our study might have influenced our results. A treatment‐free period of at least 10 days was an inclusion criterion. However, this time frame might not have been sufficient to allow any effects of therapy on lymphocyte subpopulations to subside in these dogs. Furthermore, the small sample size, especially considering the dogs evaluated pre‐ and post‐treatment, is a limiting factor for the clinical relevance of these results.
In conclusion, canine IBD seems to be associated with a different distribution of PBLs, possibly playing a role in the pathogenesis of canine IBD or reflecting a consequence of disease. Additional work in a larger cohort of dogs combining FCM results of PBLs with FCM results of lamina propria and IEL lymphocytes in similar dogs would be of interest. In addition, the sampling of IBD dogs at different stages of disease and repeated FCM analysis of PBLs in treated dogs at different time points during follow‐up could shed more light on the role of the intestinal immune system and its relationship with PBLs in canine IBD. Furthermore, it would be important to include control groups of dogs with different gastrointestinal disorders to determine the specificity of altered lymphocyte subsets in various gastrointestinal diseases.
Acknowledgments
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
The study was funded by the Start‐up grant of the Vetmeduni Vienna (project number PP13010109; title “T‐cell subpopulations in canine chronic enteropathies”).
Parts of these data have been presented as an abstract at the 2012 American College of Veterinary Internal Medicine Forum, New Orleans, Louisiana.
Footnotes
aADVIA 120, Siemens, Germany
bBio‐Rad AbD Serotec, Raleigh, NC
cBD Biosciences, San Jose, CA
dDako, Glostrup, Denmark
ePeter F. Moore, California, CA
fPAA, Pasching, Austria
gADG‐lyse, An der Grub, Bio Research, Kaumberg, Austria
hIBM Cooperation, Armonk, NY
References
- 1. Craven M, Simpson JW, Ridyard AE, Chandler ML. Canine inflammatory bowel disease: Retrospective analysis of diagnosis and outcome in 80 cases (1995–2002). J Small Anim Pract 2004;45:336–342. [DOI] [PubMed] [Google Scholar]
- 2. Washabau RJ, Day MJ, Willard MD, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010;24:10–26. [DOI] [PubMed] [Google Scholar]
- 3. German AJ, Hall EJ, Day MJ. Chronic intestinal inflammation and intestinal disease in dogs. J Vet Intern Med 2003;17:8–20. [DOI] [PubMed] [Google Scholar]
- 4. German AJ, Hall EJ, Day MJ. Immune cell populations within the duodenal mucosa of dogs with enteropathies. J Vet Intern Med 2001;15:14–25. [DOI] [PubMed] [Google Scholar]
- 5. Jergens AE, Gamet Y, Moore FM, et al. Colonic lymphocyte and plasma cell populations in dogs with lymphocytic‐plasmacytic colitis. Am J Vet Res 1999;60:515–520. [PubMed] [Google Scholar]
- 6. Stonehewer J, Simpson JW, Else RW, Macintyre N. Evaluation of B and T lymphocytes and plasma cells in colonic mucosa from healthy dogs and from dogs with inflammatory bowel disease. Res Vet Sci 1998;65:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haas E, Rütgen BC, Gerner W, et al. Phenotypic characterization of canine intestinal intraepithelial lymphocytes in dogs with inflammatory bowel disease. J Vet Intern Med 2014;28:1708–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jergens AE, Moore FM, Kaiser MS, et al. Morphometric evaluation of immunoglobulin A‐containing and immunoglobulin G‐containing cells and T cells in duodenal mucosa from healthy dogs and from dogs with inflammatory bowel disease or nonspecific gastroenteritis. Am J Vet Res 1996;57:697–704. [PubMed] [Google Scholar]
- 9. García‐Sancho M, Villaescusa A, Rodríguez‐Franco F, Sainz A. Comparative study of peripheral blood leukocytes in healthy dogs and in dogs with cancer and inflammatory diseases. J Vet Diagn Invest 2014;26:282–285. [DOI] [PubMed] [Google Scholar]
- 10. Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med 2003;17:291–297. [DOI] [PubMed] [Google Scholar]
- 11. Procoli F, Motskula PF, Keyte SV, et al. Comparison of histopathologic findings in duodenal and ileal endoscopic biopsies in dogs with chronic small intestinal enteropathies. J Vet Intern Med 2013;27:268–274. [DOI] [PubMed] [Google Scholar]
- 12. Luckschander N, Pfammatter NS, Sidler D, et al. Phenotyping, functional characterization, and developmental changes in canine intestinal intraepithelial lymphocytes. Vet Res 2009;40:58. [DOI] [PubMed] [Google Scholar]
- 13. Faldyna M, Levá L, Knötigová P, Toman M. Lymphocyte subsets in peripheral blood of dogs – a flow cytometric study. Vet Immunol Immunopathol 2001;82:23–37. [DOI] [PubMed] [Google Scholar]
- 14. Platt R, Ng T, Glover S, et al. Canine peripheral blood lymphocyte phenotyping by 7‐color multiparameter flow cytometry. Anal Quant Cytopathol Histpathol 2013;35:197–204. [PubMed] [Google Scholar]
- 15. Gauthier MJ, Aubert I, Abrams‐Ogg A, et al. The immunophenotype of peripheral blood lymphocytes in clinically healthy dogs and dogs with lymphoma in remission. J Vet Intern Med 2005;19:193–199. [DOI] [PubMed] [Google Scholar]
- 16. Bismarck D, Schütze N, Moore P, et al. Canine CD4+ CD8+ double positive T cells in peripheral blood have features of activated T cells. Vet Immunol Immunopathol 2012;149:157–166. [DOI] [PubMed] [Google Scholar]
- 17. Maeda S, Ohno K, Nakamura K, et al. Increased expression of fractalkine and its receptor CX3CR1 in canine inflammatory bowel disease and their possible role in recruitment of intraepithelial lymphocytes. Vet Immunol Immunopathol 2012;148:226–235. [DOI] [PubMed] [Google Scholar]
- 18. Giacomelli R, Parzanese I, Frieri G, et al. Increase of circulating gamma/delta T lymphocytes in the peripheral blood of patients affected by active inflammatory bowel disease. Clin Exp Immunol 1994;98:83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bucht A, Söderström K, Esin S, et al. Analysis of gamma delta V region usage in normal and diseased human intestinal biopsies and peripheral blood by polymerase chain reaction (PCR) and flow cytometry. Clin Exp Immunol 1995;99:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Söderström K, Bucht A, Halapi E, et al. Increased frequency of abnormal gamma delta T cells in blood of patients with inflammatory bowel diseases. J Immunol 1996;156:2331–2339. [PubMed] [Google Scholar]
- 21. Andreu‐Ballester JC, Amigó‐García V, Catalán‐Serra I, et al. Deficit of gammadelta T lymphocytes in the peripheral blood of patients with Crohn's disease. Dig Dis Sci 2011;56:2613–2622. [DOI] [PubMed] [Google Scholar]
- 22. Cerquetella M, Spaterna A, Laus F, et al. Inflammatory bowel disease in the dog: Differences and similarities with humans. World J Gastroenterol 2010;16:1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoffmann JC, Peters K, Henschke S, et al. Role of T lymphocytes in rat 2 #bib4 #bib6‐trinitrobenzene sulphonic acid (TNBS) induced colitis: Increased mortality after gammadelta T cell depletion and no effect of alphabeta T cell depletion. Gut 2001;48:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kühl AA, Pawlowski NN, Grollich K, et al. Aggravation of intestinal inflammation by depletion/deficiency of cd T cells in different types of IBD animal models. J Leukoc Biol 2007;81:168–175. [DOI] [PubMed] [Google Scholar]
- 25. Hoffmann JC, Pawlowski NN, Grollich K, et al. Gammadelta T lymphocytes: A new type of regulatory T cells suppressing murine 2 #bib4 #bib6‐trinitrobenzene sulphonic acid (TNBS)‐induced colitis. Int J Colorectal Dis 2008;23:909–920. [DOI] [PubMed] [Google Scholar]
- 26. Park SG, Mathur R, Long M, et al. T regulatory cells maintain intestinal homeostasis by suppressing cd T cells. Immunity 2010;33:791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yeung MM, Melgar S, Baranov V, et al. Characterisation of mucosal lymphoid aggregates in ulcerative colitis: immune cell phenotype and TcR‐ γδ expression. Gut 2000;47:215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kanazawa H, Ishiguro Y, Munakata A, Morita T. Multiple accumulation of Vdelta2+ gammadelta T‐cell clonotypes in intestinal mucosa from patients with Crohn's disease. Dig Dis Sci 2001;46:410–416. [DOI] [PubMed] [Google Scholar]
- 29. Ishikawa H, Naito T, Iwanaga T, et al. Curriculum vitae of intestinal intraepithelial T cells: Their developmental and behavioral characteristics. Immun Rev 2007;215:145–165. [DOI] [PubMed] [Google Scholar]
- 30. Erdei A, Prechl J, Isaak A, Molnar E. Regulation of B‐cell activation by complement receptors CD21 and CD35. Curr Pharm Des 2003;9:1849–1860. [DOI] [PubMed] [Google Scholar]
- 31. Mason DY, Cordell JL, Brown MH, et al. CD79a: A novel marker for B‐cell neoplasms in routinely processed tissue samples. Blood 1995;86:1453–1459. [PubMed] [Google Scholar]
- 32. Allenspach K, Steiner JM, Shah BN, et al. Evaluation of gastrointestinal permeability and mucosal absorptive capacity in dogs with chronic enteropathy. Am J Vet Res 2006;67:479–483. [DOI] [PubMed] [Google Scholar]
- 33. Garcia‐Sancho M, Rodriguez‐Franco F, Sainz A, et al. Evaluation of clinical, macroscopic, and histopathologic response to treatment in non‐ hypoproteinemic dogs with lymphocytic‐plasmacytic enteritis. J Vet Intern Med 2007;21:11–17. [DOI] [PubMed] [Google Scholar]
- 34. Allenspach K, Wieland B, Grone A, et al. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J Vet Intern Med 2007;21:700–708. [DOI] [PubMed] [Google Scholar]
- 35. Schreiner NM, Gaschen F, Gröne A, et al. Clinical signs, histology, and CD3‐positive cells before and after treatment of dogs with chronic enteropathies. J Vet Intern Med 2008;22:1079–1083. [DOI] [PubMed] [Google Scholar]
- 36. Walker D, Knuchel‐Takano A, McCutchan A, et al. A comprehensive pathological survey of duodenal biopsies from dogs with diet‐responsive chronic enteropathy. J Vet Intern Med 2013;27:862–874. [DOI] [PubMed] [Google Scholar]
- 37. Rütgen BC, König R, Essler SE, et al. Composition of lymphocyte subpopulations in normal canine lymph nodes. Vet Clin Pathol 2015;44:58–69. [DOI] [PubMed] [Google Scholar]
- 38. Mason DY, Cordell JL, Tse AGD, et al. The IgM‐associated protein mB 1 as a marker of normal and neoplastic B cells. J Immunol 1991;147:2474–2482. [PubMed] [Google Scholar]


