Abstract
Background
Prophylactic gastropexy has been promoted as a means of preventing gastric volvulus during gastric dilatation and volvulus (GDV) syndrome. Little is known about the impact of gastropexy on gastrointestinal transit time.
Hypothesis
Laparoscopic‐assisted gastropexy (LAG) will not alter gastrointestinal transit times when comparing gastric (GET), small and large bowel (SLBTT), and whole gut transit times (TTT) before and after surgery.
Animals
10 healthy client‐owned large‐breed dogs.
Methods
Prospective clinical trial. Before surgery, all dogs underwent physical examination and diagnostic evaluation to ensure normal health status. Dogs were fed a prescription diet for 6 weeks before determination of gastrointestinal transit with a wireless motility capsule. LAG was then performed, and dogs were fed the diet for 6 additional weeks. Measurement of transit times was repeated 6 weeks after surgery.
Results
Ten dogs of various breeds at‐risk for GDV were enrolled. No complications were encountered associated with surgery or capsule administration. There were no significant differences in GET 429 [306–1,370] versus 541 [326–1,298] (P = 0.80), SLBTT 1,243 [841–3,070] versus 1,540 [756–2,623] (P = 0.72), or TTT 1,971 [1,205–3,469] versus 1,792 [1,234–3,343] minutes (median, range) (P = 0.65) before and after LAG.
Conclusions and Clinical Importance
An effect of LAG on gastrointestinal transit time was not identified, and wireless motility capsule can be safely administered in dogs after LAG.
Keywords: Emptying, Endoscopy, Minimally invasive, Prophylactic
Abbreviations
- GDV
gastric dilatation volvulus
- GET
gastric emptying time
- LAG
laparoscopic‐assisted gastropexy
- MER
metabolic energy requirement
- RER
resting energy requirement
- SLBTT
small and large bowel transit time
- TTT
total transit time
Gastric dilatation and volvulus (GDV) is a life‐threatening syndrome that occurs when the stomach rotates, causing entrapment of gas, fluid, and food.1 This syndrome is most commonly seen in large‐ and giant‐breed deep‐chested dogs such as great Danes, Irish Wolfhounds, and standard poodles, although GDV can affect any breed of dog.2
Prophylactic gastropexy is highly protective against gastric volvulus,3 and there is interest in performing prophylactic gastropexies in dogs that are at risk of developing GDV. Prophylactic gastropexies are generally performed by traditional open techniques when combined with other intraabdominal procedures, or by minimally invasive techniques such as the grid approach, endoscopically assisted, laparoscopic‐assisted, or laparoscopic approaches.4, 5, 6, 7
A relatively new technique for assessing gastrointestinal transit utilizing a wireless motility capsulea has been developed which involves the ingestion of a 26 × 13 mm wireless transmitting capsule that sends signals to a portable receiver worn by the dog.8, 9 The stored signals can then be assessed by display software specifically designed for the capsule. The wireless motility capsule transmits data about pH, pressure, and temperature to the receiver that can be retrieved and analyzed. The alterations in temperature and pH are used to determine the anatomic location of the capsule within the gastrointestinal tract.
Despite the widespread acceptance of prophylactic gastropexies, very little information is available about the impact that this procedure has on gastrointestinal motility. Of concern are rare case studies of dogs developing clinical signs of vomiting and delayed gastric emptying times after prophylactic gastropexy.10 The implementation of minimally invasive gastropexy techniques makes the performance of prophylactic gastropexies ever more attractive, likely increasing the regularity with which these procedures are performed. An understanding of the impact of prophylactic gastropexy on gastric emptying and intestinal transit in apparently healthy dogs is important to determine the role gastropexies have on the development of clinical signs after surgery.
The aim of this study was to assess gastrointestinal transit with wireless motility capsules in a cohort of healthy dogs before and after prophylactic laparoscopic‐assisted gastropexy (LAG). The null hypothesis was that the performance of a LAG will not alter gastrointestinal transit when comparing gastric emptying time (GET), small and large bowel transit time (SLBTT), and total transit time (TTT) before and after surgery.
Materials and Methods
Dogs
Client‐owned giant‐ or large‐breed dogs were enrolled in the study, by a “before and after” design. The study protocol was explained to the owner, and informed consent was obtained before enrollment. The study was approved by the Institutional Animal Care and Use Committee. A thorough history was obtained, and a physical examination was performed on all enrolled dogs. A body condition score (BCS) was determined, and only dogs with BCS of 4–6 (scale 1–9) were included. Dogs were not enrolled if they were currently receiving any medications (excluding antiparasiticides) or had a history of gastrointestinal disease.
The following laboratory and imaging procedures were performed on all dogs before enrollment: complete blood count, serum biochemical profile, urinalysis, serum cobalamin/folate concentration, serum trypsinogen‐like immunoreactivity assay, fecal centrifugation floatation, baseline serum cortisol concentration, abdominal survey radiographs, and abdominal ultrasound. To be included, dogs had to have no abnormalities in the above diagnostics. Additionally, all dogs were dewormed with fenbendazole (50 mg/kg PO q24 hours for 3 consecutive days). Owners were responsible for keeping a daily diary of exercise activity, appetite, and fecal score to note any abnormalities associated with the intestinal diet or wireless motility capsule.
Diet
Once enrolled, all dogs were fed a standardized highly digestible intestinal dietb for a period of 6 weeks. The diet was fed to meet each dog's daily metabolic energy requirement (MER). Resting energy requirement was determined by the following equation: resting energy requirement (RER) = 70 × BW0.75 kcal/kg, and MER was calculated by multiplying the RER by 1.6 (neutered adult) or 1.8 (intact adult). Body weight and body condition score were reassessed every 3 weeks throughout the study to determine if any alteration to food intake was needed. Owners were also provided with hydrolyzed treatsc to aid in compliance with excluding other foods during the study period.
Wireless Motility Capsule Administration
After 6 weeks of the dogs being fed the standardized diet, the wireless motility capsule was administered PO after food was withheld overnight for 12 hours, and all dogs received the capsule between 09:00 and 12:00 hours. A test meal comprising 1/3rd of the dog's MER using the same standardized diet was fed to all dogs before the capsule was administered PO. The dogs were given the opportunity to drink 100 mL of water after which they were fitted with a vest containing the data receiver. The owners then took their dogs home to rapidly re‐establish a normal stress‐free environment and were instructed to withhold food and water until the pH had a persistent increase of at least 3 units above baseline gastric pH (pH < 3.0 in all dogs) to correspond with movement of the wireless motility capsule from the stomach into the duodenum. A diary of events was provided to the owner so that specific information about the dog's activity level, defecation episodes and times, passage of the capsule in the feces, as well as any potential adverse effects could be articulated. Adverse effects included vomiting, diarrhea, altered food intake, or altered activity (i.e. running and playing). Activity was monitored by owners who assessed the time period that the dogs spent walking, running, or playing on a weekly basis. No gastric acid suppressants or other medications were administered during the wireless motility capsule‐monitoring period.
After passage of the wireless motility capsule, a LAG was performed as described below. After surgery, the standardized intestinal diet was continued to be fed for an additional 6 weeks, at which point, a second wireless motility capsule was administered. Information obtained from the wireless motility capsule receivers including gastrointestinal transit, pH, pressure, and temperature were assessed and compared. MotiliGI version 2.2 softwared was used to analyze the data and calculate the following GI transit times: GET, SLBTT, and TTT.
LAG Procedure
All dogs were placed under general anesthesia utilizing a protocol as determined by the clinical anesthesiology service. The dogs were placed in dorsal recumbency, and the ventral abdominal region was clipped, prepared with aseptic technique, and draped. The laparoscopic‐assisted incisional gastropexy was performed as described by Rawlings et al.11 A 1‐cm incision was made on the ventral midline 2‐cm caudal to the umbilicus, and access to the abdominal cavity was obtained by the modified Hasson technique.5 A 6 mm cannula‐trocar assemblye was inserted in this location, and insufflation with CO2 f was commenced to an intraabdominal pressure of 9–12 mmHg. A 5‐mm laparoscopeg was inserted into the abdomen and aimed toward the right ventral aspect of the abdomen. A 10‐mm instrument cannulae was placed through the right ventrolateral body wall (lateral to the rectus abdominis muscle), ~3‐cm caudal to the last rib. Endoscopic Babcock forcepsh were passed through the instrument port, and the stomach was grasped and pulled toward the body wall. The port was removed from the body wall, and the gastric antrum was exteriorized. The abdominal incision of the instrument port was increased to 4 cm in length, and stay sutures were placed at the ends of the proposed gastric incision (4‐cm apart). A 4‐cm incision was made through the seromuscular layer of the stomach. The incised edges of the stomach were sutured to the incised edges of the transversus abdominis muscle with 2 lines of 2‐0 polydioxanone in simple continuous patterns. The final gastric positioning was directly visualized after gastropexy. The abdominal oblique muscles were closed with a simple continuous pattern with 3‐0 polydioxanone. The subcutaneous tissues were apposed in a simple continuous pattern with 3‐0 polydioxanone. The dermal layer was apposed using a subcuticular pattern with 3‐0 poliglecaprone. The midline incision was closed in the following manner: The linea alba layer of the midline incision was closed with simple interrupted sutures of 3‐0 polydioxanone, and the dermal layer was apposed using a subcuticular pattern with 3‐0 poliglecaprone. All dogs were discharged 1 day postoperatively, and tramadol (3 mg/kg PO q8 hours) was administered for pain control for 3 days.
Statistical Analysis
An a priori power analysis was not performed. All continuous variables were statistically conservatively considered to be not normally distributed and are described using median (min, max). The Wilcoxon signed rank test was used to compare gastrointestinal transit times for each of the gastrointestinal segments assessed (GET, SLBTT, and TTT) before and after LAG. A P‐value of <0.05 was considered significant for all comparisons. All statistical analyses were performed by a statistical software program.i
Results
Fifteen dogs were enrolled in the study. Five dogs were removed from the study for the following reasons: gastrointestinal foreign material on multiple abdominal ultrasound examinations (n = 3), splenic hemangiosarcoma (n = 1), lost to follow‐up after the first wireless motility capsule administration (n = 1). All included dogs were determined to have laboratory test results within the laboratory reference range. All dogs were giant‐to‐large breeds and were considered deep‐chested. The breeds included were great Dane (n = 3), German shepherd (2), standard poodle (2), Doberman pinscher (1), Irish wolfhound (1), and a mixed breed (1). Six dogs were intact males, 2 were intact females, 1 was a spayed female, and 1 was a castrated male. The median age was 18 months (range, 5–48 months), and the median weight was 39.8 kg (range, 21.2–68.5 kg). Six dogs had a BCS of 4/9, 2 dogs had a BCS of 5/9, and 2 dogs had a BCS of 6/9.
All wireless motility capsule administrations were successful, and no complications were encountered with capsule administration or LAG. During laparoscopic evaluation, the stomach was noted to be in an appropriate gastropexy position in all cases. Additionally, all surgical incisions healed appropriately and no incisional complications were noted. No alteration in fecal quality or exercise activity (except for a prescribed decrease for 14 days postoperatively) was recorded in any dog, and no dogs had clinical signs such as vomiting, regurgitation, or changes in appetite.
The median (range) GET, SLBTT, and TTT were 429 (306–1,370), 1,243 (841–3,070), and 1,971 (1,205–3,469) minutes, respectively, before surgery. The median (range) GET, SLBTT, and TTT were 541 (326–1,298), 1,540 (756–2,623), and 1,792 (1,234–3,343) minutes, respectively, after surgery. The median (range) difference in GET, SLBTT, and TTT when comparing before and after LAG was 23 (−587 to 583), −78 (−447 to 571), and −26 (−831 to 474) minutes, respectively (Figs 1, 2, 3). There was no significant difference in GET (P = 0.80), SLBTT (P = 0.72), or TTT (P = 0.65) after LAG.
Figure 1.
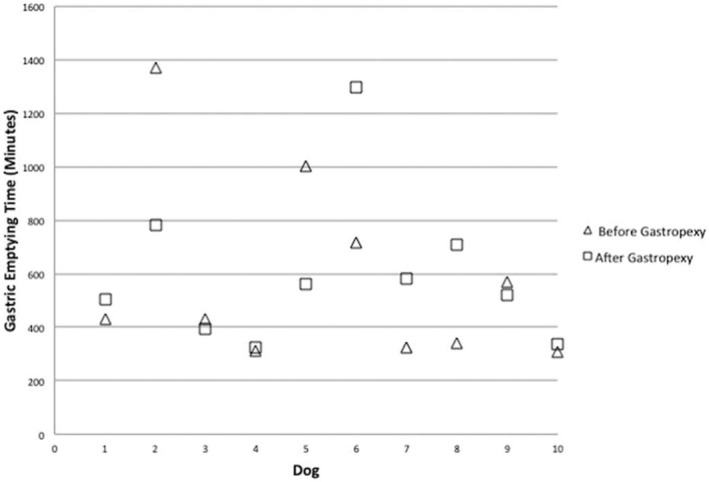
Plot of individual dog gastric emptying time (GET) before and after laparoscopic‐assisted gastropexy for 10 dogs.
Figure 2.
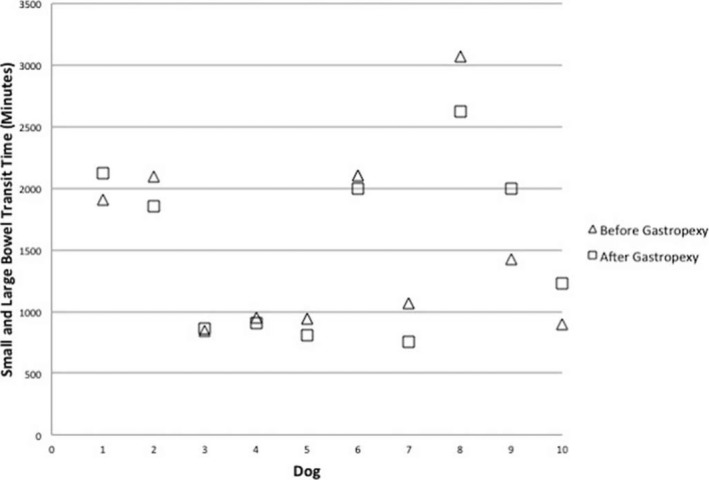
Plot of individual dog small and large bowel transit time (SLBTT) before and after laparoscopic‐assisted gastropexy for 10 dogs.
Figure 3.
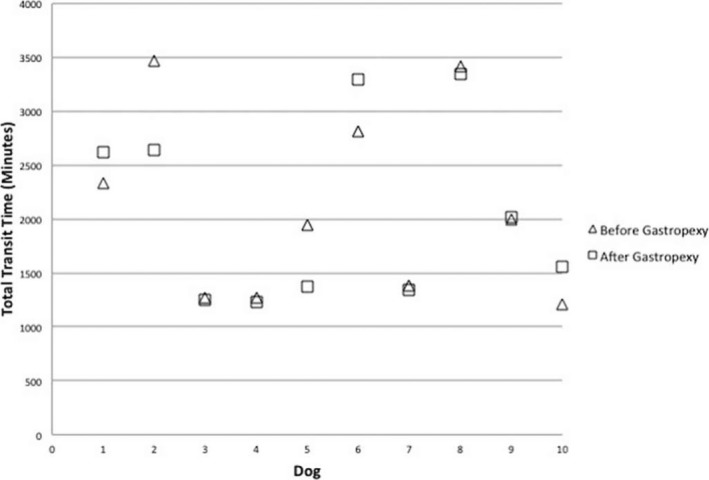
Plot of individual dog total transit time (TTT) before and after laparoscopic‐assisted gastropexy for 10 dogs.
Discussion
The performance of a gastropexy is considered standard‐of‐care in dogs that have had GDV. Further, the use of prophylactic gastropexy is being advocated as a means of reducing the incidence of GDV in dogs predisposed to developing the disorder. While many clinicians recommend the procedure in high‐risk breeds, there is little evidence describing the long‐term functional outcome of the gastrointestinal tract in those dogs with a gastropexy. This study did not demonstrate that dogs undergoing LAG have a change in GET, SLBTT, and TTT after the LAG procedure.
The main historical options for the assessment of gut transit include scintigraphy as the criterion‐referenced standard, radiopaque markers, and liquid barium upper gastrointestinal studies. With the scintigraphic method, patients ingest a meal containing a radioisotope and are then imaged with a gamma camera.12 The gastrointestinal emptying is assessed by repeating the imaging over several hours being sure to note when the radioisotope has left the stomach and entered the cecum. If colonic transit is to be assessed as part of the study, images are acquired 24–72 hours after ingestion of the radioisotope. In 1 study comparing gastric emptying of a radionuclide‐labeled test meal after gastropexy in clinically normal and GDV‐affected dogs, there was no significant difference in the gastric emptying rates and pattern.13 Although scintigraphy has historically been considered the gold standard in assessing gastrointestinal transit time, studies have shown wireless motility capsule and scintigraphy have similar variation and repeatability in dogs.14
Radiopaque markers (ROMs) can also be utilized to assess gastrointestinal motility and emptying. These nondigestible markers are ingested within a gelatin capsule which opens and releases the ROMs upon contact with gastric juices.15, 16 Serial radiographs are obtained to evaluate the location of the markers within the gastrointestinal tract. The use of ROMs was evaluated in both clinically normal dogs and dogs with GDV in 1 study.17 In that study, the performance of a circumcostal gastropexy did not alter the 90% gastric emptying time of the particles in healthy dogs; however, the gastric emptying time in dogs with GDV that had undergone circumcostal gastropexy was significantly increased.17
Several disadvantages of the scintigraphic assessment and ROMs have been identified. Scintigraphic assessment requires expensive equipment, and there is an exposure to radioactivity. Additionally, the scintigraphic studies are long in duration requiring an extended period of time that clinicians and technical staff are dedicated to a particular case.8 Furthermore, ROMs do not technically measure gastric emptying of an actual meal as these are not passed until food has left the stomach. Instead they are utilized to measure gastric emptying of the ROMs (nonphysiologic assessment). Lastly, these techniques only offer the ability to assess emptying (generally of only 1 segment of the gastrointestinal tract) and do not provide additional information about gastrointestinal pH, pressure, and temperature.
There are several advantages of the wireless motility capsule used in this study. This system allows for the assessment of motility within the entire gastrointestinal tract, whereas other tests focus mainly on 1 particular section of the gastrointestinal tract. The dogs are able to remain in their normal environment and continue their normal activity. Additionally, this system does not require multiple tests or a serial exposure to radiation. The wireless motility capsule utilized in this study is accurate for measurement of gastrointestinal transit in dogs and has been validated in several studies.14, 18, 19
All dogs in this study were safely administered a wireless motility capsule, and no complications were encountered. The smallest dog in this study weighed approximately 21 kg; the use of wireless motility capsules in small dogs was not assessed in this study. The minimum weights of dogs in other studies receiving the same wireless motility capsule used in this study were similar (19–23 kg).18, 19, 20 Investigation of the use of the capsule in smaller dogs would be useful, although not likely pertinent when referring to prophylactic gastropexy procedures, as GDV is uncommon in this cohort of dogs. As has been shown in previous studies,5, 6 the LAG procedure was performed successfully with no complications. Additionally, utilizing the described laparoscopic technique allowed the surgeons to directly evaluate the gastropexy site with the laparoscope before completion of the procedure. This visualization permitted confirmation of a successful and appropriately positioned gastropexy.
Limitations of this study should be recognized. The study included a small number of healthy dogs, although, the use of a “before and after” study design allowed each dog to serve as its own control. Regardless, a type II error is possible when utilizing a small sample size, and it should be considered that the lack of significance noted between before and after LAG values could be due to this error. Additionally, as the time frame for evaluation was short, this study lacks long‐term follow‐up in these dogs to ensure that no complications related to gastrointestinal motility occurred. Further, this study only evaluated 1 specific surgical technique performed or overseen by a board certified surgeon in a variety of dogs, and differences might be expected when utilizing different surgical techniques or evaluating different breeds. Lastly, this study did not evaluate the impact of this procedure on transit times in dogs with underlying disease processes, and extrapolation of results to this setting cannot be performed.
This study supports the use of prophylactic gastropexy in dogs at‐risk for developing GDV as there was no detected change in gastrointestinal transit as assessed by wireless motility capsule. Additional investigation of the use of wireless motility capsules to assess dogs with gastrointestinal motility disturbances after GDV is possible as this technology appears to be safe in dogs with a gastropexy.
Acknowledgment
The authors acknowledge the support of Nestlé Purina PetCare for providing the prescription intestinal diet and for use of the wireless motility capsule for the study.
Conflict of Interest Declaration
Dr. Marks is a member of the Nestlé Purina Advisory Board.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Work was performed at the Veterinary Medical Teaching Hospital, University of California‐Davis, School of Veterinary Medicine.
This study was supported by a grant from the Center for Companion Animal Health, University of California‐Davis, School of Veterinary Medicine.
This study was presented in abstract form at the 2017 Veterinary Endoscopy Society Meeting in Cabo San Lucas, Mexico.
Footnotes
aSmartPill GI Monitoring System, Medtronic, Minneapolis, MN
bPurina Pro Plan Gastroenteric EN, Nestlé Purina PetCare Company, St. Louis, MO
cPurina Pro Plan Gentle Snackers, Nestlé Purina PetCare Company, St. Louis, MO
dSmartPill Corporation, Buffalo, NY
eTernamian Endotip cannula, Karl Storz Veterinary Endoscopy, Goleta, CA
fEndoflator, Karl Storz Veterinary Endoscopy, Goleta, CA
gHopkins II, Karl Storz Veterinary Endoscopy, Goleta, CA
hBabcock Grasping Forceps, Karl Storz Veterinary Endoscopy, Goleta, CA
iStata 14.0 for Mac, Stata Corporation, College Station, TX
References
- 1. Glickman LT, Glickman NW, Schellenberg DB, et al. Incidence of and breed‐related risk factors for gastric dilatation‐volvulus in dogs. J Am Vet Med Assoc 2000;216:40–45. [DOI] [PubMed] [Google Scholar]
- 2. Ward MP, Patronek GJ, Glickman LT. Benefits of prophylactic gastropexy for dogs at risk of gastric dilatation‐volvulus. Prev Vet Med 2003;60:319–329. [DOI] [PubMed] [Google Scholar]
- 3. Eggertsdottir AV, Stigen YO, Lonaas L, et al. Comparison of the recurrence rate of gastric dilatation with or without volvulus in dogs after circumcostal gastropexy versus gastrocolopexy. Vet Surg 2001;30:546–551. [DOI] [PubMed] [Google Scholar]
- 4. Waschak MJ, Payne JT, Pope ER, et al. Evaluation of percutaneous gastrostomy as a technique for permanent gastropexy. Vet Surg 1997;26:235–241. [DOI] [PubMed] [Google Scholar]
- 5. Mayhew PD, Brown DC. Prospective evaluation of two intracorporeally sutured prophylactic laparoscopic gastropexy techniques compared with laparoscopic‐assisted gastropexy in dogs. Vet Surg 2009;38:738–746. [DOI] [PubMed] [Google Scholar]
- 6. Rawlings CA, Mahaffey MB, Bement S, et al. Prospective evaluation of laparoscopic‐assisted gastropexy in dogs susceptible to gastric dilatation. J Am Vet Med Assoc 2002;221:1576–1581. [DOI] [PubMed] [Google Scholar]
- 7. Steelman‐Szymeczek SM, Stebbins ME, Hardie EM. Clinical evaluation of a right‐sided prophylactic gastropexy via a grid approach. J Am Anim Hosp Assoc 2003;39:397–402. [DOI] [PubMed] [Google Scholar]
- 8. Maqbool S, Parkman HP, Friedenberg FK. Wireless capsule motility: Comparison of the SmartPill GI monitoring system with scintigraphy for measuring whole gut transit. Dig Dis Sci 2009;54:2167–2174. [DOI] [PubMed] [Google Scholar]
- 9. Rao SS, Camilleri M, Hasler WL, et al. Evaluation of gastrointestinal transit in clinical practice: Position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil 2011;23:8–23. [DOI] [PubMed] [Google Scholar]
- 10. Sutton JS, Steffey MA, Bonadio CM, et al. Gastric malpositioning and chronic, intermittent vomiting following prophylactic gastropexy in a 20‐month‐old great Dane dog. Can Vet J 2015;56:1053–1056. [PMC free article] [PubMed] [Google Scholar]
- 11. Rawlings CA, Foutz TL, Mahaffey MB, et al. A rapid and strong laparoscopic‐assisted gastropexy in dogs. Am J Vet Res 2001;62:871–875. [DOI] [PubMed] [Google Scholar]
- 12. Madsen JL. Scintigraphic assessment of gastrointestinal motility: A brief review of techniques and data interpretation. Clin Physiol Funct Imaging 2014;34:243–253. [DOI] [PubMed] [Google Scholar]
- 13. van Sluijs FJ, van den Brom WE. Gastric emptying of a radionuclide‐labeled test meal after surgical correction of gastric dilatation‐volvulus in dogs. Am J Vet Res 1989;50:433–435. [PubMed] [Google Scholar]
- 14. Boillat CS, Gaschen FP, Gaschen L, et al. Variability associated with repeated measurements of gastrointestinal tract motility in dogs obtained by use of a wireless motility capsule system and scintigraphy. Am J Vet Res 2010;71:903–908. [DOI] [PubMed] [Google Scholar]
- 15. Lehmann R, Borovicka J, Kunz P, et al. Evaluation of delayed gastric emptying in diabetic patients with autonomic neuropathy by a new magnetic resonance imaging technique and radio‐opaque markers. Diabetes Care 1996;19:1075–1082. [DOI] [PubMed] [Google Scholar]
- 16. Park HJ, Jung JK, Song KS, et al. Effect of erythromycin on gastric emptying in healthy individuals assessed by radio‐opaque markers and plasma acetaminophen levels. J Gastroenterol 1997;32:734–739. [DOI] [PubMed] [Google Scholar]
- 17. Hall JA, Willer RL, Seim HB 3rd, et al. Gastric emptying of nondigestible radiopaque markers after circumcostal gastropexy in clinically normal dogs and dogs with gastric dilatation‐volvulus. Am J Vet Res 1992;53:1961–1965. [PubMed] [Google Scholar]
- 18. Boillat CS, Gaschen FP, Hosgood GL. Assessment of the relationship between body weight and gastrointestinal transit times measured by use of a wireless motility capsule system in dogs. Am J Vet Res 2010;71:898–902. [DOI] [PubMed] [Google Scholar]
- 19. Gazzola KM, Nelson LL, Fritz MC, et al. Effects of prophylactic incisional gastropexy on markers of gastric motility in dogs as determined by use of a novel wireless motility device. Am J Vet Res 2017;78:100–106. [DOI] [PubMed] [Google Scholar]
- 20. Boscan P, Cochran S, Monnet E, et al. Effect of prolonged general anesthesia with sevoflurane and laparoscopic surgery on gastric and small bowel propulsive motility and pH in dogs. Vet Anaesth Analg 2014;41:73–81. [DOI] [PubMed] [Google Scholar]


