Abstract
Background
Furosemide is the only loop diuretic recommended by the ACVIM consensus guidelines for treatment of congestive heart failure (CHF) in dogs related to degenerative mitral valve disease (DMVD). Torasemide is another potent loop diuretic with a longer half‐life and a higher bioavailability.
Objectives
(1) To demonstrate that torasemide given once a day (q24h) is noninferior to furosemide given twice a day (q12h) for treating dogs with CHF; (2) and to compare the effect of the 2 drugs on the time to reach a composite cardiac endpoint “spontaneous cardiac death, euthanasia due to heart failure or CHF class worsening.”
Animals
A total of 366 dogs with CHF attributable to DMVD.
Methods
Analysis of 2 prospective randomized single‐blinded reference‐controlled trials was performed. Dogs orally received either torasemide q24h (n = 180) or furosemide q12h (n = 186) in addition to standard CHF therapy over 3 months. The primary efficacy criterion was the percentage of dogs with treatment success assessed in each study. The time to reach the composite cardiac endpoint was used as secondary criterion in the overall population.
Results
Torasemide was noninferior to furosemide (P torasemide − P furosemide = +7%; 95% CI [−8%; +22%] and P torasemide − P furosemide = +1%; 95% CI [−12%; +14%], respectively, in Study 1 and Study 2). Torasemide (median dose = 0.24 mg/kg/d q24h; range = 0.10–0.69 mg/kg/d) was associated with a 2‐fold reduction in the risk of reaching the composite cardiac endpoint (adjusted HR = 0.47; 95% CI = 0.27–0.82; P = 0.0077) as compared with furosemide (median dose = 1.39 mg/kg q12h; range = 0.70–6.30 mg/kg q12h).
Conclusions and Clinical Importance
Torasemide q24h is an effective oral diuretic in dogs with CHF.
Keywords: Canine, Clinical trial, Diuretic, Pulmonary edema
Abbreviations
- ACE
angiotensin‐converting enzyme
- AE
adverse event
- ALP
alkaline phosphatase
- ALT
alanine transaminase
- AST
aspartate aminotransferase
- CHF
congestive heart failure
- CI
confidence interval
- DMVD
degenerative mitral valve disease
- IQR
interquartile range
- IRIS
International Renal Interest Society
- HR
hazard ratio
- LA:Ao ratio
left atrium‐to‐aorta ratio
- NYHA
New York Heart Association
- P/P‐value
probability value
- γ‐GT
gamma‐glutamyl transpeptidase
Heart failure (HF) is a complex clinical syndrome resulting from structural or functional impairment of ventricular filling or blood ejection of various origins.1, 2 In both humans and dogs, a large majority of patients with HF exhibit clinical signs related to fluid retention (e.g, ascites, dyspnea, cough, and exercise intolerance resulting from pulmonary edema and pleural effusion), a condition referred to under the generic term “congestive HF” (CHF).1, 2, 3 In human and veterinary cardiology, diuretics are considered as one of the cornerstones in the management of acute and chronic CHF regardless of the underlying cause.1, 2, 3, 4, 5, 6, 7, 8 Loop diuretics are potent diuretic agents acting on the Na+:K+:2Cl− cotransporter of the thick ascending loop of Henle; they are thus usually prescribed as first‐line treatment in both small animals and humans to reduce intravascular fluid volume and so decrease preload venous/capillary pressures and relieve clinical signs of volume overload.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12
Degenerative mitral valve disease (DMVD) is the most common heart disease in the dog, representing the most frequent cause of CHF in small‐breed dogs.3, 13, 14, 15, 16, 17 According to the American College of Veterinary Internal Medicine (ACVIM) consensus statement guidelines for the diagnosis and treatment of canine DMVD, furosemide is the loop diuretic recommended for treatment of dogs with DMVD with a history of or ongoing clinical signs of CHF.3 Furosemide has a bioavailability of 77% and a half‐life of 1–2 hours in the dog.9 It causes a dose‐dependent increase in natriuresis that usually disappears 6 hours after oral administration, requiring at least 2 administrations per day for a sustained diuretic effect.18, 19, 20 Additionally, resistance to furosemide might occur, requiring progressive increase in dose to maintain the same level of diuresis.3, 19 Torasemide is a more recently developed loop diuretic21, 22, 23, 24, 25, 26, 27, 28 with more potent and long‐lasting diuretic activity than furosemide.18, 19, 29, 30, 31, 32 Torasemide is characterized by a longer half‐life (8 hours) and duration of action (12 hours), and a higher bioavailability (80–100%) than furosemide.18, 19, 32 Moreover, beyond its pure diuretic effect, torasemide has several beneficial actions that have been demonstrated in humans, animal models, and in vitro models. These include vasodilating properties33, 34 as well as improvement of cardiac function and reduction of cardiac remodeling related, at least in part, to an antialdosterone effect.35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 The TOrasemide In Congestive Heart Failure (TORIC) study demonstrated that torasemide had a more beneficial effect than furosemide and other diuretics on the mortality and morbidity of human patients suffering from chronic CHF.26 Limited data are currently available regarding the use of torasemide in veterinary cardiology, although some reports suggest its potential benefit in canine CHF.47, 48, 49
The aims of this study were therefore to evaluate the short‐Term Efficacy and Safety of Torasemide compared to furosemide (TEST study) in dogs with CHF related to DMVD, with the hypothesis that torasemide given once a day (q24h) would be noninferior to furosemide given twice a day (q12h). For this purpose, the primary efficacy criterion was based on a composite clinical score. A composite cardiac endpoint (spontaneous cardiac death, euthanasia for HF, and CHF class worsening) was assessed as a secondary endpoint.
Materials and Methods
The clinical trial process (conception, monitoring, data management, analyses, and reporting) was conducted according to Good Clinical Practice and European Medicines Agency (EMA) requirements.50, 51 In accordance with the EMA, 2 consecutive prospective, randomized, single‐blinded, and positive‐controlled clinical trials were designed. Given the absence of data regarding the optimal torasemide dosage in dogs with naturally occurring CHF at the time of the study design, a first study was planned with the intent to conduct a second one based on the first study's outcome. The first 3‐month study (Study 1) enrolled dogs from 33 veterinary practices in France (23), Spain (6), and Germany (4) between 2010 and 2011. Analysis of the results of Study 1 led to the second 3‐month study (Study 2), with a similar protocol design, but for which the dosing range and dosage change increment were slightly adjusted as described below. Study 2 recruited a new cohort of adult dogs, with similar conditions to Study 1, from 41 veterinary practices in France (27), Spain (8), and Germany (6) between 2012 and 2013. Both study protocols were approved by national authorities (i.e, Agence Nationale du Médicament Vétérinaire for France, Niedersächsischen Landesamtes für Verbraucherschutz und Lebensmittelsicherheit for Germany, and Agencia Española de Medicamentos y Productos Sanitarios for Spain).
All veterinarians included in the trial were experienced in the use of echocardiography. Both Study 1 and Study 2 were closely monitored by local study monitors (DVM, 1 for each country), who verified the inclusion criteria for all cases on site. The cases were then further verified by the clinical trial manager (SR) at a central location. A final medical review of all clinical, radiographic, and echocardiographic data was blindly performed by 2 board‐certified clinicians (VC, ECVIM‐CA cardiology, and JM, ACVECC).
Enrollment Criteria
Inclusion Criteria
Dogs of all breeds and both sexes, weighing ≥3 kg, were eligible for inclusion if they presented with current or previous episode of mild‐to‐severe CHF secondary to DMVD, if outpatient treatment was indicated, and if owners had given informed consent in their native language. Owners had the option to withdraw their dog from the study at any time. The diagnosis of DMVD was based on the following criteria: (1) left systolic apical heart murmur first detected after 1 year of age and (2) echocardiographic signs of DMVD at inclusion, including typical valvular lesions (irregular and thickened mitral valve leaflets, valve prolapse) associated with mitral valve regurgitation using color‐flow and continuous‐wave Doppler modes.52 The left atrium‐to‐aorta (LA:Ao) ratio was also assessed using the two‐dimensional method described by Hansson et al.53 from the right parasternal short‐axis view. Left‐sided CHF was defined as presence of pulmonary edema as assessed by clinical signs (e.g, tachypnea, dyspnea, or both) and thoracic radiographs, due to severe mitral valve regurgitation, as confirmed by echocardiographic evidence of moderate to severe LA enlargement (LA:Ao greater than 1.5).54 As both protocol designs were conceived in 2008, before the publication of the ACVIM consensus guidelines for diagnosis and treatment of canine DMVD,3 the modified New York Heart Association (NYHA) classification was used for both trials (Table 1).55
Table 1.
Modified New York Heart Association (NYHA) classification
| Class | Clinical Correlate |
|---|---|
| I | Asymptomatic dogs with murmur but no cardiac failure |
| II | Clinical signs of congestive heart failure (exercise intolerance, dyspnea, cough…) during intense or extended physical activity |
| III | Clinical signs of congestive heart failure during mild physical activity |
| IV | Clinical signs of congestive heart failure present when resting |
Noninclusion Criteria
Dogs were not included if they were suffering from other acquired heart disease, congenital heart disease, acute renal failure, acute CHF requiring emergency treatment with injectable drugs, or any other concurrent disease with clinical signs or treatments that could interfere with the monitoring of cardiac condition. In addition, dogs with abnormal serum sodium, potassium, or both out of the reference ranges (142–160 mmol/L and 3.90–5.60 mmol/L, respectively), known hypersensitivity to sulfonamides, pregnant or lactating, or had a planned surgery within 3 months were excluded. Finally, dogs that had received long‐acting corticosteroids within the prior month or spironolactone in the prior 24 hours were not included. Dogs receiving spironolactone could be included if treatment was stopped 24 hours before trial entry.
Postinclusion Removal
All dogs that developed a concurrent disease during the study period, which could interfere with the study parameters, as well as all dogs that required or received a nonauthorized treatment (cf. below), were withdrawn from the study. Dogs were also withdrawn from the study if they were affected by a clinically important systemic or organ‐related disease that was expected to decrease the dog's lifespan (cancer, endocrine disorder, e.g, Cushing syndrome and diabetes). They were also excluded when azotemia or electrolytic disorders required a specific medication not allowed in the study design.
Randomization and Allocation
A double‐block (strata and sites) randomization was drawn up using 2N softwarea with 1:1 allocation to maintain similar sample sizes in both treatment groups. At inclusion, investigators described whether the dog fell into 1 of 2 predefined categories, that is, either Stratum 1 or Stratum 2 defined as follows:
-
☐
Stratum 1: This included animals with ongoing CHF. This encompassed animals presented with a first CHF episode requiring a first prescription of furosemide and those suffering from a deterioration of CHF requiring a dosage adjustment of furosemide. Treatment of animals from Stratum 1 was expected to improve their clinical condition at the end of the study.
-
☐
Stratum 2: This included animals that had experienced a previous episode of CHF that were stable at the time of inclusion, that is, without clinical and radiographic signs of CHF (pulmonary edema, pleural effusion, or both). Treatment of animals from this stratum was expected at a minimum to maintain their condition.
At inclusion, the clinical investigator assigned the smallest available number to the dog from its randomization block relative to the inclusion stratum. The therapist investigator opened the treatment envelope bearing this number and delivered the corresponding treatment to the dog's owner.
Tested Treatments
All dogs received a loop diuretic either torasemideb (Torasemide group) or furosemidec (Furosemide group) for 3 consecutive months (Table 2). For dogs from Stratum 1 (i.e, presented at baseline with CHF), the initial dose of furosemide was chosen according to the severity of CHF signs, and for dogs randomized in the Torasemide group, the furosemide dosage was converted to torasemide dosage according to Table 2. For dogs from Stratum 2 (i.e, stable under furosemide treatment at time of inclusion), the dose of furosemide was maintained and was similarly converted to torasemide dosage according to Table 2 for dogs in the Torasemide group. In Study 1, torasemide dosage comprised between 0.2 mg/kg and 0.8 mg/kg q24h, adjustable as often as necessary by increments of 0.2 mg/kg/d, and furosemide was administered according to British Specific Product Characteristics,d that is, at dosage between 1 mg/kg and 5 mg/kg q12h, adjustable by increments of 1 mg/kg q12h per day. In Study 2, furosemide dosage remained the same as in Study 1 and (based on safety results obtained in Study 1) torasemide was initiated at a dosage between 0.2 mg/kg and 0.6 mg/kg q24h, adjustable if deemed necessary by 0.1 mg/kg/d increments and possibly decreased to 0.1 mg/kg q24h after treatment initiation (Table 2).
Table 2.
Dosing regimen for furosemide and torasemide in Study 1 and Study 2
| Conversion table used in Study 1 at Day 0 for determining the initial dose of torasemide# | ||||||
|---|---|---|---|---|---|---|
| Theoretical dose of furosemide (evaluated by the clinical investigator) | 1 mg/kg, 2 × /day | 2 mg/kg, 2 × /day | 3 mg/kg, 2 × /day | 4 mg/kg, 2 × /day | 5 mg/kg, 2 × /day | |
| Initial dosage (Day 0) of the study treatments distributed by the therapist investigator | Furosemidea (Furosemide group) | 1 mg/kg, 2 × /day | 2 mg/kg, 2 × /day | 3 mg/kg, 2 × /day | 4 mg/kg, 2 × /day | 5 mg/kg, 2 × /day |
| Torasemideb (Torasemide group) | 0.2 mg/kg, 1 × /day | 0.4 mg/kg, 1 × /day | 0.6 mg/kg, 1 × /day | |||
| Conversion table used in Study 2 at Day 0 for determining the initial dose of torasemide## | ||||||
|---|---|---|---|---|---|---|
| Theoretical dose of furosemide (evaluated by the clinical investigator) | 1 mg/kg, 2 × /day | 2 mg/kg, 2 × /day | 3 mg/kg, 2 × /day | 4 mg/kg, 2 × /day | 5 mg/kg, 2 × /day | |
| Initial dosage (Day 0) of the study treatments distributed by the therapist investigator | Furosemidea (Furosemide group) | 1 mg/kg, 2 × /day | 2 mg/kg, 2 × /day | 3 mg/kg, 2 × /day | 4 mg/kg, 2 × /day | 5 mg/kg, 2 × /day |
| Torasemidec (Torasemide group) | 0.2 mg/kg, 1 × /day | 0.3 mg/kg, 1 × /day | 0.4 mg/kg, 1 × /day | 0.5 mg/kg, 1 × /day | 0.6 mg/kg, 1 × /day | |
#Follow‐up from Day 0 to Day 84 ± 4 days: if judged necessary by the clinical investigator (depending on the animal's general clinical condition and all parameters recorded at each visit, i.e, physical parameters, CBC, biochemistry, and thoracic radiographs), increments of 1 mg/kg twice a day of furosemide (up to 5 mg/kg, 2x/day, according to the British Specific Product Characteristicsd) for dogs from the Furosemide group and increments of 0.2 mg/kg once a day of torasemide for dogs from the Torasemide group (up to 0.8 mg/kg/d).
##Follow‐up from Day 0 to Day 84 ± 4 days: if judged necessary by the clinical investigator (depending on the animal's clinical condition and all parameters recorded at each visit (i.e, physical parameters, CBC, biochemistry, and thoracic radiographs), increments of 1 mg/kg twice a day of furosemide (up to 5 mg/kg, 2x/day, according to the British Specific Product Characteristicsd) for dogs from the Furosemide group and increments of 0.1 mg/kg once a day of torasemide for dogs from the Torasemide group (up to 0.6 mg/kg/d). The initial dose of 0.2 mg/kg once a day could also be decreased to 0.1 mg/kg once a day after Day 7.
Bidivisible tablets containing 19 and 40 mg of furosemide.
Bidivisible tablets containing 1.5, 4.5, and 12 mg of torasemide.
Bidivisible tablets containing 0.75 mg of torasemide, and quadri‐divisible tablets containing 3.0, 7.5, and 18 mg of torasemide.
Blinding
Double blinding was not possible as the torasemide tablets were easily distinguishable from the furosemide tablets, and were to be given q24h versus q12h for furosemide. To ensure blinding regarding the assigned treatment, dual investigators were used. At each site, 2 investigators, both of whom were veterinarians, intervened in a predefined fashion. The clinical investigator was responsible for inclusion, clinical assessment, and case management throughout the study. The clinical investigator prescribed the theoretical dose of furosemide, and the therapist investigator delivered it to the owner or translated it into a torasemide prescription for dogs belonging to the Furosemide and the Torasemide group, respectively (Table 2). The clinical investigator remained blinded to which treatment was assigned to any included animal. The therapist investigator was responsible for dispensing the study drugs. Owners were instructed not to mention to the clinical investigator the name of the product administered, or the number of times per day the tablet was administered. For all questions related to treatments, the owners were instructed by the clinical investigator to discuss with the therapist investigator. Clinical investigators, study monitors, and the sponsor remained blinded for the whole duration of the studies.
Concomitant Treatments
Standard concomitant therapy for CHF that included ACE inhibitors and pimobendan ± digoxin was permitted throughout the 2 trials, provided that it was already in place at inclusion in the study and that it did not change during the 3‐month period of follow‐up (except for digoxin). The use of diuretics other than those used in the study, spironolactone, angiotensin II receptor antagonists, calcium channel blockers, nitrate therapy, β‐blockers, and type 5 phosphodiesterase inhibitors was forbidden as well as aminoglycosides, cephalosporin, sulfonamide, and corticosteroids (oral or injectable). If one of these treatments was necessary, the animal was withdrawn from the study.
General Protocol Design
As illustrated in Figure 1, dogs were examined by clinical investigators on at least 5 occasions: Day 0 (D0, inclusion day, initiation of treatment), Day 7 ± 2 days (D7), Day 28 ± 2 days (D28), Day 56 ± 4 days (D56), and Day 84 ± 4 days (D84). If a change in the diuretic treatment dosage was performed, an additional visit was scheduled 7 days later. At each visit (scheduled and additional), dogs underwent a complete physical examination. A composite clinical score was used throughout the 2 studies (Table 3). Additionally, a modified NYHA classification (Table 1) was used to score CHF severity.
Figure 1.
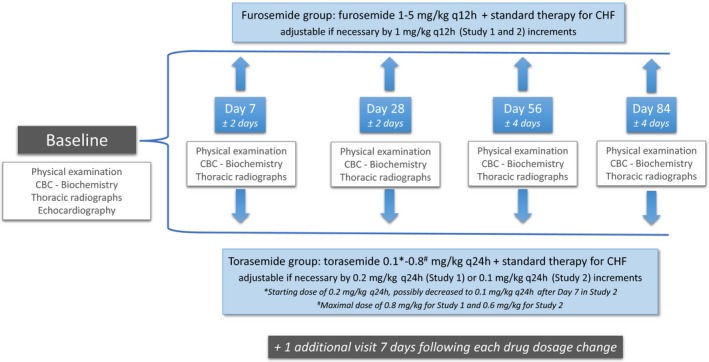
Design of the studies. The second visit (Day 7) could be performed from Day 5 to Day 9. The third visit (Day 28) could be performed from Day 26 to Day 30. The fourth visit (Day 56) could be performed from Day 52 to Day 60. The last visit (Day 84) could be performed from Day 80 to Day 88.
Table 3.
Composite clinical score scale
| Variable | Score | Clinical Correlate |
|---|---|---|
| Dyspnea | 0 | None |
| 1 | During intense physical activity (e.g, running, climbing stairs) | |
| 2 | During moderate physical activity (e.g, walking) | |
| 3 | At rest | |
| Cough (frequency) | 0 | None |
| 1 | Rare | |
| 2 | Frequent | |
| 3 | Very frequent | |
| Exercise tolerance | 0 | Normal |
| 1 | Reduced | |
| 2 | Exercise is not possible | |
| Ascites | 0 | None |
| 1 | Mild to moderatea | |
| 2 | Severea |
At each visit, the score obtained for dyspnea, cough frequency, exercise tolerance, and ascites related to hepatic venous congestion was summed to calculate the composite clinical score, based on both physical examination and complete history by the owner, which was used as treatment success assessment.
If ascites was present, maximal abdominal circumference was measured (in cm) and at the following visit, ascites was scored as follows: −1, 0, or 1 if abdominal circumference was less, equal to, or greater than circumference measured at diagnosis, respectively.
Right lateral and dorsoventral thoracic radiographs were used to investigate cardiomegaly and CHF signs at each visit as well as to exclude concurrent respiratory diseases at inclusion in the study. Clinical investigators were asked to determine whether the pulmonary edema had increased, decreased, disappeared, or had not changed compared to radiographs performed on D0.
Blood was sampled at each study visit for cell blood count, biochemistry (urea, creatinine, ALT, AST, ALP, γ‐GT, total bilirubin, total proteins), and electrolytes (sodium, potassium, chloride, calcium, phosphorus, magnesium). All blood samples were shipped to a central laboratory.e The International Renal Interest Society (IRIS) creatinine cutoff values were used to describe the renal status of patients (IRIS stages 1 to 4) over the course of the study.56
Analysis of Efficacy and Safety
Treatment success for both studies was defined as clinical improvement (Stratum 1 and Stratum 2) or clinical stabilization (Stratum 2 only) at D84 compared to D0, associated with improvement of (Stratum 1) or persistent absence of (Stratum 2) pulmonary edema on thoracic radiographs as compared to D0. Dogs were considered to have clinically improved if the clinical score decreased between D0 and D84, and were considered as clinically stable if the clinical score was equal to D0's value. Dogs that improved clinically but not radiographically (and vice versa) were considered as treatment failures. All dogs withdrawn from the studies before follow‐up completion (Day 84) were considered to be treatment failures. The percentage of dogs with treatment success as defined above was the primary criterion of efficacy and was assessed separately for each study.
During the course of the studies, when dogs died spontaneously or were euthanized, the investigator reported the reason for death or euthanasia and specified whether the cause of death was considered to be cardiac or noncardiac. Death was considered to be of cardiac origin if no evidence to support a noncardiac cause of death was identified. A composite cardiac endpoint composed of “spontaneous cardiac death, euthanasia for HF, or CHF class worsening” was used as a secondary efficacy criterion.
Adverse events were also reported by clinical investigators during the course of the studies. Cardiac events were defined as acute onset of pulmonary edema, worsening of cardiac condition (e.g, increased dyspnea despite changes in diuretic dosages), hypotension, syncope, and arrhythmias. Safety was assessed by occurrence of adverse events and changes in IRIS stage, creatinine, and potassium values over the study period.
Data Management
All clinical and dispenser forms were collected from the centers at the end of the studies. Data were double‐tabulated into Clintrial software.f Blinding was maintained during data entry and data auditing. The blind review to determine the inclusion and exclusion of the cases was performed by 2 experienced clinical trial veterinarians. Unblinding was performed by an independent biostatistician.
Statistical Analysis
Given the lack of published studies evaluating the effect of loop diuretics in spontaneous canine CHF, the power calculation was based on a presumptive 70% success rate for furosemide in accordance with the results of a published placebo‐controlled clinical trial evaluating the effect of an ACE inhibitor on survival times and clinical signs of dogs with CHF.57 In this study, 88% of the dogs in the placebo group were rated as improved or not changed concerning the global clinical condition on Day 7 (compared to Day 0) and 65.6% on Day 28 (compared to Day 7). The limit of equivalence between torasemide and furosemide was set at ‐20%, which represents the acceptable equivalence limit in the case of a bioequivalence study. It was estimated that in each study, 90 evaluable dogs would be required in each treatment group to provide a power of approximately 80% with alpha set at 2.5%.
Statistical analysis of the baseline parameters was performed to test potential differences between treatment groups at treatment initiation in both studies as well as in the overall population. Continuous variables were described as median and interquartile range (IQR), and categorical data were described as frequency and percentage. All continuous baseline variables were compared between groups using a Wilcoxon rank‐sum test. For categorical variables, a chi‐square test or Fisher's exact test was used. No adjustment was made for multiple comparisons.
As the primary objective of Studies 1 and 2 was to demonstrate that the new product (torasemide) was noninferior to a reference product (furosemide), a noninferiority approach was used to compare the rates of treatment success in each study. The hypothesis of inferiority was defined by H0: P torasemide − P furosemide ≤ −20%. The 95% confidence interval (CI) of the difference between success rates was estimated by the Newcombe method. If the lower bound (α = 2.5% in bilateral situation) of this CI was above the limit of noninferiority of −20%, it was concluded that torasemide was noninferior to furosemide.
The occurrence of the composite cardiac endpoint (secondary efficacy criterion) was compared between the 2 groups of patients by pooling Study 1 and Study 2, as (1) this secondary endpoint was not planned to be investigated when each of the 2 studies was initiated, (2) the collection of data on spontaneous cardiac death, euthanasia for HF, or CHF class worsening was performed identically in the 2 studies, and (3) there is no rationale in studying the occurrence of the composite cardiac endpoint separately in Study 1 and Study 2. The Kaplan‐Meier method was used to estimate the time from baseline (i.e, D0) to the occurrence of the composite cardiac endpoint within the first 84 days. The log‐rank test was performed to test the difference between survival curves. Dogs that died from a noncardiac death were censored at the date of death. Dogs still alive at D84 were censored at the date of D84. Dogs that were withdrawn due to adverse events or for other reasons were censored at the date of withdrawal.
Univariate Cox proportional hazards models were performed for 30 baseline variables of interest (all baseline variables with the exception of “diuretic pretrial treatment”), to determine which variables were associated with time to the composite cardiac endpoint.
The independent association between the 2 treatment groups with time to the composite cardiac endpoint was assessed using a multivariate Cox proportional hazards model. The final model was obtained using the stepwise procedure. The initial model contained the treatment group variable only; then, it was subjected to a sequential selection‐deletion cycle as follows: among all the 30 candidate variables used in the univariate analyses, 1 variable was included into the model if the P‐value of its coefficient was lower than 0.20, and 1 variable previously included was finally removed if no longer significant (i.e, P > 0.05). No interaction term was included into the stepwise procedure. The assumption of the proportional hazards was graphically checked for each variable included into the final model and by including a time‐dependent interaction (all with a P‐value > 0.057).
Chi‐square test or Fisher's exact test were used to compare the 2 groups on safety parameters.
The significance level was set at 5% for all these tests except for the primary endpoint (2.5%). All the calculations were performed using SAS/STAT version 9.2.g
Results
Baseline Data
A total of 387 dogs with DMVD and past or current CHF were initially included in the study. After a final blinded medical review by 2 board‐certified clinicians, 21 cases were not included in the statistical analysis due to equivocal echocardiographic, radiographic, or both images. Of the remaining 366 dogs, there were 228 males and 138 females with 186 allocated to the Furosemide group and 180 dogs to the Torasemide group. There were 151 evaluable cases in Study 1 (75 in the Torasemide group and 76 in the Furosemide group) and 215 in Study 2 (105 in the Torasemide group and 110 in the Furosemide group). The median number of dogs recruited per center was 4 (IQR 2–9). Regarding the whole study population, the most commonly recruited breeds were mixed breed (n = 72), Poodle (n = 53), Yorkshire Terrier (n = 49), and Cavalier King Charles Spaniel (n = 46). All dogs enrolled in the study were ≥5 years of age, with a median age at baseline (i.e, at treatment initiation) of 12.0 years (IQR 10.2–14.0 years). The median body weight was 7.8 kg (IQR 5.4–11.4 kg). All included dogs had a high‐grade heart murmur related to DMVD, that is, median grade of 4 (IQR 4–4), as well as an increased LA:Ao ratio (median ratio of 2.0, IQR 1.6–2.3; minimum–maximum 1.5–3.6). Baseline characteristics of the 2 treatment groups are presented in Table 4. A large majority of dogs (93%) had received cardiac treatment before inclusion. Diuretics were the most common (69%; furosemide, except for 1 dog that only received a combination of spironolactone and altizideh and for 2 other dogs that received this same association in addition to furosemide) followed by ACE inhibitors (47%). At baseline, duration of heart disease was significantly longer for dogs in the Furosemide group (P = 0.038); however, duration of the pretrial treatment, number of dogs with diuretic pretrial treatment, and distribution of pretrial furosemide dosage were similar between the 2 groups. Dogs in the Torasemide group also had a significantly higher dyspnea score at inclusion compared to dogs allocated to the Furosemide group (P = 0.017). All other baseline variables were not significantly different between the 2 treatment groups. Overall, dogs from the Furosemide group received a median furosemide dose of 2.78 mg/kg/d (IQR 1.98–4.71 mg/kg/d, range 1.40–12.60 mg/kg/d) during the 3‐month study period, and those from the Torasemide group received a median torasemide dose of 0.24 mg/kg/d (IQR 0.19–0.36 mg/kg/d, range 0.10–0.69 mg/kg/d). The majority of dogs (62%) received torasemide treatment in the morning.
Table 4.
Summary of baseline characteristics of the 2 treatment groups, that is, frequencies (number of dogs and percentages) or medians (interquartile range)
| Variable | Treatment group | |||
|---|---|---|---|---|
| Torasemide n = 180 | Furosemide n = 186 | P‐value | ||
| Dog characteristics | Age (years) | 12.00 [10.15–14.00] | 12.25 [10.30–14.00] | 0.43 |
| Sex (M/F/MC/FC), n (%) | 96/32/23/29 (53/18/13/16) | 81/35/28/42 (43/19/15/23) | 0.24 | |
| Cavalier King Charles, n (%) | 24 (13) | 22 (12) | 0.66 | |
| Body weight (kg) | 7.65 [5.00–11.00] | 7.95 [5.60–12.00] | 0.21 | |
| Duration of clinical signs and pretrial treatment | Duration of heart disease (days) | 126.0 [9.0–512.5] | 247.0 [28.0–703.0] | 0.038 |
| Pretrial treatment: yes/no (%) | 169/11 (94/6) | 170/16 (91/9) | 0.36 | |
| Duration of pretrial treatment (days) | 109.0 [10.0–365.0] | 129.5 [19.0–431.0] | 0.13 | |
| Diuretic pretrial treatment (furosemide and/or thiazide diuretic): yes/no (%) | 124/56 (69/31) | 129/57 (69/31) | 0.92 | |
| Pretrial furosemide dosage: <4 mg/kg/d/≥4 mg/kg/d/No furosemide (%) | 91/32/57 (50/18/32) | 104/25/57 (56/13/31) | 0.44 | |
| Duration of diuretic pretrial treatment (days) | 59 [10–299] | 117 [18–350] | 0.32 | |
| ACE inhibitors pretrial treatment: yes/no (%) | 87/93 (48/52) | 86/100 (46/54) | 0.69 | |
| Pimobendan pretrial treatment: yes/no (%) | 57/123 (32/68) | 51/135 (27/73) | 0.37 | |
| Spironolactone pretrial treatment: yes/no (%) | 34/146 (19/81) | 28/158 (15/85) | 0.33 | |
| Other pretrial treatment: yes/no (%) | 22/158 (12/88) | 18/168 (10/90) | 0.44 | |
| First diagnosis of congestive heart failure: yes/no/unknown (%) | 15/145/20 (8/81/11) | 14/160/12 (8/86/6) | 0.26 | |
| Clinical signs | Appetite: normal/decreased/anorexia (%) | 159/21/0 (88/12/0) | 159/27/0 (85/15/0) | 0.42 |
| Syncope: yes/no (%) | 22/158 (12/88) | 17/169 (9/91) | 0.34 | |
| Cough: 0/1/2/3, n (%) | 28/77/74/1 (15/43/41/1) | 25/75/85/1 (13/40/46/1) | 0.82 | |
| Dyspnea: 0/1/2/3, n (%) | 33/77/60/10 (18/43/33/6) | 61/69/48/8 (33/37/26/4) | 0.017 | |
| Exercise tolerance: 0/1/2, n (%) | 23/153/4 (13/85/2) | 34/146/6 (18/79/3) | 0.27 | |
| Ascites: 0/1/2, n (%) | 175/3/2 (97/2/1) | 181/5/0 (97/3/0) | 0.39 | |
| Composite clinical score | 3 [2–5] | 3 [2–4] | 0.14 | |
| Heart rate (beats/min) | 135 [116–151] | 139 [116–154] | 0.53 | |
| Respiratory rate (breaths/min) | 40 [28–52] | 36 [28–60] | 0.88 | |
| NYHA class (II/III/IV), n (%) | 83/74/23 (46/41/13) | 77/90/19 (42/48/10) | 0.36 | |
| Laboratory variables | Na (mmol/L) | 148 [146–151] | 148 [147–150] | 0.75 |
| K (mmol/L) | 4.70 [4.30–5.00] | 4.60 [4.30–5.00] | 0.73 | |
| Cl (mmol/L) | 108 [105–111] | 108 [104.5–111] | 0.28 | |
| Urea (g/L) | 0.51 [0.37–0.72] | 0.52 [0.36–0.77] | 0.78 | |
| Creatinine (mg/L) | 8.40 [7.20–11.60] | 9.25 [7.40–12.25] | 0.13 | |
| IRIS stage: 1/2/3/4, n (%) | 154/20/5/1 (85/11/3/1) | 157/21/7/1 (84/11/4/1) | 0.96 | |
M, male; F, female; MC, male neutered; FC, female neutered; ACE, angiotensin‐converting enzyme; NYHA, New York Heart Association; IRIS, International Renal Interest Society; Na, sodium; K, potassium; Cl, chloride. P‐values that appear in bold are < 0.05.
Baseline data from Study 1 and Study 2, separately, were similar to baseline data of the overall population (Table 5), demonstrating neither clinical nor significant differences between the Torasemide and the Furosemide group regarding the 31 tested parameters in each of the 2 studies.
Table 5.
Summary of some baseline characteristics of Studies 1 and 2, that is, frequencies (number of dogs and percentages) or medians (interquartile range)
| Variable | Study 1 | Study 2 | ||||
|---|---|---|---|---|---|---|
| Treatment group | Global (n = 151) | Treatment group | Global (n = 215) | |||
| Torasemide (n = 75) | Furosemide (n = 76) | Torasemide (n = 105) | Furosemide (n = 110) | |||
| Male, n (%) | 54 (72) | 45 (59) | 99 (66) | 65 (62) | 64 (58) | 129 (60) |
| Female, n (%) | 21 (28) | 31 (41) | 52 (34) | 40 (38) | 46 (42) | 86 (40) |
| Agea (years) | 12.00 [10.2–14.0] | 11.0 [10.0–13.6] | 12.0 [10.1–14.0] | 12.00 [10.2–13.8] | 12.7 [10.6–14.2] | 12.2 [10.3–14.0] |
| Body weighta (kg) | 7.8 [6.0–11.1] | 7.9 [5.6–10.9] | 7.9 [5.7–11.1] | 7.2 [4.6–10.9] | 8.1 [5.7–12.2] | 7.8 [5.3–11.8] |
| Composite clinical scorea | 3 [2–4] | 3 [2–4] | 3 [2–4] | 4 [3–5] | 4 [2–4] | 4 [2–5] |
| NYHA class (II/III/IV), n (%) | 37 (50)/31 (41)/7 (9) | 35 (46)/33 (43)/8 (11) | 72 (48)/64 (42)/15 (10) | 46 (44)/43 (41)/16 (15) | 42 (38)/57 (52)/11 (10) | 88 (41)/100 (46)/27 (13) |
| IRIS class (1/2/3/4), n (%) | 63 (84)/10 (13)/2 (3)/0 (0) | 69 (91)/4 (5)/2 (3)/1 (1) | 132 (87)/14 (9)/4 (3)/1 (1) | 91 (87)/10 (9)/3 (3)/1 (1) | 88 (80)/17 (15)/5 (5)/0 (0) | 179 (83)/27 (13)/8 (4)/1 (0) |
| Diuretic dosea (mg/kg/d) | 0.24 [0.19–0.40] | 2.86 [2.06–5.07] | – | 0.24 [0.19–0.34] | 2.63 [1.89–4.55] | – |
Median [1st quartile – 3rd quartile]; NYHA, New York Heart Association; IRIS, International Renal Interest Society.
Treatment Success
At D84, in Study 1, 63% of the torasemide‐treated dogs had treatment success versus 55% for the furosemide‐treated dogs. Thus, torasemide treatment was noninferior to furosemide with P torasemide − P furosemide = +7% and a 95% CI of [−8%; +22%]. Similar observations were made in the Study 2 (59% and 60 % of treatment success in the Furosemide and the Torasemide group, respectively), with P torasemide − P furosemide = +1%; 95% CI [−12%; +14%].
Composite Cardiac Endpoint (spontaneous cardiac death, euthanasia due to heart failure, or worsening of CHF class)
Of the 366 dogs of the whole study sample, the composite cardiac endpoint (i.e, secondary endpoint) occurred in 59 dogs (24 cardiac deaths and 35 dogs that had a worsening of CHF class; see Table 6). Fifty‐six dogs were withdrawn from the study before reaching the composite cardiac endpoint or D84. The median follow‐up time (IQR) from baseline until either the composite cardiac endpoint or withdrawal was 83 days (56–85 days), providing a total follow‐up time of 24,824 dog‐days, and resulting in an overall incidence rate of 2.4 events per 1,000 dog‐days. The composite cardiac endpoint was reached in a shorter time in the Furosemide group than in the Torasemide group (Fig 2; P‐log‐rank = 0.0091); the estimated percentages of dogs that presented the composite cardiac endpoint after treatment initiation were 24 and 13, respectively, in the Furosemide and the Torasemide group.
Table 6.
Numbers of dogs that reached the cardiac composite endpoint (“spontaneous cardiac death, euthanasia for heart failure, and congestive heart failure class worsening”) and the component parts of the cardiac composite endpoint
| Treatment group | ||
|---|---|---|
| Torasemide n = 180 | Furosemide n = 186 | |
| Number of dogs reaching the cardiac endpoint, n (%) | 19 (11) | 40 (22) |
| Cardiac deatha, n (%) | 8 (4) | 16 (9) |
| CHF class worsening, n (%) | 11 (6) | 24 (13) |
CHF, Congestive Heart Failure.
Spontaneous cardiac death or euthanasia for cardiac reasons.
Figure 2.
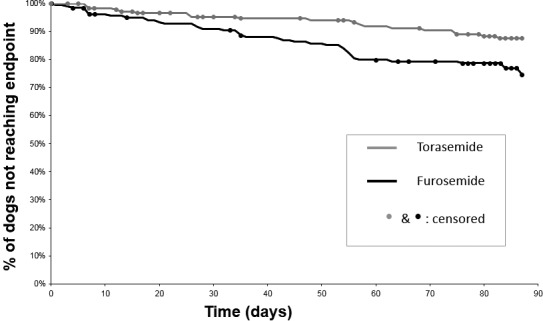
Kaplan‐Meier plot of percentage of dogs that have not yet met the composite cardiac endpoint as a function of time, in 366 dogs with congestive heart failure attributable to degenerative mitral valve disease and treated with either torasemide (n = 180) or furosemide (n = 186). The composite cardiac endpoint was a composite of spontaneous cardiac death, euthanasia for heart failure, and congestive heart failure class worsening. As compared to furosemide, torasemide was associated with a 2‐fold reduction in the risk of reaching the endpoint (adjusted HR = 0.47; 95% CI 0.27–0.82; P = 0.0077).
Univariate Cox Proportional Hazards Analyses of the Baseline Variables
The univariate analysis of treatment and of each of the 30 baseline variables assessed individually demonstrated that torasemide was associated with a longer time to the composite cardiac endpoint when compared to furosemide (HR = 0.49; 95% CI 0.29–0.85; P = 0.011). Variables associated with a worse outcome (Table 7) included the following: decreased appetite at baseline (P = 0.0010); syncope (P = 0.0089); heart rate (P = 0.0043); pretrial furosemide prescription at any dose level (P = 0.038), at <4 mg/kg/d (P = 0.021), and at ≥4 mg/kg/d (P = 0.019); and other pretrial treatment prescription (P = 0.037).
Table 7.
Univariate Cox proportional hazards analyses of baseline variables and stepwise multivariate Cox proportional hazards analyses of the effect of treatment and baseline values to determine which variables were associated with time to the composite cardiac endpoint (spontaneous cardiac death, euthanasia due to heart failure, or worsening of congestive heart failure class)
| Variable | Hazard Ratio | 95% Confidence Interval | P‐value |
|---|---|---|---|
| Univariate Cox proportional hazards analyses | |||
| Treatment group | 0.49 | [0.29; 0.85] | 0.011 |
| Age | 1.02 | [0.92; 1.12] | 0.75 |
| Sex: Female vs. Male | 1.62 | [0.97; 2.70] | 0.06 |
| Cavalier King Charles | 0.85 | [0.39; 1.87] | 0.68 |
| Body weight | 0.96 | [0.90; 1.01] | 0.13 |
| Appetite decreased vs. normal | 2.62 | [1.48; 4.66] | 0.0010 |
| Dyspnea: | |||
| 1 vs. 0 | 0.94 | [0.50; 1.76] | 0.92 |
| 2 vs. 0 | 0.95 | [0.48; 1.88] | |
| 3 vs. 0 | 0.59 | [0.14; 2.55] | |
| Exercise tolerance: | |||
| 1 vs. 0 | 0.75 | [0.39; 1.46] | 0.37 |
| 2 vs. 0 | 1.56 | [0.43; 5.60] | |
| Cough: | |||
| 1 vs. 0 | 1.52 | [0.62; 3.71] | 0.73 |
| 2 vs. 0 | 1.66 | [0.69; 4.02] | |
| Syncope yes vs. no | 2.33 | [1.24; 4.40] | 0.0089 |
| Ascites: | |||
| 1 vs. 0 | 1.40 | [0.34; 5.72] | 0.67 |
| 2 vs. 0 | 2.21 | [0.30; 16.23] | |
| Composite clinical score | 1.01 | [0.84; 1.21] | 0.92 |
| Respiratory rate | 1.01 | [1.00; 1.01] | 0.32 |
| Heart rate | 1.01 | [1.00; 1.02] | 0.0043 |
| NYHA class: | |||
| Stage III vs. stage II | 0.65 | [0.37; 1.14] | 0.21 |
| Stage IV vs. stage II | 1.17 | [0.56; 2.47] | |
| IRIS stage: | |||
| 2 vs. 1 | 0.99 | [0.43; 2.32] | 0.92 |
| 3 vs. 1 | 1.51 | [0.47; 4.85] | |
| Pretrial furosemide dosage: | 0.038 | ||
| <4 mg/kg/d vs. no furosemide | 2.28 | [1.13; 4.58] | 0.021 |
| ≥4 mg/kg/d vs. no furosemide | 2.73 | [1.18; 6.32] | 0.019 |
| Pretrial treatment | 1.50 | [0.47; 4.80] | 0.49 |
| Duration of diuretic pretrial treatment | 1.00 | [1.00; 1.00] | 0.090 |
| Duration of heart disease | 1.00 | [1.00; 1.00] | 0.84 |
| Duration of pretrial treatment | 1.00 | [1.00; 1.00] | 0.42 |
| ACE inhibitor pretrial treatment | 1.03 | [0.62; 1.71] | 0.93 |
| Spironolactone pretrial treatment | 1.35 | [0.72; 2.54] | 0.36 |
| Pimobendan pretrial treatment | 1.61 | [0.96; 2.71] | 0.07 |
| Other pretrial treatment | 2.01 | [1.05; 3.88] | 0.037 |
| First diagnosis of congestive heart failure | 0.59 | [0.18; 1.87] | 0.37 |
| Urea | 1.22 | [0.67; 2.22] | 0.52 |
| Creatinine | 1.02 | [0.97; 1.06] | 0.50 |
| Potassium | 0.68 | [0.44; 1.05] | 0.079 |
| Sodium | 0.98 | [0.95; 1.02] | 0.32 |
| Chloride | 0.99 | [0.97; 1.00] | 0.091 |
| Multivariate Cox model with stepwise selection | |||
| Treatment group: Tora vs. Furo | 0.47 | [0.27; 0.82] | 0.0077 |
| Appetite | 2.58 | [1.43; 4.65] | 0.0016 |
| Heart rate | 1.01 | [1.01; 1.02] | 0.0021 |
| Syncope | 2.19 | [1.14; 4.20] | 0.019 |
| Pretrial furosemide dosage: | 0.030 | ||
| <4 mg/kg/d vs. no furosemide | 1.94 | [0.96; 3.94] | 0.067 |
| ≥4 mg/kg/d vs. no furosemide | 3.16 | [1.35; 7.39] | 0.0081 |
P‐values that appear in bold are < 0.05.
Stepwise Multivariate Cox Proportional Hazards Analyses of the Effect of Treatment and Baseline Variables
The stepwise procedure finally selected the following variables to be included in the multivariate model: appetite, heart rate, syncope, and pretrial furosemide prescription. Independently of appetite, heart rate, syncope, and pretrial furosemide prescription, dogs in the Torasemide group remained significantly associated with a longer time to the composite cardiac endpoint within the first 84 days following treatment initiation than dogs in the Furosemide group (adjusted HR [aHR] = 0.47; 95% CI, 0.27–0.82; P = 0.0077). In this multivariate model, appetite, heart rate, syncope, and pretrial furosemide prescription ≥4 mg/kg/d were significantly associated with a shorter time to reach the composite cardiac endpoint (Table 7).
Safety and Adverse Events
The number of dogs withdrawn from the study before D84 due to clinical events other than cardiac death was overall similar in both treatment groups (Table 8). No significant difference in incidence of death (spontaneous and euthanasia) due to renal events was found between the Torasemide (4%) and the Furosemide group (2%; P = 0.22). Likewise, there was no significant difference in events (i.e, cardiac, renal, or electrolyte events) leading to withdrawal between the 2 treatment groups. Other reasons for withdrawal were higher in the Torasemide group (8%) than in the Furosemide group (3%; P = 0.036), although not clinically related to the diuretic treatment.
Table 8.
Events leading to withdrawal during the overall study follow‐up (except cardiac death)
| Treatment group | |||
|---|---|---|---|
| Torasemide n = 180 | Furosemide n = 186 | P‐value | |
| Mortality, n (%) | 12 (7) | 8 (4) | 0.32 |
| Renal, n (%) | 8 (4) | 4 (2) | 0.22 |
| Other (trauma, gunshot wound, pyometra…), n (%) | 4 (2) | 4 (2) | 1.00 |
| Adverse events, n (%) | 23 (13) | 15 (8) | 0.14 |
| Cardiaca, n (%) | 3 (2) | 1 (1) | 0.36 |
| Renal, n (%) | 2 (1) | 0 (0) | 0.24 |
| Electrolytes disorders, n (%) | 3 (2) | 8 (4) | 0.14 |
| Others (e.g, Cushing syndrome, neurological disorders, hepatic diseases (neoplasia and hepatobiliary inflammatory disorders), pyometra, arthrosis), n (%) | 15 (8) | 6 (3) | 0.036 |
Adverse cardiac events correspond to a worsening of congestive heart failure class severe enough to lead to withdrawal from the study (i.e, 4 of the 35 dogs of Table 6).
P‐values that appear in bold are < 0.05.
Table 9 depicts all the adverse events reported by clinical investigators over the course of the study. Adverse events related to discomfort due to pharmacological expected effects (polyuria‐polydipsia, urinary “incontinence,” or both, as reported by owners, related to a potent diuretic effect) were significantly more frequent in the Torasemide group (20%) than in the Furosemide group (4%, P < 0.001). Similarly, renal adverse events (ranging from mild increases in renal parameters even if still within the reference ranges to acute renal failure) were significantly more frequent in the Torasemide group (18%) compared to the Furosemide group (4%; P < 0.001). These renal adverse events in the Torasemide group were mainly observed in Study 1 (23% versus 14% in Study 2), which led to the changes in the administered doses as previously described: smaller dose adjustment (0.1 mg/kg/d), possible decrease in dosage to 0.1 mg/kg/d, and maximal dose of 0.6 mg/kg for Study 2.
Table 9.
Adverse effects reported by clinical investigators
| Observed adverse events | Treatment group | P‐valueb | |||||
|---|---|---|---|---|---|---|---|
| Torasemide | Furosemide | ||||||
| n = 180 | Study 1 n = 75 | Study 2 n = 105 | n = 186 | Study 1 n = 76 | Study 2 n = 110 | ||
| “Overpharmacology” (polyuria‐polydipsia and/or urinary “incontinence” (as reported by the owners) related to a potent diuretic effect), n (%) | 36 (20) | 9 (12) | 27 (26) | 8 (4) | 2 (3) | 6 (5) | <0.001 |
| Gastrointestinal disorders (e.g, vomiting, diarrhea, anorexia), n (%) | 31 (17) | 10 (13) | 21 (20) | 21 (11) | 6 (8) | 15 (14) | 0.10 |
| Respiratory, n (%) | 2 (1) | 1 (1) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0.24 |
| Neoplasia n (%) | 3 (2) | 1 (1) | 2 (2) | 0 (0) | 0 (0) | 0 (0) | 0.12 |
| Hepatic enzyme elevation, n (%) | 5 (3) | 2 (3) | 3 (3) | 0 (0) | 0 (0) | 0 (0) | 0.028 |
| Electrolyte disorders, n (%) | 6 (3) | 5 (7) | 1 (1) | 8 (4) | 8 (11) | 0 (0) | 0.63 |
| Urinary, n (%) | 4 (2) | 1 (1) | 3 (3) | 0 (0) | 0 (0) | 0 (0) | 0.058 |
| Neurological, n (%) | 4 (2) | 1 (1) | 3 (3) | 10 (5) | 4 (5) | 6 (5) | 0.12 |
| Ocular, n (%) | 2 (1) | 0 (0) | 2 (2) | 5 (3) | 3 (4) | 2 (2) | 0.45 |
| Dermatological (dermatitis, otitis externa), n (%) | 5 (3) | 2 (3) | 3 (3) | 4 (2) | 2 (3) | 2 (2) | 0.75 |
| Reproduction, n (%) | 5 (3) | 1 (1) | 4 (4) | 1 (1) | 0 (0) | 1 (1) | 0.12 |
| Renala, n (%) | 32 (18) | 17 (23) | 15 (14) | 8 (4) | 2 (3) | 6 (5) | <0.001 |
| Integumentary (e.g, bite wounds, lacerations, declaw), n (%) | 3 (2) | 0 (0) | 3 (3) | 3 (2) | 3 (4) | 0 (0) | 1.00 |
| Muscular disorders (muscle tremors, lameness), n (%) | 3 (2) | 1 (1) | 2 (2) | 5 (3) | 2 (3) | 3 (3) | 0.72 |
| Others, n (%) | 13 (7) | 4 (5) | 9 (9) | 15 (8) | 4 (5) | 11 (10) | 0.76 |
Renal as per VeDDRA preferred term. In other words, renal effects covered all renal events from the most severe (acute renal failure) to the least severe (elevated renal parameters when compared to baseline, even if still within the reference ranges and even if not associated with clinical signs).
Torasemide group (n = 180) versus Furosemide group (n = 186).
P‐values that appear in bold are < 0.05.
IRIS stages at D84 were statistically significantly different between the 2 groups (Table 10; P = 0.041): IRIS stage I/II was slightly more frequently observed in the Furosemide group (94%) than in the Torasemide group (89%). Serum creatinine levels were higher (P = 0.0015) and serum potassium values were lower (P = 0.0027) in the Torasemide group, although both close to the laboratory reference ranges (Table 10).
Table 10.
Frequencies (number of dogs and percentages) or medians (interquartile range) of laboratory variables measured at D84 in the 2 treatment groups (i.e, on 273 dogs that ended the study).a
| Variable | Treatment group | |||
|---|---|---|---|---|
| Torasemide n = 133 | Furosemide n = 140 | P‐value | ||
| Serum K (mmol/L) | 4.30 [3.80–4.70] | 4.50 [4.20–5.00] | 0.0027 | |
| Serum creatinine (mg/L) | 12.10 [9.40–15.90] | 9.85 [8.20–13.60] | 0.0015 | |
| Global score | IRIS stage: 1/2/3/4 (%) | 86/32/15/0 (65/24/11/0) | 109/22/8/1 (78/16/6/1) | 0.041 |
| Study 1 | IRIS stage: 1/2/3/4 (%) | 39/12/6/0 (68/21/11/0) | 43/7/1/0 (84/14/2) | 0.098 |
| Study 2 | IRIS stage: 1/2/3/4 (%) | 47/20/9/0 (62/26/12/0) | 66/15/7/1 (74/17/8/1) | 0.22 |
IRIS, International Renal Interest Society; K, potassium.
Out of the 366 dogs included in the trial, 93 dogs did not end the study, owing to 24 cardiac deaths (composite cardiac endpoint), 4 additional cardiac deaths after CHF worsening, 20 non cardiac deaths, 38 withdrawals for adverse events, and 7 withdrawals following owners' wishes.
Laboratory reference ranges: serum K = 3.90–5.60 mmol/L; serum creatinine = 4.00–12.00 mg/L.
P‐values that appear in bold are < 0.05.
Discussion
Furosemide and newer loop diuretics, such as bumetanide and torasemide, are widely known in human cardiology and commonly included in the therapeutic arsenal of CHF in conjunction with other therapeutic classes.22, 23, 24, 25, 26, 27, 28, 35, 36, 37, 38, 39, 40 In the ACVIM consensus guidelines for the diagnosis and treatment of canine DMVD, furosemide is the only recommended diuretic agent and consensus was reached to use it as the first‐line drug of both acute and chronic ACVIM stage C CHF for hospital‐based or home‐based therapy.3 However, current evidence suggests that furosemide and newer loop diuretics are not identical. Such heterogeneity among loop diuretics has been highlighted in trials and meta‐analyses in human patients suggesting, for example, a superior efficacy with improved functional status and mortality for torasemide compared to furosemide,22, 23, 24, 25, 26, 27, 28, 35, 36, 37, 38, 39, 40 recently leading some authors to propose “to revisit alternatives to furosemide.”24
Despite this fundamental and widely accepted role of loop diuretics in the treatment of CHF, there is a paucity of pharmacological and clinical data on this therapeutic class in veterinary cardiology, and little information is available on the clinical use of torasemide in the dog.18, 19, 20, 47, 48, 49 To the best of our knowledge, the TEST study is the first prospective randomized single‐blinded clinical trial designed to comparatively assess the efficacy and safety of 2 loop diuretics, that is, torasemide and furosemide, in the management of CHF in a large canine population including more than 360 dogs with mild‐to‐severe CHF caused by DMVD.
Benefits of ACE inhibitors,57, 58, 59, 60 pimobendan,54, 61 and spironolactone62, 63 in DMVD dogs with CHF have been demonstrated. In all these prospective randomized controlled clinical trials, furosemide was authorized and prescribed in most (66%) to all recruited dogs.54, 57, 58, 60, 62 The present trial demonstrates that torasemide q24h is noninferior to furosemide q12h when considering a composite clinical score endpoint associated with radiographic confirmation of improvement of (Stratum 1) or persistent absence of (Stratum 2) pulmonary edema as compared to baseline. Further analysis of the results also showed that torasemide was associated with a 2‐fold reduction in the risk of reaching the secondary composite cardiac endpoint “spontaneous cardiac death, euthanasia for HF, or CHF class worsening” over a 3‐month period, after adjusting for potential confounders. These results offer evidence of efficacy of torasemide for the treatment of canine CHF, with the advantage of a single‐daily oral dosage versus a twice‐daily administration for furosemide.
Torasemide is a long‐acting loop diuretic characterized by a duration of action twice as long as furosemide, with a higher bioavailability.10, 18, 19 From a practical point of view, these pharmacokinetic properties are of great interest as they allow a single oral administration per day, as chosen in the present protocol design. Dogs with CHF are often prescribed numerous drugs, and decreasing their administration frequency could help to increase owners' compliance, and therefore contribute to the treatment success.3 Such single‐daily administration has already been reported in a small number of dogs,47 although a twice‐daily administration has also been described in a few others.48, 49
In the present studies, noninferiority was determined using a composite clinical score based on dyspnea, cough, exercise tolerance, and ascites, combined with radiographic assessment of pulmonary edema. Such composite clinical score represents a semiobjective measurement of dogs' response to therapy, based on the most common clinical signs of CHF assessed by owners and investigators. Additionally, to take into account the wide pleomorphic presentation of dogs in CHF, animals were further allocated into 2 groups (Stratum 1 and Stratum 2), and definition of treatment success was tailored to the presentation at inclusion. Dogs with an ongoing episode of CHF were allocated to Stratum 1, and the aim of the diuretic treatment for those dogs was to improve both clinical and radiographic signs of CHF. Dogs with previous episodes of CHF but stable on their current cardiac therapy were allocated to Stratum 2, and the aim of diuretic treatment for those cases was to maintain the clinical and radiographic signs over time with the possibility of some clinical improvement (e.g, improved exercise tolerance), probably related to decrease in pulmonary arterial hypertension, a common DMVD complication detected even in treated animals.64, 65 The strict requirement of such dual efficacy criteria provided a more robust and objective assessment of treatment success than the use of 1 single score (either radiographic or clinical), and in these studies, it permitted to conclude that torasemide was noninferior to furosemide.
To determine the impact of the 2 studied loop diuretics on outcome of DMVD dogs with CHF, a secondary efficacy criterion was used. This criterion was a composite cardiac endpoint comprised of cardiac death (spontaneous or euthanasia for cardiac reasons) and worsening of the CHF class. Similar composite criteria have already been used in clinical trials assessing efficacy of cardiac drugs in dogs with CHF related to DMVD.54, 61, 62 Variables highly suggestive of advanced heart disease and decreased clinical condition (i.e, decreased appetite, heart rate, syncope, and pretrial furosemide prescription ≥4 mg/kg/d) were associated with a shorter time to the composite cardiac endpoint. Conversely, torasemide was associated with a 2‐fold reduction in the risk of reaching the endpoint (aHR = 0.47; 95% CI 0.27–0.82; P = 0.0077). These results are similar to those obtained in the TORIC study.26 In this open‐label, nonrandomized, postmarketing surveillance trial involving 1,337 human patients with NYHA class II–III CHF, patients treated with torasemide had a 51.5% reduction in the risk of death compared to those receiving furosemide or other diuretics. This mortality benefit was associated with a greater functional improvement in NYHA class and a significantly lower incidence of abnormally low serum potassium levels.26 A meta‐analysis of several studies including the TORIC trial also suggested an improved functional status and mortality with torasemide as compared to furosemide, with a higher benefit of quality of life and reduced frequency and duration of CHF‐related hospitalizations.22, 24, 25, 26, 27, 28 It has been proposed that part of this mortality and morbidity benefit over furosemide might be related to the above‐mentioned pharmacokinetic differences and also to the pharmacodynamic properties of torasemide. Although not demonstrated in the dog, beyond its action on the thick loop of Henle, torasemide exerts vasodilating and antialdosterone effects, which are both beneficial in hypervolemic states.33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 In vitro and in vivo studies have suggested that torasemide has antialdosterone properties, either by blocking the mineralocorticoid receptor binding of aldosterone43 (although recent data suggested that torasemide does not act as a mineralocorticoid receptor antagonist)66 or by inhibition of aldosterone synthase, with these effects being associated with prevention of atrial fibrosis and atrial fibrillation in mice as well as improvement of cardiac function and survival rate in rats with CHF.42, 44, 45 Whether or not the mechanism underlying the risk reduction of reaching the composite cardiac endpoint observed in the Torasemide group of the present trial is related to an antialdosterone effect remains to be determined by further studies.
In the present studies, clinical investigators were permitted to determine the dosage of furosemide of each included dog at each visit. The therapist investigator was then responsible for converting each furosemide dosage into a torasemide dosage using a conversion table. Based on the published literature, the 1/10 ratio was used in Study 1,18, 49 and given the increase in renal values for the torasemide‐treated group, this ratio was further questioned leading to the second protocol with a lower increment change in torasemide (0.2 mg/kg versus 0.1 mg/kg per day) and the ability to decrease the dosage to 0.1 mg/kg q24h if needed. A recent studyi with a crossover design performed on healthy dogs receiving 0.1 and 0.6 mg/kg torasemide q24h or 5 dosages of furosemide ranging from 0.5 to 40 mg/kg q12h demonstrated that the ratio between furosemide and torasemide is closer to 1/20 when using urine output as the pharmacodynamic endpoint, compared to the previously published and used 1/10 ratio.18, 49 Based on these recent pharmacological data together with the results of the present trial confirming the potent diuretic effects of torasemide, we therefore recommend starting torasemide at a low dosage (with a 1/10–1/20 furosemide ratio) and closely monitoring the patient for clinical signs, renal values, and electrolytes to find the lowest effective dosage with minimal renal effects.
The present trial presents several limitations. The 3‐month follow‐up period was relatively short, which led to a small number of cardiac events during this time frame. A longer study period would have been of interest to assess the long‐term benefit of torasemide compared to furosemide. Dogs from the Furosemide group had been diagnosed with DMVD for a longer duration than those from the Torasemide group. Lastly, dogs from the Torasemide group had a significantly higher dyspnea score at baseline. However, this did not affect the composite clinical score, which was similar between groups at inclusion, and the stepwise multivariate analysis did not show any influence of these baseline differences as an increased risk of reaching the composite cardiac endpoint.
In conclusion, torasemide q24h is an effective diuretic in DMVD dogs with mild‐to‐severe CHF. Compared to dogs receiving furosemide twice daily, torasemide‐treated dogs showed a similar treatment success as a first diuretic treatment or as a replacement of furosemide and were even associated with a decreased risk in reaching the composite cardiac endpoint comprised of cardiac death and worsening of the CHF class. However, given its potent diuretic effects, the lowest effective dosage should always be determined and, as recommended by the ACVIM consensus guidelines, dogs under such diuretic treatment should be closely monitored for renal and electrolyte abnormalities.3 Further studies are now required to explore the potential beneficial antifibrotic effect of torasemide on dogs with CHF, and its potential benefit over furosemide on long‐term survival.
Acknowledgment
The authors thank all the TEST study investigators and owners of the dogs included in the study.
Conflict of Interest Declaration:
Five authors (Menard, Blanc, Rougier, Lucats, and Woehrle) were employed by Vetoquinol SA which sponsored and monitored these studies. The clinical trial process (conception, monitoring, data management, analyses, and reporting) was conducted according to Good Clinical Practice and European Medicines Agency (EMA) requirements. In accordance with the EMA, 2 consecutive prospective, randomized, single‐blinded, and positive‐controlled clinical trials were designed. These conditions were considered to have reduced the risk of bias.
Chetboul and Pouchelon were consulted for protocol design and results evaluation. Desquilbet was consulted for results evaluation. Petit participated to the trials monitoring under Vetoquinol sponsoring.
Off‐label Antimicrobial Declaration:
The authors declare no off‐label use of antimicrobials.
This article was published online on 4 October 2017. After online publication, minor revisions were made to the text and the Figure 2. This notice is included in the online version to indicate that has been corrected on 10 October 2017.
Footnotes
2N software, Martin‐Hauer Jensen, Program for Calculation of Sample Size for a Clinical or Experimental Study, University of Arkansas for Medical Sciences, 1992
Upcard, Vetoquinol SA, Lure, France
Dimazon, MSD Santé Animale, Beaucouze, France
Frusedale, Dechra Veterinary Products Limited, Shrewsbury, UK
Laboratoire Billiemaz, Toulon, France
Clintrial software version 4.5, Oracle Health, Redwood Shores
SAS 9.2, SAS Institute, Cary
Aldactazine, Pfizer Holding France, Paris, France
Schneider M, Bonavaud S, Menard J et al. Diuretic dosage equipotency between torasemide and furosemide in healthy dogs. Southern European Veterinary Conference 2015. Barcelona, Spain
References
- 1. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to revise the 1995 guidelines for the evaluation and management of heart failure). J Am Coll Cardiol 2001;38:2101–2113. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 3. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 4. Atkins CE, Häggström J. Pharmacologic management of myxomatous mitral valve disease in dogs. J Vet Cardiol 2012;14:165–184. [DOI] [PubMed] [Google Scholar]
- 5. Erling P, Mazzaferro EM. Left‐sided congestive heart failure in dogs: Treatment and monitoring of emergency patients. Compend Contin Educ Vet 2008;30:94–104. [PubMed] [Google Scholar]
- 6. DeFrancesco TC. Management of cardiac emergencies in small animals. Vet Clin North Am Small Anim Pract 2013;43:817–842. [DOI] [PubMed] [Google Scholar]
- 7. Levy PD, Bellou A. Acute heart failure treatment. Curr Emerg Hosp Med Rep 2013;1:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Futterman LG, Lemberg L. Diuretics, the most critical therapy in heart failure, yet often neglected in the literature. Am J Crit Care 2003;12:376–380. [PubMed] [Google Scholar]
- 9. El‐Sayed MG, Atef M, El‐Gendi AY, Youssef SA. Disposition kinetics of furosemide in dogs. Arch Int Pharmacodyn Ther 1981;253:4–10. [PubMed] [Google Scholar]
- 10. Roush GC, Kaur R, Ernst ME. Diuretics: A review and update. J Cardiovasc Pharmacol Ther 2014;19:5–13. [DOI] [PubMed] [Google Scholar]
- 11. Wargo KA, Banta WM. A comprehensive review of the loop diuretics: Should furosemide be first line? Ann Pharmacother 2009;43:1836–1847. [DOI] [PubMed] [Google Scholar]
- 12. Buggey J, Mentz RJ, Pitt B, et al. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J 2015;169:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thrusfield MV, Aiken CGC, Darke PGG. Observations on breed and sex in relation to canine heart valve incompetence. J Small Anim Pract 1985;26:709–717. [Google Scholar]
- 14. Kvart C, Häggstrom J. Acquired valvular heart disease In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 6th ed Philadelphia: WB Saunders; 2005:1022–1039. [Google Scholar]
- 15. Beardow AW, Buchanan JW. Chronic mitral valve disease in Cavalier King Charles spaniels: 95 cases (1987–1991). J Am Vet Med Assoc 1993;203:1023–1029. [PubMed] [Google Scholar]
- 16. Serfass P, Chetboul V, Carlos Sampedrano C, et al. Retrospective study of 942 small sized‐dogs: Prevalence of left apical systolic heart murmur and left‐sided heart failure, critical effects of breed, and sex. J Vet Cardiol 2006;8:1–8. [DOI] [PubMed] [Google Scholar]
- 17. Chetboul V, Tissier R, Villaret F, et al. Epidemiological, clinical, echo‐doppler characteristics of mitral valve endocardiosis in Cavalier King Charles in France: A retrospective study of 451 cases (1995 to 2003). Can Vet J 2004;45:1012–1015. [PMC free article] [PubMed] [Google Scholar]
- 18. Uechi M, Matsuoka M, Kuwajima E, et al. The effects of the loop diuretics furosemide and torasemide on diuresis in dogs and cats. J Vet Med Sci 2003;65:1057–1061. [DOI] [PubMed] [Google Scholar]
- 19. Hori Y, Takusagawa F, Ikadai H, et al. Effects of oral administration of furosemide and torsemide in healthy dogs. Am J Vet Res 2007;68:1058–1063. [DOI] [PubMed] [Google Scholar]
- 20. Harada K, Ukai Y, Kanakubo K, et al. Comparison of the diuretic effect of furosemide by different methods of administration in healthy dogs. J Vet Emerg Crit Care (San Antonio) 2015;25:364–371. [DOI] [PubMed] [Google Scholar]
- 21. Ishido H, Senzaki H. Torasemide for the treatment of heart failure. Cardiovasc Hematol Disord Drug Targets 2008;8:127–132. [DOI] [PubMed] [Google Scholar]
- 22. DiNicolantonio JJ. Should torsemide be the loop diuretic of choice in systolic heart failure? Future Cardiol 2012;8:707–728. [DOI] [PubMed] [Google Scholar]
- 23. Pitt B, Nicklas J. Loop diuretics in patients with heart failure: Time to change to torsemide? J Cardiovasc Pharmacol 2009;53:435–437. [DOI] [PubMed] [Google Scholar]
- 24. Bikdeli B, Strait KM, Dharmarajan K, et al. Dominance of furosemide for loop diuretic therapy in heart failure: Time to revisit the alternatives? J Am Coll Cardiol 2013;61:1549–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patterson JH, Adams KF, Applefeld MM, et al. Oral torsemide in patients with chronic congestive heart failure: Effects on body weight, edema, and electrolyte excretion. Torsemide Investigators Group. Pharmacotherapy 1994;14:514–521. [PubMed] [Google Scholar]
- 26. Cosín J, Díez J, TORIC investigators . Torasemide in chronic heart failure: Results of the TORIC study. Eur J Heart Fail 2002;4:507–513. [DOI] [PubMed] [Google Scholar]
- 27. Díez J, Coca A, de Teresa E, et al. TORAFIC study protocol: Torasemide prolonged release versus furosemide in patients with chronic heart failure. Expert Rev Cardiovasc Ther 2009;7:897–904. [DOI] [PubMed] [Google Scholar]
- 28. Muller K, Gamba G, Jaquet F, Hess B. Torasemide vs furosemide in primary care patients with chronic heart failure NYHA II to IV – efficacy and quality of life. Eur J Heart Fail 2003;5:793–801. [DOI] [PubMed] [Google Scholar]
- 29. Sogame Y, Okano K, Hayashi K, et al. Urinary excretion profile of torasemide and its diuretic action in dogs. J Pharm Pharmacol 1996;48:375–379. [DOI] [PubMed] [Google Scholar]
- 30. Uchida T, Ohtaki Y, Kido H, Watanabe M. Diuretic profile of a novel loop diuretic torasemide in rats and dogs. Drugs Exp Clin Res 1991;17:293–298. [PubMed] [Google Scholar]
- 31. Uchida T, Hayashi K, Suzuki Y, Matsumura Y. Effects of torasemide on renal haemodynamics and function in anaesthetized dogs. Clin Exp Pharmacol Physiol 1991;18:497–504. [DOI] [PubMed] [Google Scholar]
- 32. Plumb DC. In: Plumb DC. ed. Torasemide. Plumb's Veterinary Drug Handbook, 8th ed Wisconsin: Wiley‐Blackwell; 2015:1431–1433. [Google Scholar]
- 33. Uchida T, Yamanaga K, Kido H, et al. Diuretic and vasodilating actions of torasemide. Cardiology 1994;84:14–17. [DOI] [PubMed] [Google Scholar]
- 34. Uchida T, Kido H, Yamanaga K, et al. A novel loop diuretic, torasemide, inhibits thromboxane A2‐induced contraction in the isolated canine coronary artery. Prostaglandins Leukot Essent Fatty Acids 1992;45:121–124. [DOI] [PubMed] [Google Scholar]
- 35. Yamato M, Sasaki T, Honda K, et al. Effects of torasemide on left ventricular function and neurohumoral factors in patients with chronic heart failure. Circ J 2003;67:384–390. [DOI] [PubMed] [Google Scholar]
- 36. Harada K, Izawa H, Nishizawa T, et al. Beneficial effects of torasemide on systolic wall stress and sympathetic nervous activity in asymptomatic or mildly symptomatic patients with heart failure: Comparison with azosemide. J Cardiovasc Pharmacol 2009;53:468–473. [DOI] [PubMed] [Google Scholar]
- 37. López B, González A, Beaumont J, et al. Identification of a potential cardiac antifibrotic mechanism of torasemide in patients with chronic heart failure. J Am Coll Cardiol 2007;5:859–867. [DOI] [PubMed] [Google Scholar]
- 38. López B, Querejeta R, González A, et al. Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J Am Coll Cardiol 2004;43:2028–2035. [DOI] [PubMed] [Google Scholar]
- 39. Kasama S, Toyama T, Hatori T, et al. Effects of torasemide on cardiac sympathetic nerve activity and left ventricular remodelling in patients with congestive heart failure. Heart 2006;92:1434–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tsutamoto T, Sakai H, Wada A, et al. Torasemide inhibits transcardiac extraction of aldosterone in patients with congestive heart failure. J Am Coll Cardiol 2004;44:2252–2253. [DOI] [PubMed] [Google Scholar]
- 41. Han LN, Guo SL, Lin XM, et al. Torasemide reduces dilated cardiomyopathy, complication of arrhythmia, and progression to heart failure. Genet Mol Res 2014;13:7262–7274. [DOI] [PubMed] [Google Scholar]
- 42. Adam O, Zimmer C, Hanke N, et al. Inhibition of aldosterone synthase (CYP11B2) by torasemide prevents atrial fibrosis and atrial fibrillation in mice. J Mol Cell Cardiol 2015;85:140–150. [DOI] [PubMed] [Google Scholar]
- 43. Uchida T, Yamanaga K, Nishikawa M, et al. Anti‐aldosteronergic effect of torasemide. Eur J Pharmacol 1991;205:145–150. [DOI] [PubMed] [Google Scholar]
- 44. Veeraveedu PT, Watanabe K, Ma M, et al. Comparative effects of torasemide and furosemide in rats with heart failure. Biochem Pharmacol 2008;75:649–659. [DOI] [PubMed] [Google Scholar]
- 45. Veeraveedu PT, Watanabe K, Ma M, et al. Torasemide, a long‐acting loop diuretic, reduces the progression of myocarditis to dilated cardiomyopathy. Eur J Pharmacol 2008;581:121–131. [DOI] [PubMed] [Google Scholar]
- 46. Goodfriend TL, Ball DL, Oelkers W, Bähr V. Torsemide inhibits aldosterone secretion in vitro. Life Sci 1998;63:PL45–PL50. [DOI] [PubMed] [Google Scholar]
- 47. Caro‐Vadillo A, Ynaraja‐Ramírez E, Montoya‐Alonso JA. Effect of torsemide on serum and urine electrolyte levels in dogs with congestive heart failure. Vet Rec 2007;160:847–848. [DOI] [PubMed] [Google Scholar]
- 48. Oyama MA, Peddle GD, Reynolds CA, Singletary GE. Use of the loop diuretic torsemide in three dogs with advanced heart failure. J Vet Cardiol 2011;13:287–292. [DOI] [PubMed] [Google Scholar]
- 49. Peddle GD, Singletary GE, Reynolds CA, et al. Effect of torsemide and furosemide on clinical, laboratory, radiographic and quality of life variables in dogs with heart failure secondary to mitral valve disease. J Vet Cardiol 2012;14:253–259. [DOI] [PubMed] [Google Scholar]
- 50.VICH Topic GL9, Good Clinical Practices (CVMP/VICH/595/98‐FINAL).
- 51. EMA/CVMP/EWP/81976/2010 , Guideline on statistical principles for clinical trials for veterinary medicinal products (pharmaceuticals).
- 52. Chetboul V, Tissier R. Echocardiographic assessment of canine degenerative mitral valve disease. J Vet Cardiol 2012;14:127–148. [DOI] [PubMed] [Google Scholar]
- 53. Hansson K, Häggström J, Kvart C, Lord P. Left atrial to aortic root indices using two‐dimensional and M‐mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet Radiol Ultrasound 2002;43:568–575. [DOI] [PubMed] [Google Scholar]
- 54. Häggström J, Boswood A, O'Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: The QUEST study. J Vet Intern Med 2008;22:1124–1135. [DOI] [PubMed] [Google Scholar]
- 55. Autran de Morais H. In: Ettinger SJ, Feldman EC, eds. Pathophysiology of Heart Failure and Clinical Evaluation of Cardiac Function. Textbook of Veterinary Internal Medicine, 5th ed. Philadelphia, PA: WB Saunders; 2000:692–713. [Google Scholar]
- 56. Polzin DJ. In: Ettinger SJ, Feldman EC, eds. Chronic Kidney Disease. Textbook of Veterinary Internal Medicine, 7th ed. Philadelphia, PA: WB Saunders; 2010:1990–2021. [Google Scholar]
- 57. Pouchelon JL, Martignoni L, King JN, et al. The effect of benazepril on survival times and clinical signs of dogs with congestive heart failure: Results of a multicenter, prospective, randomised, double‐blinded, placebo‐controlled, long‐term clinical trial. J Vet Cardiol 1999;1:7–18. [DOI] [PubMed] [Google Scholar]
- 58. The IMPROVE Study Group . Acute and short‐term hemodynamic, echocardiographic, and clinical effects of enalapril maleate in dogs with naturally acquired heart failure: Results of the Invasive Multicenter PROspective Veterinary Evaluation of Enalapril study. J Vet Intern Med 1995;9:234–242. [DOI] [PubMed] [Google Scholar]
- 59. The COVE Study Group . Controlled clinical evaluation of enalapril in dogs with heart failure: Results of the Cooperative Veterinary Enalapril Study Group. J Vet Intern Med 1995;9:243–252. [DOI] [PubMed] [Google Scholar]
- 60. Ettinger SJ, Benitz AM, Ericsson GF, et al. Effects of enalapril maleate on survival of dogs with naturally acquired heart failure. The Long‐Term Investigation of Veterinary Enalapril (LIVE) Study Group. J Am Vet Med Assoc 1998;213:1573–1577. [PubMed] [Google Scholar]
- 61. Häggström J, Boswood A, O'Grady M, et al. Longitudinal analysis of quality of life, clinical, radiographic, echocardiographic, and laboratory variables in dogs with myxomatous mitral valve disease receiving pimobendan or benazepril: The QUEST study. J Vet Intern Med 2013;27:1441–1451. [DOI] [PubMed] [Google Scholar]
- 62. Bernay F, Bland JM, Häggström J, et al. Efficacy of spironolactone on survival in dogs with naturally occurring mitral regurgitation caused by myxomatous mitral valve disease. J Vet Intern Med 2010;24:331–341. [DOI] [PubMed] [Google Scholar]
- 63. Lefebvre HP, Ollivier E, Atkins CE, et al. Safety of spironolactone in dogs with chronic heart failure because of degenerative valvular disease: A population‐based, longitudinal study. J Vet Intern Med 2013;27:1083–1091. [DOI] [PubMed] [Google Scholar]
- 64. Borgarelli M, Abbott J, Braz‐Ruivo L, et al. Prevalence and prognostic importance of pulmonary hypertension in dogs with myxomatous mitral valve disease. J Vet Intern Med 2015;29:569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Serres FJ, Chetboul V, Tissier R, et al. Doppler echocardiography‐derived evidence of pulmonary arterial hypertension in dogs with degenerative mitral valve disease: 86 cases (2001–2005). J Am Vet Med Assoc 2006;229:1772–1778. [DOI] [PubMed] [Google Scholar]
- 66. Gravez B, Tarjus A, Jimenez‐Carino R, et al. The diuretic torasemide does not prevent aldosterone mineralocorticoid receptor activation in cardiomyocytes. PLoS ONE 2013;8:e73737. [DOI] [PMC free article] [PubMed] [Google Scholar]


