Abstract
Background
Mycophenolate mofetil (MMF), the prodrug of mycophenolic acid (MPA), is becoming increasingly popular as an alternative immunosuppressant in feline medicine. Pharmacokinetic information is not available for cats.
Objective
The purpose of this study was to determine whether MMF is biotransformed into the active metabolite MPA and to evaluate the disposition of MPA after a 2‐hour constant rate intravenous (IV) infusion of MMF in healthy cats.
Animals
Healthy cats (n = 6).
Methods
This was a prospective pilot study. All cats were administered MMF at 20 mg/kg every 12 hours over a 2‐hour constant rate infusion for 1 day. The concentrations of MPA and its derivatives in blood were determined using a validated UHPLC–UV method.
Results
All cats biotransformed MMF into MPA. The mean AUC0‐14 h ranged from 6 to 50 h*mg/L after IV dosing of MMF. Transient large bowel diarrhea was recorded in 2 of 6 cats after medication administration.
Conclusion and Clinical Importance
The disposition of MPA in plasma was highly variable, which could result in high interindividual variability in the safety and efficacy of treatment with MMF in cats.
Keywords: Cats, Immunosuppressant, Mycophenolate mofetil, Pharmacokinetics
Abbreviations
- AUC0–14 h
observed area under the drug concentration vs. time curve from zero to 14 hours after finishing the infusion of mycophenolate mofetil
- CBC
complete blood count
- IV
intravenous
- LLOD
lower limit of detection
- LLOQ
lower limit of quantification
- MMF
mycophenolate mofetil
- MPAGls
MPA phenol glucoside
- MPAG
MPA phenol glucuronide
- MPA
mycophenolic acid
- PK
pharmacokinetics
- UHPLC–UV
ultra‐high‐performance liquid chromatography ultraviolet
Cats suffer from multiple autoimmune diseases, such as hemolytic anemia, thrombocytopenia, inflammatory bowel disease, pemphigus foliaceus, and polyarthritis.1, 2, 3, 4 Unfortunately, some of these autoimmune diseases are progressive, unpredictable, and fatal if left untreated in cats. The first‐line medications used to treat immune‐mediated diseases are glucocorticoids, in particular, prednisolone.1, 2, 3, 4, 5 However, glucocorticoids are contraindicated in animals suffering from certain endocrine diseases, and in those cats that are sensitive to their adverse effects. Furthermore, there are animals that suffer from glucocorticoid refractory immune conditions or experience severe adverse effects, requiring alternative immune‐suppressive therapies to survive.1, 2, 3, 4
MMF is a fermentation product of the species Penicillium and the prodrug of the active moiety MPA.6 After oral administration, MMF undergoes presystemic de‐esterification by carboxylesterases.6, 7, 8 Tissue de‐esterification of MMF is wide spread and rapid.6 Once absorbed, MMF enters the liver via the portal vein and within the hepatocytes, gets converted into MPA (the active metabolite). In humans, MPA is eliminated from the body primarily by liver biotransformation (glucuronidation) into MPA phenol glucuronide (MPAG), a stable and pharmacologically nonactive metabolite, and MPA phenol glucoside (MPAGls). Some inactive MPA derivatives are then excreted from the body in the feces and urine, and some MPA undergoes enterohepatic recycling.6, 9 In humans, phase II glucuronidation of MPA constitutes a major metabolic pathway mediated by intestinal and liver UDP glucuronosyl transferases. Recent ex vivo studies revealed that the total conjugation capacity of MPA seems to be similar in dogs and cats, meaning that although cats typically do not glucuronidate medications efficiently, they adequately metabolize MPA utilizing glucosidation versus glucuronidation as done in dogs and humans.10
In human medicine, MMF is favored in cases of refractory immune‐mediated conditions and is used to prevent organ transplant rejection.6, 11, 12 MPA is an attractive immunosuppressant option for treating immune‐mediated conditions in veterinary medicine because of its T and B lymphocyte antiproliferative effect.13, 14, 15, 16 MPA inhibits inosine monophosphate dehydrogenase, a regulator of the intracellular guanine nucleotide pool, preventing lymphocyte proliferation, antibody production, cellular adhesion, and T and B lymphocyte migration.6 An in vitro cat study showed that MPA's T and B lymphocyte antiproliferative effect is concentration dependent,13 indicating that adequate levels of immune suppression should be achievable using an optimal dosage regimen. Unfortunately, there is a paucity of literature supporting MPA's use in cats, which limits the administration of safe and effective MMF. Therefore, the purpose of this study was to determine whether MMF is biotransformed into the active metabolite MPA and to evaluate the disposition of MPA after a 2‐hour constant rate IV infusion of MMF in healthy cats.
Materials and Methods
The research protocol was approved by the Washington State University Institutional Animal Care and Use Committee (ASAF # 04665‐005).
Study Population
Six healthy cats 1–4 years of age (mean, 2.1 years) with body weights 3.5–5.4 kg (mean 4.2 kg) were studied. There were 3 spayed females and 3 neutered males. A complete physical examination, a FeLV/FIV screening test, urinalysis, CBC, and serum chemistry were performed on each cat before enrollment in the study.
Experimental Protocol
The cats were treated with MMF at 20 mg/kg, IV, twice, 12 hours apart. The dose was selected based on clinical experience and use of MMF, and information available in the literature in people,9 dogs,11, 12, 14 and preliminary data in catsb (not reported). We selected a constant rate infusion over a bolus to avoid phlebitis, thrombosis, and potential allergic reactions to the drug or components of the formulation.9, 15 MMF1 IV solution was diluted with 5% dextrose in water to a final concentration of 6 mg/mL solution and then administered over 2 hours via syringe pump. Foodc was withheld 2 hours before and postdrug administration. Water was always freely available to the cats. A repeat CBC and serum chemistry were performed in all study cats within 24 hours post‐MMF IV infusion.
Blood Collection
Twenty‐four hours before administration of IV MMF, a dual port sampling jugular catheter was aseptically placed after sedation with ketamined (5 mg/kg IM), acepromazinee (0.02 mg/kg IM), and butorphanolf (0.4 mg/kg IM). The jugular catheters were bandaged and flushed with 1 mL of heparinized saline, IV, every 6–8 hours. Blood was collected before and at the end of the first 2‐hour infusion of MMF and at 0.75, 1.5, 3, 6, 9, 12 hours postinitial infusion. Additionally, blood samples were taken immediately after the end of the second infusion and at 12 and 24 hours after stopping the second infusion of MMF. A total of less than 5% of circulating blood volume was obtained for analysis. Blood samples were obtained and transferred to glass tubes containing citrate. The tubes were then centrifuged at 1800 × g for 8 minutes. The plasma was separated and placed into 200 μL aliquots in plastic Eppendorf tubes and stored at −80°C until analysis. The samples were analyzed in 1 batch for this study.
Determination of MPA, MPAG, and MPAGls
Plasma MPAg and its derivatives MPAGh and MPAGlsh were quantified using a chromatographic method validated in our laboratory.17 The method was validated according to the Guidelines for Bioanalytical Method Validation published by the Food and Drug Administration.18 Briefly, analysis of the metabolites was carried out using liquid chromatography with ultraviolet detection. The mobile phase consisted of solvent A, 0.05% phosphoric acidi in H2O and solvent B, acetonitrilei 100%, with a linear gradient elution from 77% A (4 minutes hold) to 39% B during 6 minutes, and returned to initial conditions over 5 minutes. The system was left for an additional 5 minutes to re‐equilibrate before injection of a new sample. The flow rate was 0.25 mL/min. The column and auto‐sampler temperatures were set at 32 and 25°C, respectively. Analytes were detected at 215 nm, and injection volume was 1 μL.
The extraction procedure was carried out by a method developed in our laboratory as reported by Rivera et al.19 The extraction and chromatographic methods used in this study allowed us to obtain LLOQs of 0.2, 0.25, and 0.2 μg/mL for MPA, MPAG, and MPAGls in feline plasma.
Pharmacokinetic Analysis
Pharmacokinetic parameters were determined using noncompartmental analyses.j The arithmetic mean of pharmacokinetic parameters was obtained by averaging the individual parameter estimates. Observed maximum plasma concentration (Cmax) and time of maximum plasma concentration (Tmax) of MPA correspond to the maximal concentration observed for each cat.
Results
The IV administration of MMF was tolerated in all cats. Serum biochemical values remained in the reference range after treatment of MMF. Self‐limiting transient loose stool (large bowel diarrhea with mucus) was noted in 2 of 6 study cats. One of the cats had 2 episodes of diarrhea within 12 hours of the first drug infusion. The other cat had 1 episode of diarrhea within 24 hours after the second administration of MMF. No housing or food intake was altered in any of the cats during the study period. The disposition of MPA was evaluated after a short intravenous infusion of MMF (Figs 1 and 2), and estimated pharmacokinetic parameters are presented in Table 1.
Figure 1.
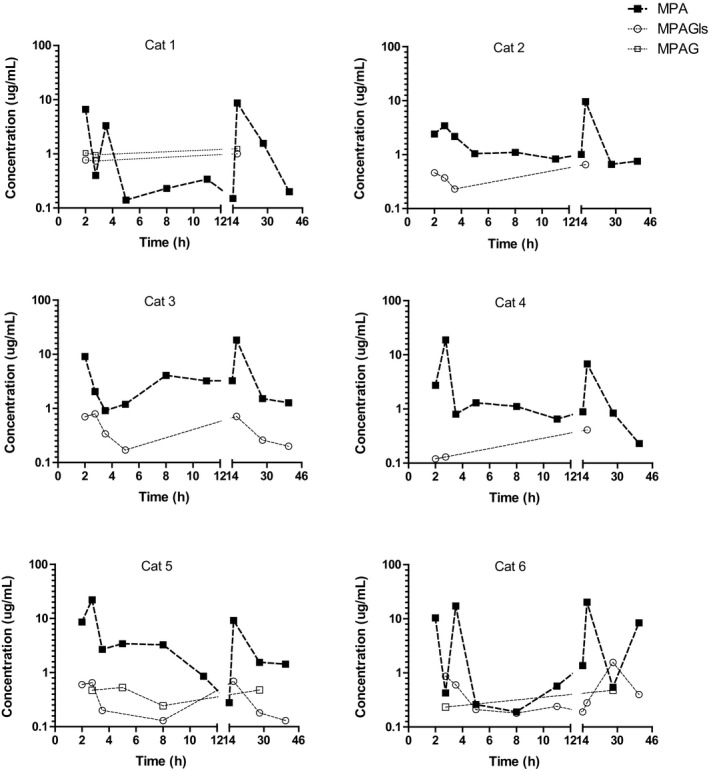
Disposition of MPA and its derivatives in plasma from individual cats after 2‐hour infusion of MMF at 20 mg/kg 12 hours apart (semilogarithmic plot).
Figure 2.
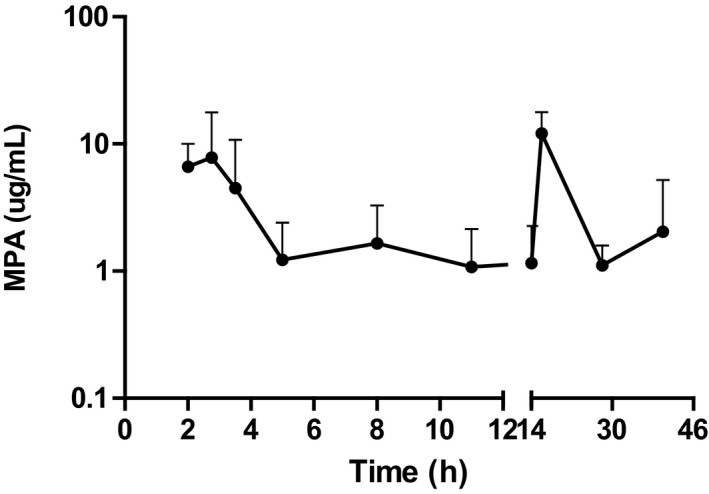
Disposition of MPA plasma concentration (mean ± standard deviation) cats (n = 6) after 2‐hour infusion of MMF at 20 mg/kg 12 hours apart (semilogarithmic plot).
Table 1.
Pharmacokinetic parameters of MPA in plasma from cats (n = 6)
| Pharmacokinetics Parameters | Mean | SD | Min | Max |
|---|---|---|---|---|
| First Infusion of MMF | ||||
| Time to maximal concentration after starting infusion (h) | 2.4 | 0.6 | 2.0 | 3.5 |
| Maximal concentration (μg/mL) | 12.9 | 7.5 | 3.4 | 22.1 |
| Concentration at the end of the dose interval (μg/mL) | 1.2 | 1.1 | 0.2 | 3.2 |
| AUC0‐14 h (h*mg/L) | 32 | 14 | 6 | 50 |
| Second Infusion of MMF | ||||
| Concentration at the end of the infusion (μg/mL) | 12.1 | 5.7 | 6.8 | 20.2 |
| Concentration at the end of the dose interval (μg/mL) | 2.05 | 3.16 | 0.2 | 8.41 |
All cats biotransformed MMF into MPA (Fig 1). The plasma concentration of MPA decreased relatively quickly during the first 4 hours after stopping the administration of MMF. The fast decay of MPA in plasma was followed by a slow decrease in the plasma concentration in all the cats (Figs 1 and 2). After the second infusion, MMF was also metabolized rapidly into MPA.
The metabolite MPAG was detected in 3 of 6 cats (Fig 1; cats 1, 5, and 6). This metabolite was not detected in any of the cats 6 hours after stopping the MMF infusion. In contrast, the metabolite MPAGls was detected in all 6 cats. The concentration of this metabolite ranged between the LLOQ 0.22 and 1 μg/mL. The maximum concentration of MPAGls was detected 24 hours after stopping the second administration MMF. We were not able to detect the metabolite acyl glucuronide in any of the cats. As mentioned in the material and methods section, MPA and its derivatives were quantified using a validated method. The accuracy and precision of the analytical method during the evaluation of the study samples were monitored using quality controls (QC). All the QC met the acceptance criteria and had a relative low variability (CV < 10%), reflecting a precise and accurate determination of MPA and its derivatives in the study samples.
Discussion
This is the first study describing the disposition of MPA in plasma after 2 constant rate intravenous infusions of MMF separated by 12 hours in cats (Fig 2). After infusion, all cats metabolized MMF into the active moiety MPA.
We expected to observe the maximal concentration of MPA right at the end of the infusion of MMF. However, in 3 of the cats (Fig 1; cats 4, 5, 6), the maximum concentration of MPA was detected at 0.75 and 1.5 hours after stopping the infusion of MMF. The rate of MPA elimination from the body and distribution into tissues could have played a role in this finding. However, the rate of formation of MPA is probably the most important contributing factor to this observation. A rate of biotransformation of MMF into MPA that was slower than the rate of infusion of MMF could have resulted in the continued synthesis of MPA at conclusion of the MMF infusion. This hypothetical “mismatch” between the rate of infusion and the rate of biotransformation of MMF into MPA could have been confirmed by evaluating the disposition of MMF in plasma during and after the infusion of the prodrug. Unfortunately, we did not assess the disposition of MMF in plasma from these cats, as an additional alternative IV catheter would have been necessary to accurately measure MMF independent of the infusion site. However, we acknowledge that studying the disposition of MMF and establishing the rate of biotransformation of MMF into MPA would provide valuable information for designing and optimizing constant rate infusions of MMF for cats.
The plasma concentration of MPA decreased at 2 different rates. A relatively fast decay of MPA occurred in plasma initially, followed by a slower decrease of the plasma concentration in 3 of the cats (2, 4, and 5). In the remaining cats, the plasma concentration of MPA slightly increased around 8–10 hours after starting the administration of MMF (Fig 1). Enterohepatic recycling of MPA could explain the very slow decay and the increments of the plasma concentration of MPA.6, 9 Interestingly, a relatively similar profile of MPA in plasma has been reported in humans.20 Overall, MPA was eliminated from the body rapidly, likely by hepatic biotransformation. MPA was metabolized into at least 2 metabolites; MPAGls and MPAG. The metabolite MPAGls was quantifiable in plasma from all the cats (n = 6) whereas MPAG was only quantifiable in 3 of 6 cats, suggesting that glucosidation of MPA is a key route for MPA elimination in cats, instead of glucuronidation. This is in agreement with a recently published in vitro study.10
One of the most remarkable aspects of the plasma disposition of MPA in cats is the relatively high interindividual variability of its plasma concentrations. This point does not seem to be feline specific, as it has also been a concern in human patients.9, 21 The high variability of MPA concentration in plasma can be translated into unpredictable drug responses, which raises a reasonable concern in patients that require sustained suppression of the immune system and are not being co‐treated with other immune suppressants. In our study, the variability of the analytical method was very low (~10%) and within the conventional standard limits.18 Therefore, it would not fully explain the variability observed in the plasma disposition of MPA. Interindividual variability in the rate of biotransformation of MMF and MPA, and enterohepatic recycling, could explain the large interindividual variability in the disposition of MPA (Table 1). The reasons for this variability remain to be determined. In human medicine, several factors were associated with high interindividual variability in the disposition of MPA, including sex, body weight, serum albumin, and renal and hepatic health.9 The cats in our study, had normal albumin, and renal and hepatic parameters. It is unknown whether abnormalities of these factors impact on the kinetics of MPA in cats.
The disposition of MPA after the administration of a second infusion of MMF suggests an apparent intra‐individual variability in the disposition of MPA. We expected that the concentrations of MPA after the first and second infusion of MMF were going to be relatively similar. However, 4 of 6 cats had a higher concentration of MPA at the end of the second infusion than after the first administration of MMF (Fig 1; cats 1, 2, 3, and 6). The reasons for the intra‐individual variability of MPA are unclear. At least 2 factors could have contributed to these findings; 1. interoccasion variability in the distribution or 2. interoccasion variability in the rate of biotransformation of MMF or MPA. Changes in the distribution of MMF or MPA are unlikely. In our study, all cats received the first infusion during the morning (8 am) and the second infusion 12 hours later. It is possible to speculate that the rate of biotransformation of MMF to MPA could have been influenced by the circadian cycle (chrono‐pharmacokinetics), considering that in human patients a circadian effect on MPA exposure has been reported.22 The potential effect of the circadian cycle on the exposure of MPA is intriguing and also deserves investigation.
We assessed whether MMF is biotransformed into MPA using 2 infusions of MMF because we wanted to explore the biotransformation using a dosage regimen that has been used for humans and dogs.9, 11, 12, 14 Unfortunately, the small sizes of the cats enrolled in this study did not allow us to safely collect additional blood samples and use a more intensive sampling design, which is ideal for estimation of additional pharmacokinetic parameters and a comprehensive characterization of the disposition of MPA in cats.
The results of this study did not permit us to establish whether the disposition of MPA in plasma is linear or nonlinear in cats. However, the results suggest that the disposition of MPA in plasma from cats after the administration of MMF is likely to be highly variable and unpredictable. In clinical settings, unpredictable exposure to MPA could be translated into uncertainty about the safety and efficacy of the treatment, putting the patient at risk for toxicity in the case of overexposure or poor immune suppression in the case of underexposure.
Several studies report different optimal plasma concentrations for humans. One recent study suggests that dosing MMF to achieve a target MPA AUC12 >35 mg.h/L is likely to lead to better efficacy and outcomes in patients with autoimmune diseases.9 Other studies set therapeutic targets at AUC0‐12 h of 30–60 mg.h/L23 or trough levels ranging between 1.0 and 5.0 μg/mL depending on the condition being treated.24, 25 The dose used in this study resulted in a comparable exposure to MPA (mean AUC0‐14 h 32 mg.h/L). Nevertheless, the exposure was highly variable (range AUC0‐14 h 6–50 mg.h/L9 and trough levels ranged from 0.2 to 3.2 μg/mL) (Table 1), suggesting that therapeutic drug monitoring and pharmacokinetic‐based dosing of MMF will probably be necessary for cats, particularly for those patients suffering from life‐threatening conditions unresponsive to other immune suppressants. The relationship between MPA exposure and its effect on T and B lymphocytes in cats requires further investigation to determine optimal dosage regimens.
A potential limitation of this study is the dosing choice of MMF. In this study, we used a relatively high dose of MMF in comparison with the dose of 10 mg/kg BID reported in the 1 published case study in cats.26 The oral dose used in the case report of MMF in cats was extrapolated from published human and canine literature.9, 11, 12, 14 We selected a dose of 20 mg/kg of MMF in our study based on dosing for humans (aCellcept dosing recommendations) and preliminary data about the MPA exposure obtained from 3 healthy cats.g MMF is used via the intravenous route in clinical settings for hospitalized patients that are not eating well or that are too debilitated for oral medications. One of the main concerns with using high doses of MMF is the risk of adverse gastrointestinal effects. Our results suggest that some cats tolerate 20 mg/kg of IV MMF. It is important to remark that we enrolled 6 healthy cats, and they were treated for only 24 hours. Therefore, the safety of this dosage regimen remains to be determined. Oral administration of MMF would likely be used for long‐term well‐designed safety studies to determine the absorption and distribution of the oral MMF. Additionally, using a target population of cats would provide more accurate information about feline tolerance of this drug.
In conclusion, this study reveals that cats biotransform MMF into the active metabolite MPA, and provides relevant novel information that can be used for designing and studying dosage regimens of MMF for cats. Clinicians should be aware that marked inter‐ and intra‐individual variability in the disposition of MPA in plasma can occur. The information generated in this study is valuable for clinicians treating critical hospitalized feline patients with immune‐mediated diseases. An in hospital constant rate infusion of MMF may be an option to treat cats with immune‐mediated disease; however, another study would be necessary to fully characterize the safety of MMF and disposition of MPA after the oral route in cats.
Acknowledgments
The authors thank the Humane Society of the Palouse for their support of this research.
Conflict of Interest Declaration:
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration:
Authors declare no off‐label use of antimicrobials.
All work for this project was performed at Washington State University, Pullman, WA.
This study was supported by the 2015 WSU Intra‐mural Research Fund, Beardsley‐Blanco Endowment Fund, and the Dorothy Shea Brink Memorial Fund. Michael Court is funded by the National Institutes of Health National Institute of General Medical Sciences [Grant R‐01‐GM102130 (to M.H.C.)] and the William R. Jones Endowment to Washington State University College of Veterinary Medicine.
The abstract for this study was presented at the 2016 ACVIM Forum, Denver, Colorado.
Footnotes
Cellcept intravenous mycophenolate mofetil hydrochloride for injection (6 mg/mL), Genetech USA Inc., South San Francisco, CA
Slovak JE, Rivera SM, Hwang JK, et al. Evaluation of intravenous mycophenolate mofetil use in healthy cats. ACVIM Forum Proceedings; 2016 June 8‐11 Denver, CO
Purina Cat Chow indoor formula, Purina Animal Nutrition Gray Summit, MO
Ketaset injectable (100 mg/mL), Zoetis Inc., Kalamazoo, MI
Promace injectable (10 mg/mL), Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO
Torbugesic injectable (100 mg/mL), Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO
MPA, Sigma‐Aldrich Fine Chemicals, St. Louis, MO
MPAG and MPAGls, Toronto Research Chemicals Inc., Toronto, Ontario
Phosphoric acid and acetonitrile, Fisher Scientific, Pittsburgh, PA
PK Solutions 2.0.x v. 2, Montrose, CO
References
- 1. Swann JW, Szladovis B, Glanemann B. Demographic characteristics, survival and prognostic factors for mortality in cats with primary immune‐mediated hemolytic anemia. J Vet Intern Med 2016;30:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Viviano K. Update on immunosuppressive therapies for dogs and cats. Vet Clin Small Animal. St. Louis, MO: Elsevier; 2013; 43: 1149–1170. [DOI] [PubMed] [Google Scholar]
- 3. Kohn B, Weingart C, Eckmann V, et al. Primary immune‐mediated hemolytic anemia in 19 cats: Diagnosis, therapy, and outcome (1998–2004). J Vet Intern Med 2006;20:159–166. [DOI] [PubMed] [Google Scholar]
- 4. Viviano K, Webb J. Clinical use of cyclosporine as an adjunctive therapy in the management of feline idiopathic pure red cell aplasia. J Feline Med Surg 2011;13:885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Court M. Feline drug metabolism and disposition. Vet Clin Small Animal. St. Louis, MO: Elsevier; 2013; 43:1039–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bullingham R, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Drug Metab Disp 1998;6:429–455. [DOI] [PubMed] [Google Scholar]
- 7. Fujiyama N, Masatomo M, Shotaro K, et al. Involvement of carboxylesterase 1 and 2 in the hydrolysis of mycophenolate mofetil. Drug Metab Disp 2010;12:2210–2217. [DOI] [PubMed] [Google Scholar]
- 8. Satoh T, Taylor P, Bosron WF, et al. Current progress on esterases: From molecular structure to function. Drug Metab Disp 2002;5:488–493. [DOI] [PubMed] [Google Scholar]
- 9. Abd Rahman A, Tett S, Staatz C. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in patients with autoimmune disease. Clin Pharm 2013;52:303–331. [DOI] [PubMed] [Google Scholar]
- 10. Slovak JE, Mealey K, Court MH. Comparative metabolism of mycophenolic acid by glucuronide and glucoside conjugation in human, dog and cat liver microsomes. J Vet Pharm Ther 2016;2:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lange S, Mueller SC, Altmann S, et al. Pharmacokinetics of oral mycophenolate mofetil in combination with CsA in dogs after nonmyeloablative allogeneic hematopoietic stem cell transplantation. Bio Bone Marrow Trans 2008;41:667–674. [DOI] [PubMed] [Google Scholar]
- 12. Lupu M, McCune J, Kuhr C, et al. Pharmacokinetics of oral mycophenolate mofetil in dogs: Bioavailability studies and the impact of antibiotic therapy. Bio Blood Marrow Transpl 2006;12:1352–1354. [DOI] [PubMed] [Google Scholar]
- 13. Kyles AE, Gregory CR, Craigmill AL. Comparison of the in vitro anti‐proliferative effects of five immunosuppressive drugs on lymphocytes in whole blood from cats. Am J Vet Res 2000;8:906–909. [DOI] [PubMed] [Google Scholar]
- 14. Wang A, Smith J, Creevy K. Treatment of canine idiopathic immune‐mediated haemolytic anemia with mycophenolate mofetil and glucocorticoids: 30 cases (2007–2011). J Small Anim Pract 2013;54:399–404. [DOI] [PubMed] [Google Scholar]
- 15. Jeong H, Kaplan B. Therapeutic monitoring of mycophenolate mofetil. Clin J Am Soc Nephrol 2007;2:184–191. [DOI] [PubMed] [Google Scholar]
- 16. Whitley NT, Day MJ. Immunomodulatory drugs and their application to the management of canine immune‐mediated disease. J Small Anim Pract 2011;52:70–85. [DOI] [PubMed] [Google Scholar]
- 17. Rivera SM, Slovak J, Court MH, et al. Simultaneous determination of mycophenolic acid and its glucuronide and glycoside derivatives in canine and feline plasma by UHPLC‐UV. Biom Chrom 2017;31:e3942. [DOI] [PubMed] [Google Scholar]
- 18. US Department of Health and Human Service, Food and Drug Administration, Center for Drug Evaluation and Research and Center for Veterinary Medicine . Guidance for Industry. Bioanalytical Method Validation, May 2001; www.fda.gov/downloads/Drugs/Guidance.
- 19. Rivera SM, Morassi A, Court MH, et al. Development and validation of an ultrafast chromatographic method for detection and quantification of the immunosuppressant mycophenolic acid in canine, feline and human plasma. J Pharm Bio Anal 2016;131:94–102. [DOI] [PubMed] [Google Scholar]
- 20. Bullingham R, Monroe S, Nicholls A, et al. Pharmacokinetics and bioavailability of mycophenolate mofetil in healthy subjects after single‐dose oral and intravenous administration. J Clin Pharm 1996;4:315–324. [DOI] [PubMed] [Google Scholar]
- 21. Todorova EK, Huang SH, Kobrzynski MC, et al. What is the intrapatient variability of mycophenolic acid trough levels? Ped Transplant 2015;7:669–674. [DOI] [PubMed] [Google Scholar]
- 22. Tedesco‐Silva H Jr, Felipe CR, Slade A, et al. Chronopharmacokinetics of mycophenolic acid and its glucuronide and acyl glucuronide metabolites in kidney transplant recipients converted from cyclosporine to everolimus. Ther Drug Monit 2012;6:652–659. [DOI] [PubMed] [Google Scholar]
- 23. Martial LC, Jacobs BA, Cornelissen EA, et al. Pharmacokinetics and target attainment of mycophenolate in pediatric renal transplant patients. Ped Transplant 2016;4:492–499. [DOI] [PubMed] [Google Scholar]
- 24. Gajarski RJ, Crowley DC, Zamberlan MC, et al. Lack of correlation between MMF dose and MPA level in pediatric and young adult cardiac transplant patients: Does the MPA level matter? Am J Transplant 2004;9:1495–1500. [DOI] [PubMed] [Google Scholar]
- 25. Dipchand AI, Pietra B, McCrindle BW, et al. Mycophenolic acid levels in pediatric heart transplant recipients receiving mycophenolate mofetil. J Heart Lung Transplant 2001;10:1035–1043. [DOI] [PubMed] [Google Scholar]
- 26. Bacek L, Macintire D. Treatment of primary immune‐mediated hemolytic anemia with mycophenolate mofetil in two cats. J Vet Emerg Crit Care 2011;21:45–49. [DOI] [PubMed] [Google Scholar]


