Abstract
Background
Canine diffuse large B‐cell lymphoma (DLBCL) is a common and aggressive hematologic malignancy. The lack of conventional therapies with sustainable efficacy warrants further investigation of novel therapeutics. The Janus kinase (JAK) and signal transducer and activator of transcription (STAT) pathways play important roles in the pathogenesis of hematologic malignancies in humans including DLBCLs. AZD1480 and CYT387 are novel JAK1/2 inhibitors that have been used in clinical trials for treating various hematologic cancers in humans. No studies have characterized the antitumor effects of JAK inhibitors on DLBCL in dogs.
Hypothesis/Objectives
We hypothesize that JAK1/2 inhibitors AZD1480 and CYT387 can effectively inhibit growth of canine DLBCL in vitro. We aim to assess the antitumor activity of AZD1480 and CYT387 in canine DLBCL and to determine the underlying mechanisms of action.
Methods
In vitro study of canine lymphoma cell growth, proliferation, and apoptosis by viability, proliferation and apoptosis assays.
Results
A significant decrease in viable canine lymphoma cells was observed after AZD1480 and CYT387 treatments. In addition, AZD1480 and CYT387 treatment resulted in decreased lymphoma cell proliferation and increased early apoptosis.
Conclusion and Clinical Importance
AZD1480 and CYT387 inhibit canine lymphoma cell growth in a dose‐dependent manner. Our findings justify further phase I/II clinical investigations of the safety and efficacy of JAK1/2 inhibitors in canine DLBCL and suggest new opportunities for novel anticancer therapies.
Keywords: Apoptosis, Canine DLBCLs, Cell cycle, JAK inhibitors
Abbreviations
- AE
adverse event
- AML
acute myeloid leukemia
- BCA
bicinchoninic acid
- DLBCL
diffuse large B‐cell lymphoma
- DPBS
Dulbecco's phosphate‐buffered saline
- ECL
enhanced chemiluminescence
- FBS
fetal bovine serum
- FDA
Food and Drug Administration
- FITC
fluorescein isothiocyanate
- Gapdh
glyceraldehyde 3‐phosphate dehydrogenase
- IC50
the half maximal inhibitory concentration
- IMDM
Iscove's Modified Dulbecco's Medium
- JAK
Janus kinase
- MDCK
Madin‐Darby canine kidney
- MFI
median fluorescence intensity
- MF
myelofibrosis
- MPN
myeloid proliferative neoplasia
- OD490
optical density at 490 nm
- PBS
phosphate‐buffered saline
- PCR
polymerase chain reaction
- PMSF
phenylmethylsulfonyl fluoride
- rh
recombinant human
- RIPA
radioimmunoprecipitation assay
- RPMI 1640 medium
Roswell Park Memorial Institute 1640 medium
- RPM
revolutions per minute
- SDS‐PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SDS‐PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- STAT
signal transducer and activator of transcription
- TBST
tris‐buffered saline with tween
- ΔΔCT
delta‐delta cycle threshold
The Janus kinase (JAK) and signal transducer and activator of transcription (STAT) pathways play important roles in proliferation and pathogenesis of hematologic malignancies.1 The JAK family of cytoplasmic tyrosine kinases includes JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2) in vertebrates. The JAK kinases are activated by tyrosine phosphorylation of the cytoplasmic domains of cytokine receptors after cytokine binding. Thrombopoietin, erythropoietin, granulocyte macrophage colony stimulating factor (GM‐CSF), interleukin 5 (IL‐5) and interleukin 3 (IL‐3), and other factors signal through JAK2.2 Activation of JAK2 promotes recruitment and phosphorylation of the transcription factor STAT3, which then leads to STAT3 dimerization and translocation into the nucleus where they activate downstream target genes important in cell proliferation, differentiation, and apoptosis.3 JAK2‐mediated STAT3 activation promotes growth and survival of a variety of human lymphomas.4, 5, 6 In diffuse large B‐cell lymphoma (DLBCL) of humans, JAK2‐STAT3 is constitutively activated and promotes lymphoma cell proliferation and survival.6 We have observed that STAT3 expression and activity are upregulated in dogs with DLBCL (unpublished data). Canine DLBCL is 1 of the most common cancers in dogs in the United States and serves as a comparative model for DLBCL in humans.7 Because of the poor prognosis with the current standard of care cyclophosphamide‐doxorubicin‐vincristine‐prednisone (CHOP), new therapies that may benefit both species are needed.
Identification of the JAK2 V617F mutation in human myeloid proliferative neoplasia (MPN) has led to the development of small‐molecule JAK inhibitors that target the deregulated JAK signaling pathway specifically. Many compounds with JAK inhibitory activity have been generated, with several currently being assessed in clinical trials of human patients diagnosed with MPN and acute myeloid leukemia (AML).8 AZD14801 is a pyrazolopyrimidine ATP competitive inhibitor of JAK1 and JAK2, with some activity against JAK3, TYK2, and Aurora‐A kinase at high maximal inhibitory concentration (IC) values. AZD1480 inhibits TEL‐JAK2 fusion in AML of humans,9, 10 but clinical evaluation of AZD1480 in patients with lymphoma has yet to be reported in either human or veterinary oncology. CYT387 (Momelotinib2) is a JAK1/2 inhibitor that has clinical activity in humans with myelofibrosis.11, 12 In veterinary medicine, the first JAK1/2 inhibitor Oclacitinib (Apoquel3) was approved by the Food and Drug Administration (FDA) in 2013 for treating dogs with atopic dermatitis.9, 13, 14, 15 Although success has been achieved in with this JAK1 inhibitor to treat dogs with dermatologic disease, no study has evaluated the potential therapeutic effects of JAK inhibitors in dogs with DLBCL. Our study aimed to evaluate the in vitro effects of JAK inhibitors AZD1480 and CYT387 in canine DLBCL cells and to elucidate the underlying mechanisms of AZD1480‐ and CYT387‐ mediated antitumor effects.
Materials and Methods
Cell Lines and Cell Culture Conditions
The canine diffuse large B‐cell lymphoma (DLBCL) cell line CLBL‐110 was cultured in Iscove's modified Dulbecco's medium (IMDM) containing 20% fetal bovine serum (FBS), 1 × penicillin/streptomycin, and 1 × L‐glutamine. Madin‐Darby canine kidney (MDCK), normal canine kidney cells, was maintained in Dulbecco's modified Eagle medium (DMEM) containing 10% FBS, 1 × penicillin/streptomycin, and 1 × nonessential amino acids. The human DLBCL cell line OCL‐LY7 was cultured in Roswell Park Memorial Institute (RPMI) 1640 medium containing 20% FBS, 1 × penicillin/streptomycin, 1 × nonessential amino acids, 1 mm sodium pyruvate, and 1 × L‐glutamine. All cell lines were cultured at 37°C with 5% CO2 and were passaged every 48 hours.
Canine Large B‐cell Lymphoma Samples and Fine Needle Aspirates
Lymph node samples from 3 client‐owned dogs with a cytologic diagnosis of naïve multicentric DLBCL were collected by the Oncology Service of the UW‐Madison Teaching Hospital. The immunophenotype was confirmed by flow cytometry evaluation at Colorado State University. All canine lymphoma cells were aspirated with a 20 ga needle into C10 medium containing RMPI1640 with 10% FBS, 1 × Penicillin/streptomycin, 1 × nonessential amino acids, 1 × L‐glutamine, 1 mm sodium pyruvate, and 1 × HEPES. Cells were spin down at 1.5 × 103 revolutions per minute (RPM) for 5 minutes at 4°C and were resuspended in ACK lysis buffer for RBC lysis. Lymphoma cells were used for a viability assay after RBC lysis.
Trypan Blue Exclusion Assay
The CLBL‐1 and MDCK cells were seeded at concentrations of 0.1 × 106/mL and 0.5 × 106/mL, respectively in 6‐well plates. Both groups of cells were treated with AZD1480 (Chemietek4) or CYT387 (Chemietek4) at concentrations of 1 μm, 2 μm, and 5 μm on the next day after cell plating. Dimethyl sulfoxide (DMSO, 100%) without AZD1480 or CYT387 was used as a control. Each cell line was seeded in triplicate for each treatment group and the entire experiment was repeated 3 times. The JAK inhibitors or DMSO‐treated cells were harvested after 24, 48, or 72 hours of drug treatment. The trypan blue assay was used to determine the number of viable cells present in a cell suspension. The viable cells were counted by trypan blue exclusion assay before the drug treatment, or with 24‐, 48‐, or 72‐hour drug treatment. The percentage of viable cells was normalized to the predrug treatment count. The trypan blue dye was mixed with the cell suspension at a ratio of 1 : 1. Viable cells were counted with a hemocytometer.
3‐(4,5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐Tetrazolium (MTS) Assay and the Half Maximal Inhibitory Concentration (IC50)
Cell growth in vitro was measured by the CellTiter96 AQueous One Solution Cell Proliferation assay (Pormega5). In brief, canine lymphoma cell line CLBL‐1, human lymphoma cell line OCL‐LY7, or primary canine lymphoma cells were suspended to a final concentration of 4 × 105 cells/mL in their culture medium. 100 μL (40,000 cells) was dispensed into each well of 96‐well plates and quadruplicates were made for each treatment group. The cells were treated with AZD1480 or CYT387 at concentrations of 1 μm, 2 μm, or 5 μm continuously for 24, 48, or 72 hours, and 20 μL 3‐(4,5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium/phenazine ethosulfate solution was added per well after drug treatments. Plates were incubated at 37°C for 1–4 hours in a humidified, 5% CO2 atmosphere. The absorbance at 490 nm was recorded by a 96‐well plate reader. The optical density at 490 nm (OD490) of JAK inhibitor‐treated groups was normalized by the OD490 of a DMSO control, and results were plotted as the normalized OD490 (Y axis) versus the concentration of JAK inhibitors (X axis). The IC50 was defined as the concentration of JAK inhibitors necessary to give one‐half of the inhibitory response and was calculated with GraphPad Prism v6.05.6
Reverse Transcription and Real‐Time PCR
The CLBL‐1 cells were treated with DMSO (control), AZD1480 (2.5 μm), or CYT387 (1.5 μm) in 6‐well plates (1 × 106/well) for 48 hours. Total RNA was isolated with RNeasy Mini Kit (Qiagen 741047) according to the manufacturer's protocol. Complementary DNA (cDNA) was synthesized with a SuperScript III First‐Strand Synthesis System Kit (Life Technologies, 18080‐518) with oligo (dt) primers. Jack1 and Jack2 transcripts were detected by Roche Lightcycler 969 with the cycle setting at 95°C (10 minutes), 95°C (10 seconds), 60°C (10 seconds), and 72°C (20 seconds) for a total of 40 cycles. Glyceraldehyde 3‐phosphate dehydrogenase (Gapdh) was used as an internal control for normalization, and quantitation was determined by the delta‐delta cycle threshold (ΔΔCT) method. The primer sequences are listed below: Jak1 forward: 5′‐CCCCCATTGATCGTCCACAA‐3′; Jak1 reverse: 5′‐CACATACATCCCCTCCTCGC‐3′, Jak2 forward: 5′‐TAGGGTTTCCTGGTGCTT‐3′, Jak2 reverse: 5′‐TGTTGTCTTGTAGAGGGTCAT‐3′, Gapdh forward: 5′‐TAGTGAAGCAGGCATCGGAG‐3′ and Gapdh reverse: 5′‐CGAAGGTGGAAGAGTGGGTG‐3′.
Western Blot
The CLBL‐1 cells were treated with control DMSO, AZD1480 (1–5 μm), or CYT387 (1–5 μm) for 12 or 24 hours and then were lysed in radioimmunoprecipitation assay (RIPA) buffer (Tris‐HCL 25 mm, NaCL 150 mm, NP‐40 1%, sodium dodecyl sulfate 0.1%, and Na deoxycholate 1%) with 1 × phenylmethylsulfonyl fluoride (PMSF), 1 × Halt protease inhibitor cocktail,10 and 1 × Halt phosphatase inhibitor cocktail10 and incubated on ice for 20 minutes. The supernatant was collected after full‐speed centrifugation at 13,000 RPM for 15 minutes at 4°C. The protein concentrations were verified by a standard bicinchoninic acid (BCA) method (Pierce BCA Protein Assay Kit11). Total cell lysates were mixed with 4 × Laemmli Sample Buffer12, and 30 μg of protein was loaded into each well. Because the endogenous expression level of p‐JAK2 was very low (Figure S1), for detection of phosphorylated JAK2 and phosphorylated STAT3, cells were stimulated with 25 ng/mL of recombinant human (rh) IL‐6 (PeproTech13) for 10 minutes before cell lysate preparations were made. For detection of total JAK1, total JAK2, and total STAT3, CLBL‐1 cells were not stimulated with any cytokine. All samples were boiled at 100°C for 5–10 minutes before loading on SDS‐polyacrylamide gels. Samples were electrophoresed on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE) (Bio‐Rad Mini gel system12). Transfer of proteins to nitrocellulose membrane was performed at 4°C for 1.5–2 hours at 110 V using a 25 mm Tris, 192 mm glycine, 20% (v/v) methanol, pH 8.3 transfer buffer (Bio‐Rad Mini Trans‐Blot system12). After blocking for 2 hours in 5% milk at 37°C, blots were incubated with the following commercial antibodies: p‐JAK2 (1 : 500, Santa Cruz Biotechnology14, sc‐16566‐R), p‐STAT3 (1 : 500, Cell Signaling Technology15, Y705 D3A), JAK1 (1 : 500, Cell Signaling Technology15, 6G4), JAK2 (1 : 500, Cell Signaling Technology15, D2E12), and β‐actin (1 : 3000, Sigma‐Aldrich16, AC‐15) overnight at 4°C. Blots were washed 5 times with tris‐buffered saline with tween (TBST) buffer and developed with the enhanced chemiluminescence (ECL) detection system (Bio‐Rad12) according to the manufacturer's protocol.
Flow Cytometry Analysis of p‐STAT3
Flow cytometry analysis of p‐STAT3 was performed as previously described.16 Briefly, CLBL‐1 cells were plated in 6‐well plates (1 × 106/well) treated with control (DMSO), AZD1480 (2.5 μm), or CYT387 (1.5 μm). After 24‐hour JAK inhibitor treatment, the cells were harvested and washed with phosphate‐buffered saline (PBS). Cells were resuspended in 1 mL of IMDM with 1% BSA and incubated for 90 minutes at 37°C for serum starvation. The cells were incubated with DMSO control or JAK inhibitors for another 10 minutes and then stimulated with 25 ng/mL rhIL‐6 (PeproTech13) for 10 minutes at 37°C. Cells were fixed with 2% paraformaldehyde and permeabilized by 95% methanol. p‐STAT3 was detected by Alexa Fluor 647‐conjugated primary antibody (pY705, BD Biosciences17, 562673). Samples were analyzed on the following day by BD LSRFortessa (BD Biosciences17). Results were analyzed by FlowJo v10.0.7 software18.
Apoptosis Assay
CLBL‐1 and MDCK cells were treated with AZD1480 and CYT387 as described in the cell viability assay. JAK inhibitor‐treated CLBL‐1 and MDCK cells were stained with Annexin V and SYTOX Red dead cell staining and were evaluated by flow cytometry. After 72 hours of drug treatment, cells treated with DMSO, AZD1480 (2 μm and 5 μm), or CYT387 (1 μm and 5 μm) were collected and washed once with Dulbecco's phosphate‐buffered saline (DPBS) and once with 1 × Annexin V binding buffer (0.1 m Hepes/NaOH, 1.4 m NaCl, and 5 mm CaCl2). Cells at a concentration of 1 × 106 cells/mL were resuspended in the binding buffer solution and stained with fluorescein isothiocyanate (FITC)‐conjugated Annexin V (eBioscience19; 1 : 40) and SYTOX DNA binding dye (Thermo Fisher Scientific10; 1 : 1000). Samples were analyzed with BD LSRII flow cytometry machine (BD Biosciences17), and results were analyzed by FlowJo v10.0.7 software18.
Cell Proliferation Assay
After 72‐hour JAK inhibitor treatment, 0.5 × 106 CLBL‐1 cells were harvested. Cells first were stained with the fixable viability dye, Ghost Dye Red 780 (Tonbo Biosciences20; 1 : 1000). For intracellular Ki67 staining, cells were fixed and permeabilized with the BD Cytofix/Cytoperm kit (BD Biosciences17) and further labeled with FITC anti‐Ki67 (BD Biosciences17; 1 : 20). DAPI solution (Thermo Fisher Scientific10; 1 : 250) then was added and incubated overnight at 4°C. Samples were analyzed the next day on a BD LSRFortessa (BD Biosciences17) and results were analyzed with FlowJo v10.0.7 software18.
Statistical Analysis
All statistical analyses were conducted by GraphPad Prism v6.05.6 One‐way analysis of variance (ANOVA) followed by Tukey's post hoc test was used to compare the changes in the proportion of cells undergoing apoptosis, as measured by Annexin V and SYTOX Red flow cytometric analysis. Differences between the vehicle control and selected drug concentrations at different time points after treatment were determined by 1‐way ANOVA followed by Dunnett's multiple comparisons. Upon normalization of cell viability data to DMSO control, 2‐tailed unpaired Student's t tests were used to determine the significance of difference between the CLBL‐1 and MDCK cell lines at different concentrations of AZD1480 or CYT387. P‐values ≤0.05 were considered significant.
Results
JAK1/2 Inhibitors AZD1480 and CYT387 Decrease Canine DLBCL Cell Growth In Vitro
To test the potential therapeutic effects of AZD1480 and CYT387 on canine DLBCLs, canine diffuse large B‐cell lymphoma cell line (CLBL‐1 cells10) was utilized to test whether AZD1480 or CYT387 can inhibit canine DLBCLs cell growth in vitro. The normal canine kidney cell line, MDCK, was used to compare the effect of AZD1480 and CYT387 on lymphoma cells versus normal cells. Compared with the DMSO control group, there was a significant decrease in viable CLBL‐1 cells in both AZD1480‐ and CYT387‐treated cells. This inhibitory effect in CLBL‐1 cells was dose‐dependent as there was a gradual decrease in viable cells with increasing drug concentrations (Fig 1A & B). In contrast, both AZD1480 and CYT387 only had mild inhibitory effects on normal canine kidney cell (MDCK) even at the high concentrations of 5 μm (Fig 1C & D). To further investigate if the cell growth inhibition effects caused by AZD1480 and CYT387 were more significant in canine lymphoma cells, we compared the percentage of cell growth reduction in JAK inhibitor‐treated CLBL‐1 cells to MDCK. CLBL‐1 and MDCK cells treated by DMSO only were used as control. With 48 or 72‐hour drug treatment, both AZD1480 and CYT387 caused significantly greater reduction of cell growth in canine DLBCL cells than in normal canine kidney cells, especially at higher concentrations of 2 μm and 5 μm (Fig 1E–G). Therefore, JAK inhibitors AZD1480 and CYT387 cause more growth inhibition in canine lymphoma cell line as compared to normal kidney cells.
Figure 1.
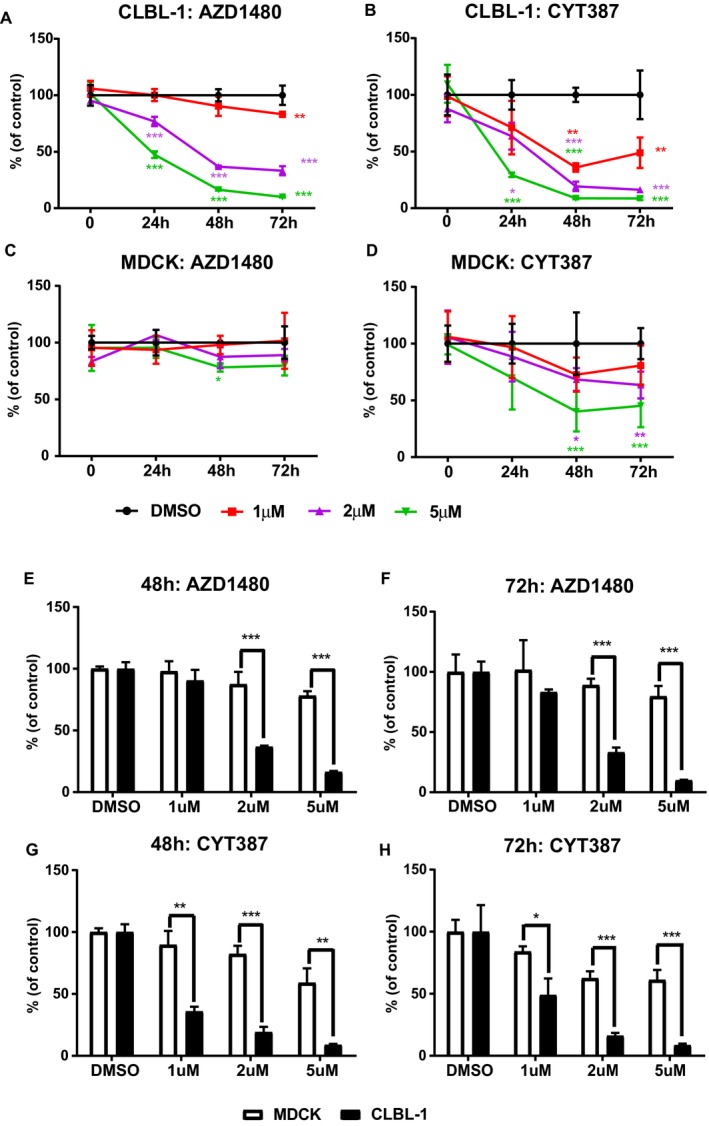
(A–D) Trypan blue exclusion assay show that JAK inhibitors AZD1480 and CYT387 inhibit canine DLBCL cell growth in vitro. Canine diffuse large B‐cell lymphoma cell line CLBL‐1 and normal canine kidney cells (MDCK) were treated with AZD1480 or CYT387 at variable concentrations. DMSO‐treated cells were used as an internal control. The percentage of growth was normalized to the control treatment with DMSO. (E–H) Canine DLBCL cells CLBL‐1 are more sensitive to JAK inhibitors AZD1480 and CYT387 compared with canine normal kidney cells MDCK. The reduction of cell growth in CLBL‐1 cells was more significant compared to MDCK cells with 48 and 72‐hour drug treatments. The percentage of growth was normalized to the control treatment with DMSO (*P < 0.05; **P < 0.01; ***P < 0.001). Data were presented as mean ± SD.
In addition to the trypan blue exclusion assay, CellTiter96 AQueous One Solution Cell Proliferation assay was conducted. Consistent with the result of the trypan blue exclusion assay, there was a significant dose‐dependent reduction in CLBL‐1 cell growth after 24, 48, or 72 hours of drug treatment (Fig 2A). To further quantify the concentration of JAK inhibitors necessary for IC50, CLBL‐1 cells were treated with AZD1480 or CYT387 at concentrations of 0–6 μm for 72 hours. MTS assay was conducted and the OD490 of JAK inhibitor‐treated groups was normalized by the OD490 of the DMSO control. The IC50 of AZD1480 with 72‐hour treatment was 2.58 μm, and the IC50 of CYT387 with 72‐hour treatment was 1.56 μm (Fig 2B).
Figure 2.
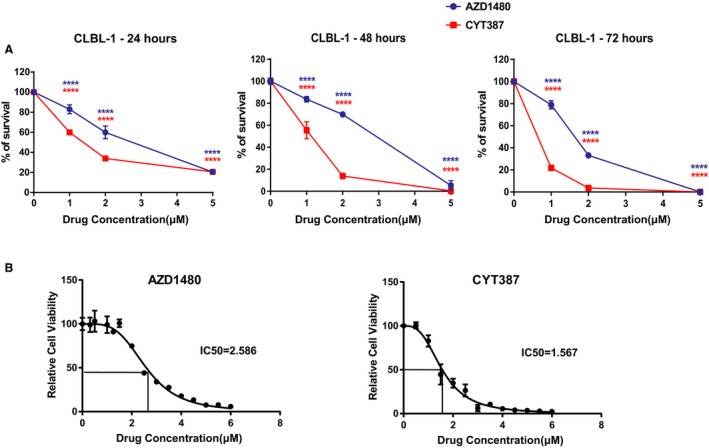
(A) CellTiter96 AQueous One Solution Cell Proliferation assays show that JAK inhibitors AZD1480 and CYT387 reduce the viability of canine CLBL‐1 cells. (B) IC50 of AZD1480 and CYT387 in CLBL‐1 cells after 72‐hour drug treatment. The percentage of growth was normalized to the control treatment with DMSO (***P < 0.001). Data were presented as mean ± SD.
JAK1/2 Inhibitors AZD1480 and CYT387 Decrease the Viability of Primary Canine DLBCL Cells and Human Lymphoma Cell Line
In addition to CLBL‐1 cells, we tested whether AZD1480 and CYT387 can decrease the viability of canine primary DLBCL cells. Three canine DLBCL samples were aspirated from the lymph nodes of canine DLBCL patients at the Oncology Service of UW‐Madison Teaching Hospital. Lymphoma diagnosis and immunophenotype were confirmed by cytology evaluation and flow cytometry, respectively. Approximately 40,000 primary canine lymphoma cells were plated per well of a 96‐well plate and were treated with AZD1480 and CYT387 at concentrations of 1 μm, 2 μm, or 5 μm. CellTiter96 AQueous One Solution Cell Proliferation assays were performed after 48 hours of drug treatment. Compared with the DMSO control group, all 3 canine primary lymphoma cells had a significant reduction in cell growth with AZD1480 or CYT387 treatment (Fig 3A–C). Consistent with the CLBL‐1 cell line result, these data show that AZD1480 and CYT387 decrease primary canine lymphoma cell growth. As DLBCL in dogs is a well‐established animal model for non‐Hodgkin lymphoma of humans,7 we extended our study to test whether AZD1480 and CYT387 can decrease the viability of human DLBCL cell line OCL‐LY7. At concentrations of 2 μM and 5 μM, 48‐hour drug treatment with AZD1480 and CYT387 resulted a significant reduction in viability of human lymphoma cells compared with the DMSO control. At a concentration of 1 μm, CYT387 also significantly decreased cell viability, but no significant effect was observed in the AZD1480‐treated group (Fig 3D). By utilizing a panel of both canine and human lymphoma samples, we conclude that AZD1480 and CYT387 decrease the viability of DLBCL cells.
Figure 3.
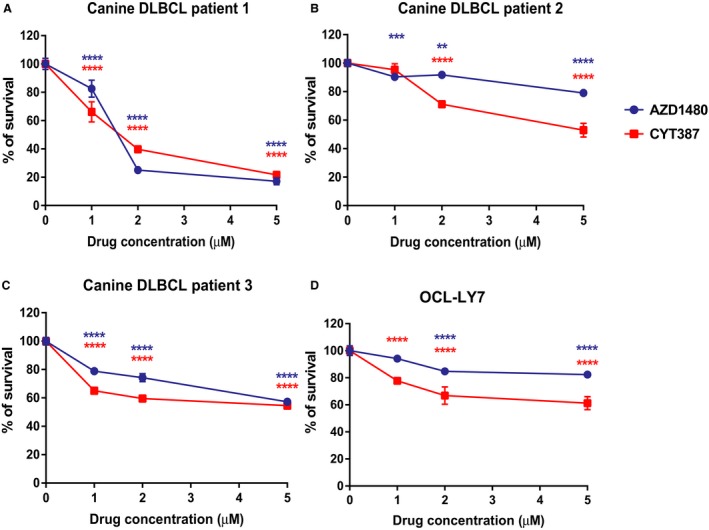
CellTiter96 AQueous One Solution Cell Proliferation assays show that JAK inhibitors AZD1480 and CYT387 reduce the viability of primary canine lymphoma cells (A‐C) and human DLBCL cell line OCL‐LY7 (D). The percentage of growth was normalized to the control treatment with DMSO (*P < 0.05; **P < 0.01; ***P < 0.001). Data were presented as mean ± SD.
JAK1/2 Inhibitors AZD1480 and CYT387 Inhibit Phosphorylation of STAT3 in a Dose‐Dependent Manner In Vitro
To evaluate how AZD1480 and CYT387 impact JAK2 and its downstream target STAT3 expression and activation, CLBL‐1 cells were treated with DMSO, AZD1480 (2.5 μm), or CYT287 (1.5 μm) at their respective IC50 concentrations for 48 hours. Total RNA and cell lysates were harvested. JAK1 and JAK2 mRNA levels were evaluated by quantitative real‐time PCR. AZD1480 and CYT387 treatment did not decrease JAK1 or JAK2 mRNA expression (Fig 4A). Instead, we detected a slight increase in JAK1 and JAK2 mRNA expression in the CYT387‐treated group. To evaluate the protein expression of JAK1 and JAK2, total cell lysates from DMSO‐, AZD1480‐, and CYT387‐treated groups were fractionated by SDS‐PAGE, and Western blot was conducted with primary antibodies against total JAK1, JAK2, and STAT3. Beta actin was used as a loading control. Compared with the DMSO control group, total JAK1, JAK2, and STAT3 had similar protein expression levels in AZD1480‐ or CYT387‐treated group (Fig 4B left panel). Thus, AZD1480 and CYT387 do not inhibit JAK1 and JAK2 expressions at either the mRNA transcript or protein level. Because JAK2 and STAT3 are activated by phosphorylation of tyrosine residues, we further assessed whether AZD1480 and CYT387 can inhibit activation of the JAK2‐STAT3 pathway by decreasing phosphorylation of JAK2 and STAT3. Compared with the DMSO‐treated control cells, both p‐JAK2 and p‐STAT3 were decreased in CYT387 or AZD1480‐treated cells at a concentration of 5 μM (Fig 4B right panel). In addition, inhibition of STAT3 phosphorylation was dose‐dependent as there was a gradual decrease in p‐STAT3 protein expression with increasing concentrations of AZD1480 and CYT387. To further support this conclusion that STAT3 phosphorylation was decreased by AZD1480 and CYT387 treatments, we evaluated STAT3 phosphorylation by flow cytometry analysis (Fig 4C & 4D). In DMSO‐treated CLBL‐1 cells, p‐STAT3 expression increased significantly upon cytokine IL‐6 stimulation. However, in AZD1480 (2.5 μm)‐ and CYT387 (1.5 μm)‐treated cells, p‐STAT3 expression was not responsive to IL‐6 stimulation and remained at the basal level (Fig 4C). There was a significant increase in p‐STAT3 median fluorescence intensity (MFI) in the DMSO control group (2.4‐fold increase) upon IL‐6 stimulation, but MFI did not change in AZD1480 or CYT387‐treated cells even with cytokine stimulation (Fig 4D). We conclude that AZD1480 and CYT387 treatment do not inhibit JAK1/2 transcript or protein expressions in DLBCL cells, instead, they inhibit JAK2 and STAT3 phosphorylation and activation.
Figure 4.
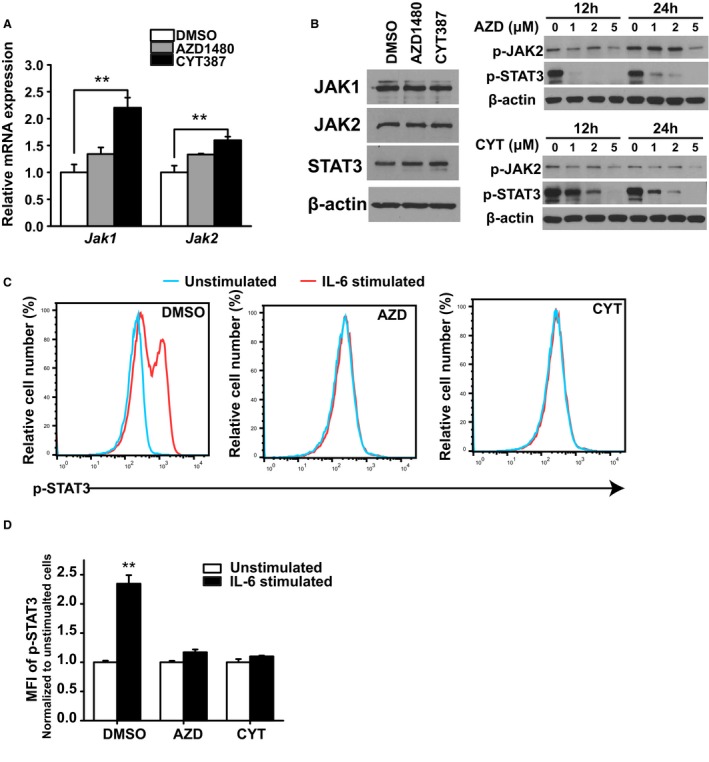
(A) JAK1 and JAK2 mRNA expressions in DMSO‐, AZD1480‐, or CYT387‐treated CLBL‐1 cells. Gapdh was used as an internal control. Relative mRNA expression level was normalized to DMSO control group. (B) Total JAK1, JAK2, p‐JAK2, STAT3, and p‐STAT3 protein expressions in DMSO (0 μm)‐, AZD1480 (1–5 μm)‐, or CYT387 (1–5 μm)‐ treated CLBL‐1 cells. β‐actin was used as a loading control for Western blot. (C) Flow cytometry analysis of p‐STAT3 expressions upon IL‐6 stimulation in DMSO‐, AZD1480‐, or CYT387‐treated CLBL‐1 cells. (D) Quantification of median fluorescence intensity (MFI) of p‐STAT3 in DMSO‐, AZD1480‐, or CYT387‐treated CLBL‐1 cells. The fold increase was normalized to unstimulated control. **P < 0.01
AZD1480 and CYT387 Inhibit Canine DLBCL Cell Growth by Increasing Apoptosis
To explore the underlying mechanism of JAK inhibitors AZD1480 and CYT387 on canine DLBCL cell growth, we performed an apoptosis assay to determine if the decrease in cell number occurred as a consequence of increased apoptosis. Both CLBL‐1 and MDCK cells were treated with DMSO, AZD1480 (2 μm or 5 μm), or CYT387 (1 μm or 5 μm). At 72‐hour post‐treatment, CLBL‐1 and MDCK cells were stained with Annexin V and SYTOX Red dead cell stain and were subjected to flow cytometry analysis. Early apoptotic cells were defined as Annexin V‐positive and SYTOX Red dead cell stain‐negative. Healthy cells were defined as double negative for both Annexin V and SYTOX Red dead cell stain (Fig 5A & D). Although the percentage of healthy CLBL‐1 cells decreased with increasing concentrations of AZD1480 and CYT387, the percentage of early apoptotic cells was increased (Fig 5B & E). There was no significant change in the percentage of early apoptotic cells in AZD1480‐treated MDCK cells or CYT387‐treated MDCK cells at lower concentrations (1 μm; Fig 5C & F). Therefore, treatment with JAK‐2 inhibitors AZD1480 and CYT387 induces apoptosis in DLBCL cells in a dose‐dependent manner. This proapoptotic effect of AZD1480 and CYT387 is more pronounced in canine lymphoma cells compared with normal kidney cells (MDCK).
Figure 5.
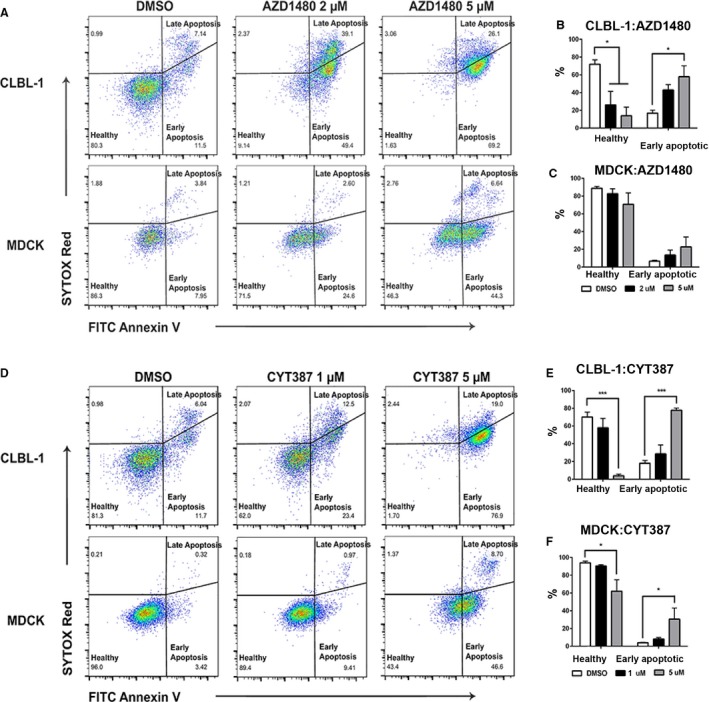
JAK inhibitors AZD1480 and CYT387 increase apoptosis in canine diffuse large B‐cell lymphoma cells (CLBL‐1). Representative flow cytometric analysis of AZD1480‐treated (A–C) and CYT387‐treated cells (D‐F) for early apoptosis (Annexin V+, SYTOX Red −), late apoptosis (Annexin V+, SYTOX Red+), and healthy cells (Annexin V−, SYTOX Red−). Compared with DMSO controlled treated cells, there was a significant increase in early apoptosis in canine DLBCL cell line CLBL‐1 treated with either AZD1480 or CYT387 (B, E). The increase in apoptosis in normal canine kidney cell line MDCK was minimal and not statistically significant (C, F). Data were presented as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
AZD1480 and CYT387 Disrupt Canine Lymphoma Cell Proliferation Cycle
Inhibition of cell growth can be achieved by increasing apoptosis, by decreasing cell proliferation, or by both mechanisms. We further assessed the impact of AZD1480 and CYT387 on cell cycle in canine lymphoma cells. The CLBL‐1 cells were treated with AZD1480 at 2 μm, CYT387 at 1 μm, or DMSO as negative control. After 72 hours of JAK inhibitor treatment, CLBL‐1 cells were fixed and stained with proliferation markers Ki67 and DAPI. Cells stained negative for both Ki67 and DAPI (Ki67−DAPI−) were defined as cells in the resting phase (G0). Cells in active proliferation stage were cells in the G1 (Ki67+DAPI−) or S/G2/M (Ki67+DAPI+) phase (Fig 6A). Compared with DMSO‐treated control, AZD1480‐ or CYT387‐treated canine lymphoma cells were less proliferative with increased percentage of cells in G0 phase and decreased percentages of cells in G1 and S/G2/M phases. Thus, AZD1480 and CYT387 effectively inhibited canine lymphoma cell proliferation.
Figure 6.
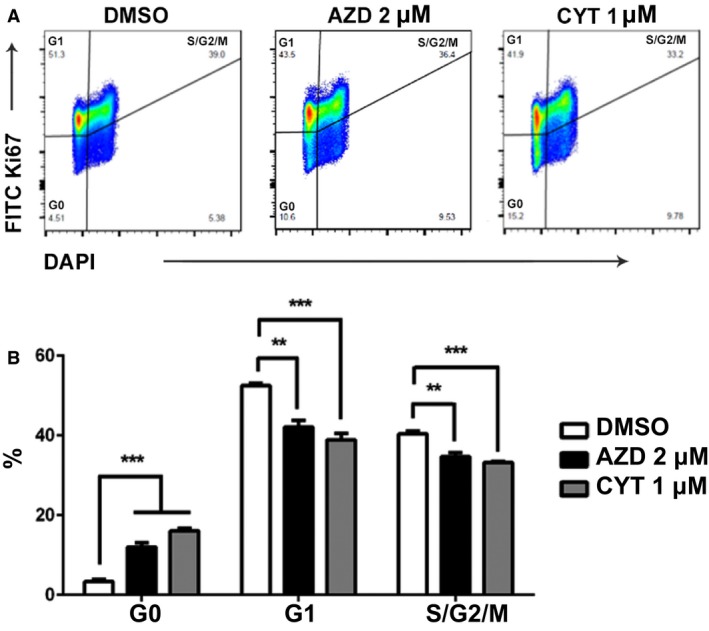
AZD1480 and CYT387 inhibit CLBL‐1 cell proliferation. (A) Representative gating strategy for Ki67/DAPI cell proliferation assay. Cells in G0 phase were defined as Ki67−DAPI−. Cells in G1 phase were defined as Ki67+DAPI−. Cells in S/G2/M phase were defined as K67+DAPI+. (B) Quantification of cells in G0, G1, and S/G2/M phases. Data were presented as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Although CHOP‐based chemotherapy can prolong survival in dogs with DLBCL, complete cure is rare. Management of dogs with DLBCL remains challenging, and effective targeted therapies are much needed. Several JAK1/2 ATP competitive type I kinase inhibitors, including AZD1480 and CYT387, have been tested in clinical trials in humans for various solid and hematologic malignancies.17 AZD1480 was first described as pyrazol‐3‐yl pyrimidin‐4‐amine, a potent inhibitor for both JAK1 and JAK2.18 The AZD1480 can inhibit STAT3 phosphorylation, nuclear translocation, and tumor growth in a dose‐dependent manner in vitro.19 In a mouse xenograft model, AZD1480 can inhibit cell proliferation of neuroblastomas and sarcomas and induce apoptosis by inhibition of the STAT3 pathway.20 Similar results are observed in human myeloma cell lines.21 CYT387 is a novel JAK1/2 inhibitor that was first developed to treat myelofibrosis (MF) in humans. This drug was well tolerated in a nonrandomized study with clinical benefit in intermediate or high‐risk MF with response in anemia and splenomegaly.12 In addition to its therapeutic effects in MF, in vitro studies showed that CYT387 can inhibit multiple myeloma cell proliferation by interrupting the cell cycle.22 Our study is the first to evaluate the in vitro activity of AZD1480 and CYT387 in DLBCL of dogs. These results show that both drugs can inhibit canine DLBCL cell growth in a dose‐dependent manner by inducing apoptosis and inhibiting cell proliferation. These findings support the feasibility of a future phase I/II clinical trial with AZD1480 and CYT387 in dogs with DLBCL.
CYT 387 is a JAK1/2 inhibitor that also has activity against JAK3 and TYK2 at high IC values. Although in vitro kinase assays indicate that CYT387 inhibits the activity of recombinant JAK1, JAK2, JAK3, and TYK2 kinases domains with IC50 values of 11, 18, 155, and 17 nm, respectively, cell‐based studies show growth suppression and apoptosis in JAK‐2 dependent hematopoietic cell lines with IC50 between of 0.5 and 1.5 μm.23 The IC50 (1.5 μm) in our study is consistent with what has been reported previously in human hematopoietic cell lines. Similarly, nanomolar IC50 concentrations of AZD1480 are generated from the none cell‐based kinase assays. In cell‐based assay, AZD1480 showed a 50% inhibitory effect on murine myeloid leukemia cells at a concentration of 5 μm,16 and of human DLBCL cell lines and solid tumor cell lines at concentrations from 0.5 to 1 μm.19, 24 The IC50 of AZD1480 in our study was approximately 2.5 μm and comparable to what has been previously published. Above 1 μM, AZD1480 has been shown to inhibit Ba/F3 cell lines driven by the JH1 catalytic domains of JAK3 or TYK2 fused to the oligomerization domain of translocation‐Ets‐leukemia virus protein.19, 25 The IC50 of AZD1480 in CLBL‐1 cells is >1 μm. Furthermore, although statistical differences were found between treated and untreated cells, it appears that in 67% patient samples and in the OCL‐LY7 cell line that >50% of the cells survived even at the highest drug concentration for both AZD1480 and CYT387 (Fig 3). Thus, AZD1480‐mediated lymphoma‐specific inhibition in our study may not entirely be regulated by inhibition of the JAK2‐STAT3 pathway. Instead, AZD1480 regulatory functions in TYK3, JAK3, FLT‐3, and Aurora‐A‐kinase may impact development of B‐cell lymphoma in dogs at multiple levels. Although broad spectrum inhibitory effects may be beneficial in treating several hematologic malignancies, the off‐target effects of AZD1480 indicate caution for risk of severe adverse events (AE) in future clinical trials. Dose‐limiting toxicities (DLT) of AZD1480 in phase I studies of humans consist of pleiotropic neurologic adverse events including dizziness, anxiety, ataxia, hallucinations, and behavior changes.26, 27 In vivo evaluation of AZD1480 and CYT387 functions in a canine xenograft lymphoma mouse model28 may provide invaluable safety and efficacy data before a phase I/II canine lymphoma clinical trial. In addition, future phase I clinical trial in DLBCL in dogs should incorporate a dose escalation design, and investigators should be aware of the risk of severe, unexpected AE with AZD1480.
JAK2‐STAT3 is a point of convergence for multiples oncogenic signaling pathways. The link between STAT3 activation and regulation of Bcl‐2 family protein expression has been extensively studied in human lymphoid cells.29, 30 STAT3 inhibition is associated with increased apoptosis and decreased Mcl‐1 expression in large granular lymphocytic leukemia.30 In human myeloma cells, STAT3 activation and nuclear translocation promote IL‐6 responsive gene expression.29 Although IL‐6 promotes expression of the prosurvival protein Bcl‐xl, the JAK‐2 inhibitors can inhibit the expression of Bcl‐xl.31 In addition, STAT3 inhibition induces apoptosis by decreasing expression of survivin, an antiapoptotic protein, in primary effusion lymphoma of humans.32 Future studies focusing on gene regulation of the Bcl‐2 family proteins may be important to identify the underlying mechanisms of AZD1480‐ and CYT387‐induced apoptosis. Because STAT3 inactivation by JAK2 inhibitors is associated with deregulation of the critical cell cycle protein, Cyclin D1,33 it is also important to evaluate the effects of AZD1480 and CYT387 on CyclinD, Ras/Map kinase, and the β‐catenin‐Tcf/LEF pathways. Other future directions include evaluating the impact of AZD1480 and CYT387 on tumor angiogenesis and metastasis because AZD1480 has antiangiogenic and antimetastatic activities via inhibition of vascular endothelial growth factor and matrix metallopeptidase 9.31
Conclusion
Our study determined that JAK1/2 inhibitors AZD1480 and CYT387 effectively inhibit canine lymphoma cell growth by promoting apoptosis and interrupting the normal cell cycle. AZD1480 and CYT387 inhibit JAK2‐STAT3 activation by inhibiting its tyrosine phosphorylation in a dose‐dependent manner.
Supporting information
Figure S1: Western blot for p‐JAK2 expressions in CLBL‐1 cells stimulated with no cytokine, IL‐3, IL‐6, or combination of IL‐3 and IL‐6. β‐actin was used as a loading control.
Acknowledgments
We thank Dr. Barbara Rutgen and Dr. Jaime Modiano for providing the canine CLBL‐1 cell line. We thank Dr. David Vail for providing the canine MDCK cell line. We thank Lixin Rui (Carbon Cancer Center, UW‐Madison) for providing the human OCL‐LY7 cell line.
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
The work was conducted at the University of Wisconsin‐Madison and was supported by startup funds from UW‐Madison, NIH grants K01OD020153‐01A1 and T35OD011078. We would like to thank the University of Wisconsin Carbone Comprehensive Cancer Center (UWCCC) for use of its Shared Services (Flow Core Lab) to complete this research. The data were presented in part as a research report at the 2016 ACVIM Forum, Denver, CO.
Footnotes
AstraZeneca, Inc, Cambridge, England
Gilead Sciences, Inc, Foster City, CA
Zoetis, Parsippany‐Troy Hills, NJ
Chemietek, Indianapolis, IN
Promega Corporation, Fitchburg, WI
GraphPad Software, La Jolla, CA
QIAGEN, Hilden, Germany
Life Technologies Corporation, Carlsbad, CA
Roche Diagnostics, Indianapolis, IN
Thermo Fisher Scientific, Madison, WI
Thermo Scientific Pierce Protein Biology, Madison, WI
Bio‐Rad Laboratories, Hercules, CA
PeproTech, Rocky Hill, NJ
Santa Cruz Biotechnology, Dallas, TX
Cell Signaling Technology, Danvers, MA
Sigma‐Aldrich Corporation, St. Louis, Missouri
BD Biosciences, San Jose, CA
FlowJo, LLC, Ashland, Oregon
eBioscience, Inc, San Diego, CA
Tonbo Biosciences, San Diego, CA
References
- 1. Younes A. Beyond chemotherapy: New agents for targeted treatment of lymphoma. Nat Rev Clin Oncol 2011;8:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quintas‐Cardama A, Verstovsek S. Molecular pathways: Jak/STAT pathway: Mutations, inhibitors, and resistance. Clin Cancer Res 2013;19:1933–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinberg RA. Cytoplasmic Signaling Circuitry Programs Many of the Traits of Cancer. Biology of Cancer, Second Edition 2014:175–229.
- 4. Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non‐Hodgkin's lymphoma and multiple myeloma to chemotherapeutic drug‐mediated apoptosis. Clin Cancer Res 2003;9:316–326. [PubMed] [Google Scholar]
- 5. Amin HM, McDonnell TJ, Ma Y, et al. Selective inhibition of STAT3 induces apoptosis and G(1) cell cycle arrest in ALK‐positive anaplastic large cell lymphoma. Oncogene 2004;23:5426–5434. [DOI] [PubMed] [Google Scholar]
- 6. Ding BB, Yu JJ, Yu RY, et al. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B‐cell subtype of diffuse large B‐cell lymphomas. Blood 2008;111:1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seelig DM, Avery AC, Ehrhart EJ, et al. The comparative diagnostic features of canine and human lymphoma. Vet Sci. 2016;3. pii: 11. doi: 10.3390/vetsci3020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meyer SC, Levine RL. Molecular pathways: Molecular basis for sensitivity and resistance to JAK kinase inhibitors. Clin Cancer Res 2014;20:2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cook AM, Li L, Ho Y, et al. Role of altered growth factor receptor‐mediated JAK2 signaling in growth and maintenance of human acute myeloid leukemia stem cells. Blood 2014;123:2826–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rutgen BC, Hammer SE, Gerner W, et al. Establishment and characterization of a novel canine B‐cell line derived from a spontaneously occurring diffuse large cell lymphoma. Leuk Res 2010;34:932–938. [DOI] [PubMed] [Google Scholar]
- 11. Tefferi A, Pardanani A. JAK inhibitors in myeloproliferative neoplasms: Rationale, current data and perspective. Blood Rev 2011;25:229–237. [DOI] [PubMed] [Google Scholar]
- 12. Pardanani A, Laborde RR, Lasho TL, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia 2013;27:1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gadeyne C, Little P, King VL, et al. Efficacy of oclacitinib (Apoquel(R)) compared with prednisolone for the control of pruritus and clinical signs associated with allergic dermatitis in client‐owned dogs in Australia. Vet Dermatol 2014;25:512–518 e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cosgrove SB, Wren JA, Cleaver DM, et al. A blinded, randomized, placebo‐controlled trial of the efficacy and safety of the Janus kinase inhibitor oclacitinib (Apoquel(R)) in client‐owned dogs with atopic dermatitis. Vet Dermatol 2013;24:587–597 e141‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gonzales AJ, Bowman JW, Fici GJ, et al. Oclacitinib (APOQUEL((R))) is a novel Janus kinase inhibitor with activity against cytokines involved in allergy. J Vet Pharmacol Ther 2014;37:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kong G, Wunderlich M, Yang D, et al. Combined MEK and JAK inhibition abrogates murine myeloproliferative neoplasm. J Clin Invest 2014;124:2762–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene 2013;32:2601–2613. [DOI] [PubMed] [Google Scholar]
- 18. Ioannidis S, Lamb ML, Wang T, et al. Discovery of 5‐chloro‐N2‐[(1S)‐1‐(5‐fluoropyrimidin‐2‐yl)ethyl]‐N4‐(5‐methyl‐1H‐pyrazol‐3‐yl) pyrimidine‐2,4‐diamine (AZD1480) as a novel inhibitor of the Jak/Stat pathway. J Med Chem 2011;54:262–276. [DOI] [PubMed] [Google Scholar]
- 19. Hedvat M, Huszar D, Herrmann A, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell 2009;16:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yan S, Li Z, Thiele CJ. Inhibition of STAT3 with orally active JAK inhibitor, AZD1480, decreases tumor growth in Neuroblastoma and Pediatric Sarcomas In vitro and In vivo. Oncotarget 2013;4:433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scuto A, Krejci P, Popplewell L, et al. The novel JAK inhibitor AZD1480 blocks STAT3 and FGFR3 signaling, resulting in suppression of human myeloma cell growth and survival. Leukemia 2011;25:538–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monaghan KA, Khong T, Burns CJ, et al. The novel JAK inhibitor CYT387 suppresses multiple signalling pathways, prevents proliferation and induces apoptosis in phenotypically diverse myeloma cells. Leukemia 2011;25:1891–1899. [DOI] [PubMed] [Google Scholar]
- 23. Tyner JW, Bumm TG, Deininger J, et al. CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood 2010;115:5232–5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rui L, Drennan AC, Ceribelli M, et al. Epigenetic gene regulation by Janus kinase 1 in diffuse large B‐cell lymphoma. Proc Natl Acad Sci USA 2016;113:E7260–E7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gozgit JM, Bebernitz G, Patil P, et al. Effects of the JAK2 inhibitor, AZ960, on Pim/BAD/BCL‐xL survival signaling in the human JAK2 V617F cell line SET‐2. J Biol Chem 2008;283:32334–32343. [DOI] [PubMed] [Google Scholar]
- 26. Plimack ER, Lorusso PM, McCoon P, et al. AZD1480: A phase I study of a novel JAK2 inhibitor in solid tumors. Oncologist 2013;18:819–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verstovsek S, Hoffman R, Mascarenhas J, et al. A phase I, open‐label, multi‐center study of the JAK2 inhibitor AZD1480 in patients with myelofibrosis. Leuk Res 2015;39:157–163. [DOI] [PubMed] [Google Scholar]
- 28. Rutgen BC, Willenbrock S, Reimann‐Berg N, et al. Authentication of primordial characteristics of the CLBL‐1 cell line prove the integrity of a canine B‐cell lymphoma in a murine in vivo model. PLoS ONE 2012;7:e40078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Catlett‐Falcone R, Landowski TH, Oshiro MM, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 1999;10:105–115. [DOI] [PubMed] [Google Scholar]
- 30. Epling‐Burnette PK, Liu JH, Catlett‐Falcone R, et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl‐1 expression. J Clin Invest 2001;107:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xin H, Herrmann A, Reckamp K, et al. Antiangiogenic and antimetastatic activity of JAK inhibitor AZD1480. Can Res 2011;71:6601–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 2003;101:1535–1542. [DOI] [PubMed] [Google Scholar]
- 33. Guo R, Overman M, Chatterjee D, et al. Aberrant expression of p53, p21, cyclin D1, and Bcl2 and their clinicopathological correlation in ampullary adenocarcinoma. Hum Pathol 2014;45:1015–1023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Western blot for p‐JAK2 expressions in CLBL‐1 cells stimulated with no cytokine, IL‐3, IL‐6, or combination of IL‐3 and IL‐6. β‐actin was used as a loading control.


