Abstract
Background
Chronic gingivostomatitis in cats (FCG) is a debilitating disease with potentially deleterious effects on overall health.
Hypothesis/Objectives
Little is known about the pathophysiology and overall impact of FCG. The aims of our study were to investigate whether gingivostomatitis occurs concurrently with esophagitis, if FCG treatment contributes to esophagitis and if esophagitis exacerbates signs of FCG.
Animals
Fifty‐eight cats with clinical signs of FCG and 12 healthy control cats exhibiting no signs of oral disease, all client‐owned.
Methods
Prospective study. Physical, oral and endoscopic examinations were performed on all cats. Measurements of salivary and esophageal lumen pH were obtained from both groups. Biopsies were acquired from sites of esophageal inflammation in cats with FCG and from normal‐appearing esophageal mucosa in control cats.
Results
The majority of cats with clinical signs of FCG exhibited some degree of esophagitis especially in the proximal (44/58) and distal (53/58) parts (P < 0.001) with or without columnar metaplasia, compared to controls. All cats lacked signs related to gastrointestinal disease. Salivary and esophageal lumen pH were not statistically different compared to controls.
Conclusions and Clinical Importance
Feline chronic gingivostomatitis seems to occur concurrently with esophagitis. Esophagitis also should be managed in cats with chronic gingivostomatitis because it may aggravate the existing condition.
Keywords: Cat, Esophageal metaplasia, Esophagitis, Oral mucosa inflammation
Abbreviations
- FCG
feline chronic gingivostomatitis
- GT
gastrointestinal tract
- GER
gastroesophageal reflux
In clinical practice, there may be advantages to grouping oral inflammatory conditions in the cat together. However, feline chronic gingivostomatitis (FCG) is a distinctive, but ill‐defined, painful, and often debilitating disease. The syndrome is characterized by inflammation as well as, erosive or proliferative lesions or both.1 Etiology remains obscure, but it has been suggested that microbial factors and alterations in the innate immune response may play roles in the pathogenesis of the syndrome.2, 3
The oral cavity is the primary interface between the host and the external environment and is inhabited by a plethora of native and exogenous microorganisms. The oral mucosa of healthy cats harbors a variety of immune cells such as T‐cells, intraepithelial lymphocytes (IELs), dentritic cells and mast cells responsible for maintaining homeostasis.4 Failure to maintain homeostasis between microorganisms and the mucosa predisposes to oral disease.4
On the other hand, the esophagus is not merely a conduit of food. Transient and native bacteria are consistently detected on the surface of the esophageal epithelium. A first line of defense mechanisms of the innate immune system maintains a microbial homeostasis at the surface of the esophageal epithelium. Several innate immune factors such as Toll‐like receptor 2 (TLR‐2) and β‐defensins have been identified within the feline esophageal epithelium, identifying the esophagus as an immunologically active organ.5 Although esophageal disease has been reported rarely in cats, various factors, including hiatal hernia, have been acknowledged as causative factors.6 Characteristic signs of esophagitis include regurgitation, hypersalivation, odynophagia and avoidance of food.7
A certain number of FCG cases remain refractory to all treatments. On the other hand, similarity in signs of gastrointestinal tract (GT) disorders may mask concurrent pathology. The aim of our study was to investigate whether FCG occurs concurrently with esophagitis, whether medications play a part in the interaction between the diseases, and if signs of esophagitis overlap with those of FCG. An additional aim was to propose pathophysiologic mechanisms for the interactions between these 2 diseases.
Materials and Methods
The study was conducted at the Surgery and Obstetrics Unit, Companion Animal Clinic, Aristotle University Thessaloniki (March 2015‐December 2016). The protocol was approved by the Ethics committee, School of Veterinary Medicine (Approval No: 880/1/12/2015). Fifty‐eight client‐owned cats with FCG of various breeds, age and both sexes and 12 healthy cats admitted for ovariohysterectomy or orchiectomy met the inclusion criteria. The owners gave written consent for their pets to receive general anesthesia and participate in the study.
Study Population
A thorough history was recorded for each cat with emphasis on clinical signs such as the various patterns of exhibition of oral pain and salivation, and on prescribed medications: antibiotics, steroids, nonsteroidal anti‐inflammatory drugs (NSAIDs), or other drugs prescribed for diseases of the GT such as proton pump inhibitors. Animals of both groups underwent detailed physical and oral examination, esophageal endoscopy and salivary and esophageal lumen pH measurements. Health status was evaluated by means of CBC and serum biochemical profile.
Inclusion Criteria
Control animals were young adult healthy cats with no apparent oral pathology or previous history of GT disease. Animals with FCG exhibiting oral signs at the time of admission and also before endoscopy, must not have received medications for ≥10 days (≥30 days for methylprednisolone acetate).These animals had to have exhibited no other signs but those relevant to the syndrome, whereas gingivostomatitis had to have been macroscopically or histologically confirmed or both. Cats with endoscopic evidence of diverticula, strictures or hiatal hernias and animals that had undergone anesthesia in the past ≥30 days also were excluded from the study.
Study Design
Endoscopy always was performed by the same experienced endoscopist (TSR) before major surgery in both groups.1 Food and water were withheld for 12 hours before general anesthesia. Animals were anesthetized in the endoscopy room, taking care not to exert unnecessary manipulations and to avoid reflux. The anesthetic protocol was identical for all cats: acepromazine maleate2 (0.05 mg/kg) with butorphanol3 (0.1 mg/kg) IM for premedication, propofol4 IV titrated to effect for induction and isoflurane5 delivered in oxygen for maintenance after intubation. Salivary pH was obtained before intubation with a portable single sensor pH meter using an antimony pH probe designed for recording gastroesophageal pH6 (pH‐day2, Medica S.p.A., Modena‐Italy). The pH meter was placed between the molar salivary gland and the tongue, a site where saliva normally pools. After intubation and under endoscopic guidance, 3 pH measurements were obtained consecutively at 3‐minute intervals from the proximal (cervical) esophagus, middle (around the base of the heart) and distal (2 cm from the cardia) parts of the esophagus using the same portable pH meter, and mean pH values were estimated for each part. A combined total for mean salivary and esophageal (per part) pH value for the 2 groups separately is depicted in Table 1. Macroscopic signs of esophagitis (e.g. erosion, strictures or other pathological features) were concurrently recorded for each part of the esophagus. Esophagitis was scored on a scale 0‐3 (0: [normal] mucosa is smooth, glistening, pale pink, superficial, and submucosal vessels are normally visible, 1: [mild] mucosal hyperhemia and erythema, 2: [moderate] mucosal erythema and loss of clarity of vascular markings, slight mucosal fragility upon passage of endoscope, 3: [severe] mucosal hyperhemia and ulcers, absence of clarity of vascular markings, presence of exudates, pseudomembranes and longitudinal furrows, severe mucosal fragility upon passage of endoscope and strictures) based upon the degree of inflammation, according to Table 2. Subsequently, partial thickness biopsy specimens were endoscopically obtained with a biopsy forceps7 from sites of inflammation from the FCG group, wherever possible. Biopsy specimens from the distal part of the esophagus also were obtained from randomly chosen controls. The formalin‐fixed specimens were embedded in paraffin, cut at 4 μm and stained with hematoxylin and eosin.
Table 1.
Mean salivary and esophageal pH values for FCG (F) and Control (C) animals
| FCG Mean (SD) | Controls Mean (SD) | P‐value | |
|---|---|---|---|
| Salivary pH (F = 26, C = 12) | 8.9 (0.8) | 8.6 (0.7) | 0.317 |
| Proximal pH (F = 26, C = 11) | 8.5 (0.8) | 8.6 (0.5) | 0.680 |
| Middle pH (F = 26, C = 11) | 8.5 (1.1) | 8.3 (0.5) | 0.556 |
| Distal pH (F = 26, C = 11) | 8.3 (1.2) | 8.4 (0.6) | 0.715 |
Table 2.
Number of animals with FCG (F[n = 58]) exhibiting macroscopic signs of some degree of esophagitis (scale 0‐3) per part (proximal, middle, distal) and the statistical correlation of esophagitis per part: (Es. part) with controls (C[n = 12], P (τ)] and with the severity (scale a‐c) of the oral inflammation [SV(n = 58)] (P‐value [Kendall's τ])
| Es. part | F (n = 58) N(%) | C (n = 12) N(%) | P (τ) | SV (n = 58) N(%) | |||
|---|---|---|---|---|---|---|---|
| a | b | c | |||||
| 5 (100.0) | 28 (100.0) | 25 (100.0) | P‐value (Kendall's τ) | ||||
| Proximal | <0.001 (−0.431) | 0.390 (0.098) | |||||
| 0 | 14 (24.1) | 12 (100.0) | 3 (60.0) | 6 (21.4) | 5 (20.0) | ||
| 1 | 23 (39.7) | 1 (20.0) | 11 (39.3) | 11 (44.0) | |||
| 2 | 16 (27.6) | 1 (20.0) | 9 (32.1) | 6 (24.0) | |||
| 3 | 5 (8.6) | 0 (0.0) | 2 (7.1) | 3 (12.0) | |||
| Middle | 0.035 (−0.167) | 0.918 (−0.014) | |||||
| 0 | 41 (70.1) | 12 (100.0) | 4 (80.0) | 19 (67.9) | 18 (72.0) | ||
| 1 | 9 (15.5) | 0 (0.0) | 5 (17.9) | 4 (16.0) | |||
| 2 | 8 (13.8) | 1 (20.0) | 4 (14.3) | 3 (12.0) | |||
| Distal | <0.001 (−0.519) | 0.724 (0.040) | |||||
| 0 | 5 (8.6) | 12 (100.0) | 1 (20.0) | 2 (7.1) | 2 (8.0) | ||
| 1 | 16 (27.6) | 0 (0.0) | 9 (32.1) | 7 (28.0) | |||
| 2 | 27 (46.6) | 3 (60.0) | 14 (50.0) | 10 (40.0) | |||
| 3 | 10 (17.2) | 1 (20.0) | 3 (10.7) | 6 (24.0) | |||
After endoscopy, all FCG cats received thorough dental examination including a modified estimation of the stomatitis disease activity index (SDAI)5 and underwent proper treatment: partial or full dental extractions, NSAIDs, opioids, antimicrobials, CO2 laser treatment, as well as proton pump inhibitors, prokinetics and sucralfate as needed. The SDAI enables evaluation of the intensity of the oral inflammation quantified by the veterinarian (score vet, a‐c scale), the owners’ perception concerning the syndrome's impact on their pets, and a combination of the 2 indices scored on a tabulation sheet (Table 3). Table 3 displays a modified version of the original SDAI, so as to understand how the score was calculated. However, for the purpose of our study, only the index for oral inflammation (score vet) was utilized (evaluated by MK and SP in all cases). Thus, Table 3 illustrates only questions relevant to score vet.
Table 3.
Modified Stomatitis Disease Activity Index (SDAI). Macroscopic inflammation of the oral mucosa in a 0‐3 scale is marked. The sum is grouped under an a‐c scale (a:0‐8, b:9‐16, c:17‐24) to aid comparisons
| SDAI | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Maxillary buccal mucosal inflammation | ||||
| Mandibular buccal mucosal inflammation | ||||
| Maxillary attached gingival inflammation | ||||
| Mandibular attached gingival inflammation | ||||
| Inflammation lateral to palatoglossal folds | ||||
| Molar salivary gland inflammation | ||||
| Oropharyngeal inflammation | ||||
| Lingual and/or sublingual inflammation | ||||
| Total maximum score | 0 | 8 | 16 | 24 |
Statistical Analysis
Comparisons between groups were performed using Student's t‐tests or Mann‐Whitney U‐tests as appropriate. Especially for the pH measurements, a linear mixed model was used to take into account the potential correlation of multiple measurements on the same animal. Kendall's τ (τ‐b or τ‐c, as appropriate) coefficients along with the corresponding P‐values were used to assess associations between ordinal variables (e.g. esophagitis with oral inflammation). Nonparametric tests for trends and Fisher's exact test were used to compare levels of quantitative and categorical data across levels of other variables (e.g. salivary pH with oral inflammation), respectively. The level of significance was set at 0.05. A software package was used for statistical analysis.6
Results
pH and SDAI
Esophageal pH measurements were taken from 26 cats with clinical signs of FCG and 11 control cats due to pH‐metric errors. Salivary pH measurements were taken from 26 FCG cats and 12 controls. Neither salivary (FCG [Mean ± SD] 8.9 ± 0.8, control 8.6 ± 0.7; P = 0.317) nor esophageal (per part proximal: FCG [Mean ± SD]; 8.5 ± 0.8, control 8.6 ± 0.5 P = 0.680; middle: FCG [Mean ± SD]: 8.5 ± 1.1, control 8.3 ± 0.5; P = 0.556), distal: FCG [Mean ± SD]: 8.3 ± 1.2, control 8.4 ± 0.6; P = 0.715) pH comparisons between groups were significantly different. Statistical analysis determined that oral inflammation (score vet) did not correlate with esophagitis (proximal: P = 0.390; Kendall's [τ = 0.098], middle: P = 0.918 [τ = −0.014], distal: P = 0.724 [τ = 0.040], respectively) or with variations in salivary pH (pH [Mean ± SD)] 8.88 ± 0.77; P = 0.151). Finally, salivary pH did not correlate with esophageal pH per part (proximal: P = 0.680, mid: P = 0.556, distal: P = 0.715).
Esophagoscopy
Endoscopic findings were recorded for 58 cats with FCG and 12 controls.
Control group: None exhibited macroscopic signs of esophagitis.
FCG group: 57/58 (98%) animals exhibited some degree of esophagitis in ≥1 part of the esophagus, especially in the proximal (44/58, 76%) or distal part (53/58, 91%) or both. A total of 39.7% of the FCG cats exhibited 1st‐degree esophagitis in the proximal, 70.7% of the FCG cats exhibited no signs of esophagitis in the middle, and 46.6% of the FCG cats exhibited 2nd‐degree esophagitis in the distal part of the esophagus (Fig 1). The different degree of esophagitis per part of the esophagus in the FCG group was compared to controls. As shown in Table 2, the results were significantly different for all parts of the esophagus (P < 0.001, 95% confidence interval [CI]: proximal 5.27, 32.56; distal: 20.13, 173.88). Moreover, esophagitis was not related to prior medications such as antibiotics, corticosteroids (PO, SC or both) or NSAIDs (Table 4). Finally, neither salivation nor chronicity of the FCG signs reached statistical significance when compared to the degree of esophagitis per part for salivation: proximal esophagus (P = 0.329 [Kendall's τ = −0.143]), mid‐esophagus (P = 0.370 [Kendall's τ = −0.111]), distal esophagus (P = 0.796 [Kendall's τ = −0.038]) and for the chronicity of FCG: P = 0.826 (0.026), P = 0.255 (0.118), P = 0.120 (0.179), respectively.
Figure 1.
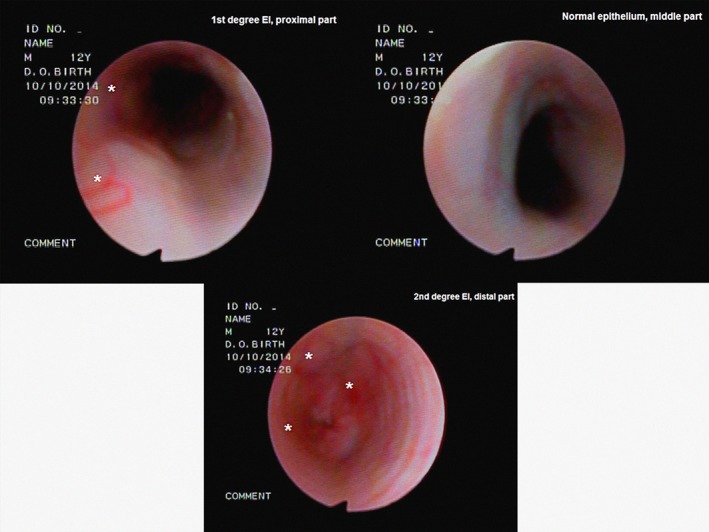
Endoscopic appearance of esophagitis:1st, normal and 2nd degree in the proximal, middle and distal part, respectively (same animal). Pathological sites are noted with an asterisk (*).
Table 4.
Esophagitis (ES) per part compared to prior medication intake. Number of animals with FCG (n) that had received the medications is shown for each type of medication
| Antibiotics (n = 32) | Steroids p.os (n = 9) | Steroids s.c. (n = 16) | Steroids both (n = 5) | NSAIDs (n = 9) | |
|---|---|---|---|---|---|
| P‐value (Kendall's τ) | |||||
| Proximal ES | 0.587 (−0.080) | 0.273 (−0.117) | 0.956 (−0.008) | 0.641 (−0.039) | 0.692 (−0.043) |
| Middle ES | 0.125 (0.188) | 0.639 (−0.043) | 0.397 (0.094) | 0.769 (−0.021) | 0.193 (0.117) |
| Distal ES | 0.398 (0.121) | 0.881 (−0.017) | 0.503 (0.087) | 0.177 (0.109) | 0.255 (−0.119) |
Oral re‐examination was scheduled 6 months and 1 year postoperatively for the cats with clinical remission, and as often as needed if relapse was noticed by the owner. Interestingly, 2 cats with clinical remission that were endoscopically re‐examined 6 months postoperatively also exhibited macroscopic resolution of the esophagitis. On the contrary, 1 cat with repeated clinical relapses was endoscopically re‐examined 5 and 21 months after the first admittance and, despite appropriate treatment for esophagitis, the animal also exhibited esophagitis.
Histopathological Examination
Effort was made to obtain biopsies from all parts of the esophagus in all 58 FCG cats. However, only 25 biopsy specimens obtained from 19 animals with clinical signs of FCG were finally evaluated, because of technical difficulties during the biopsy7 and fixation process. All specimens were examined by the same pathologist (DP). Normal squamous epithelium with lymphocytic, plasmacytic, polymononuclear or mixed infiltration of the lamina propria was noted in 20 cases (Fig 2). Additionally, macrophages also were noted in 1 case, and extensive endothelial edema (esophagitis grade 2) and necrotic tissue (esophagitis grade 2) were noted in 2 others. In the remaining 5 specimens, squamous epithelium was replaced by metaplastic columnar epithelium, and the lamina propria was infiltrated by lymphoplasmacytic population with or without polymononuclear cells (Fig 3). Two specimens exhibited metaplasia: 1 specimen was obtained from the middle part of the esophagus, and the remaining ones from the distal part. Rare fundic gastric glands also were present in all specimens with metaplasia. In total, 4 cats (3 with squamous epithelium, 1 metaplastic) with specimens that had inflammatory infiltration did not exhibit signs of macroscopic esophagitis.
Figure 2.
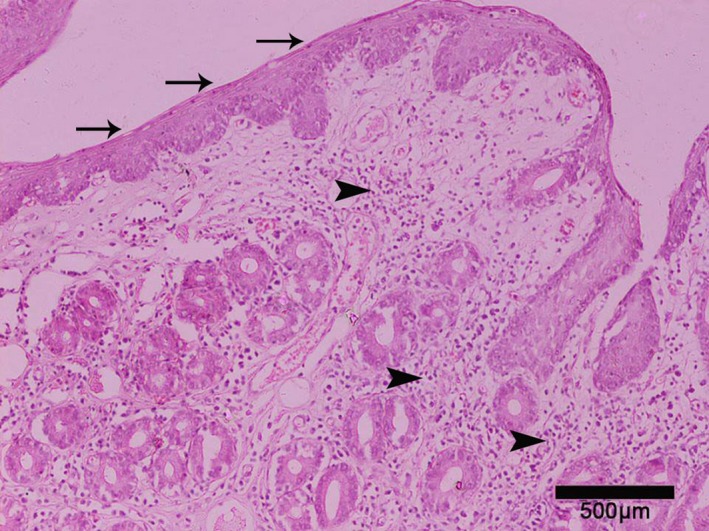
Upper esophagus, lined by squamous epithelium (arrows). Lamina propria is severely infiltrated by mixed inflammatory population (arrowheads) (neutrophils, lymphocytes, plasma cells). Hematoxylin‐eosin, magnification ×400.
Figure 3.
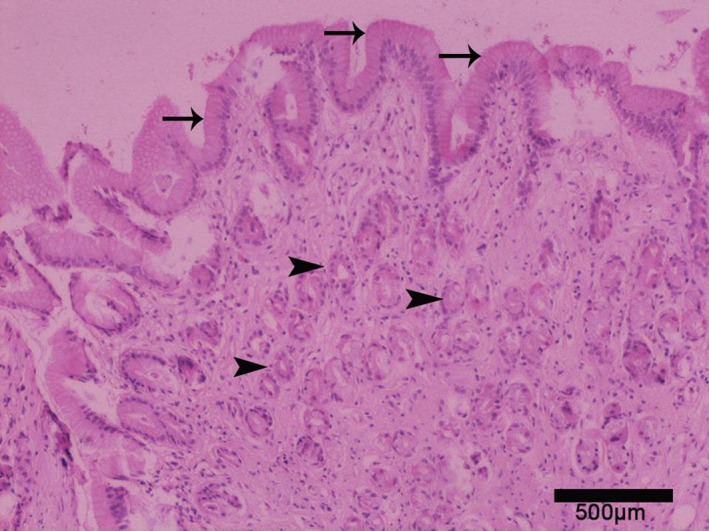
Replacement of the normal squamous by columnar epithelium (arrows) in the distal esophagus. Gastric glands (arrowheads) are also present. Hematoxylin‐eosin, magnification ×100.
Biopsy samples were obtained from 6 controls, but only 4 samples were evaluated because of technical difficulties during the biopsy collection and fixation process. No signs of microscopic inflammation were observed.
Discussion
The etiology of FCG remains elusive, and current treatment options are unrewarding because of its multifactorial nature. Furthermore, the occurrence of esophagitis among cats with FCG has not been investigated before. To the authors’ knowledge, our is the largest prospective study concerning both esophagoscopic evaluation and biopsy acquisition in cats and the first to consider cats affected by FCG.
In our study, naturally occurring esophagitis was recorded in 98% of the FCG cases. Despite the fact that only 25 biopsies eventually were microscopically evaluated, 5 specimens exhibited metaplasia, raising more questions concerning the pathophysiology of FCG. In humans, esophageal metaplasia can progress to dysplasia and cancer (Barrett's esophagus).8 According to their owners, no cat exhibited typical signs of esophageal disease or signs of esophageal disease were masked by FCG.
The majority of the examined FCG cats (75.9%) exhibited some degree of esophagitis in the proximal third of the esophagus. Excessive salivation in FCG cats could result in accumulation of saliva in the proximal esophagus. Deglutition is accomplished in stages. Not every bolus is accompanied by a peristaltic wave. Repeated boluses result in their accumulation in the proximal esophagus until the boluses are carried by a primary wave or a larger bolus.9 Microbes carried by the saliva may directly affect the proximal esophageal mucosa. Lipopolysaccharides of the Gram‐negative bacterial wall can actively affect epithelial cells, which in turn secrete proinflammatory cytokines.10 In lieu of bacteria, epithelial cells also can actively initiate inflammation by secreting proinflammatory mediators, upregulating immune cells and also causing epithelial damage.11 The putative pathogens responsible for stimulating the host immune response in FCG can significantly increase the production of cytokines including Interleukin‐1β (IL‐1β) and Interleukin‐6 (IL‐6),1 which in an experimental feline esophagitis model contributed to decreased esophageal contraction.12 Decreased esophageal contraction could delay esophageal peristalsis causing prolonged contact of the oral microbiome‐containing saliva with the proximal mucosa. Local immune dysregulation combined with prolonged contact could lead to esophagitis. On the other hand, the fact that the proximal third of the esophagus is elevated compared to the rest of the lumen could mechanically propel the saliva by gravity, minimizing the effect of potentially decreased esophageal contraction, thus causing only mild esophagitis in the majority of cases.
Most of the FCG cats (70.7%) had no macroscopic signs of esophagitis in the mid‐esophagus. However, microscopic signs of inflammation and metaplasia respectively were found when 2 biopsies of normal‐appearing mucosa were examined histologically. This finding is not surprising: Cats with chronic reflux esophagitis may have a grossly normal‐appearing esophagus, but still exhibit microscopic submucosal inflammation.13 The middle part of the esophagus is under the influence of cardiac contraction and relaxation. Saliva and transient microbes are not only actively (by peristaltic waves), but also passively carried to the distal esophagus because of gravity and cardiac contraction and relaxation. When clinical esophagitis is encountered, it may be related to exacerbated individual immune response, delayed esophageal clearance which alters contact time, or individual variations in the microbial flora that may give rise to more potent immune‐stimulating species of bacteria. Although endoscopic examination of the esophageal mucosa is the most sensitive method for the diagnosis of esophagitis, the diagnosis of reflux esophagitis cannot be based solely on visual examination because microscopic inflammation sometimes occurs beforehand.14 Extending this observation, a portion of the grossly normal‐appearing esophagi studied could in fact have submucosal inflammation.
As far as the distal part of the esophagus was concerned, inflammation was observed in 91.4% of the cases. Swallowed boluses of food and saliva accumulate orad to the cardiac sphincter. In the cat, swallowing causes relaxation of the sphincter.15 In humans, gastroesophageal reflux (GER) is believed to be caused by transient relaxations of the lower esophageal sphincter rather than a sustained decrease in the sphincter tone.16 Although GER is discussed in a limited fashion in the veterinary literature, few cases have been reported.13
Transient relaxation of the sphincter could be responsible for the esophageal mucosal injury. The central dogma that esophagitis develops from chemical injury on the surface has been challenged. It has been suggested that refluxed gastric fluid may stimulate esophageal epithelial cells to secrete chemokines that attract and activate immune cells, causing damage to the squamous epithelium.7 It has been shown that a profound change in the composition of the microbial community in the esophagus might engage innate immune functions of the epithelial cells.17 Altered transient microbial flora swallowed with saliva in FCG cats may provoke such immune‐mediated activity. Gram‐negative bacteria induce abnormal relaxation of the sphincter by activation of the nitric oxide synthase (NOS) pathway in the cat.18 Nitric oxide synthase can induce relaxation of the esophageal sphincter and also affects blood flow, facilitating the inflammatory process.19 Normal human subjects without reflux‐related signs can have significant acid reflux.20 Exposure to acid can further decrease sphincter pressure at the same time as injury to the mucous membrane, and the low pressure can increase the probability of additional reflux, which initiates a vicious cycle. At the time of recording, visual laxity of the esophageal sphincter was not evident in our study, even though acepromazine has been incriminated previously to affect its pressure21 Additionally, esophageal pH measurements did not indicate GER at the time of recording, based on previously reported literature.22 However, gastric pH was not recorded for the 2 groups. Taken together, visual laxity of the sphincter, abnormal pΗ measurements or refluxed material was not observed in our study, making the hypothesis of typical GER disease rather unlikely. Nevertheless, silent GER causing disruption of the esophageal barrier and inflammation of the mucosa cannot be ruled out.
On the other hand, the intermediate type of innate flora of the lower esophagus, combined with possible inability of the saliva to buffer normally occurring regurgitations could promote selection of virulent bacterial strains. Such bacteria could cause immune‐mediated inflammation to the lower esophagus in cats with FCG.
Esophageal inflammation is not driven by immune cells alone. It has been proposed that essentially all nonimmune cell types of the esophagus can actively secrete proinflammatory mediators such as proinflammatory cytokines and reactive oxygen species (ROS).11 Supposing that FCG cats experience such insults, a potentially exaggerated nonimmune‐nonimmune or immune‐nonimmune inflammatory response can result in esophagitis at the lower part of the feline esophagus. Saliva is known to regulate homeostasis of the oral cavity3 and the esophageal lumen.23 In humans, it has been shown that salivary pH varies depending on inflammatory conditions of the oral cavity24 and mastication in patients with reflux esophagitis.25 Compared to controls, salivary and esophageal pH were normal in the FCG group. Salivary immunoglobulin A (IgA) concentration is lower in cats with FCG,3 suggesting local immunoincompetence or dysregulation. Additionally, affected cats tend to swallow food without chewing and salivation. Local immune dysregulation, inflammation and lack of chewing may account for the loss of buffering ability.
Esophageal injury is rarely reported in cats. According to the results of our study, esophagitis did not correlate with any of the previously prescribed medications. The active ingredients, route and period of administration of the medications could account for this finding. When antibiotics, NSAIDs and corticosteroids were administered, it was for a short period, and in most cases their active ingredients are not considered harmful to the esophageal mucosa.
It has been shown that oral microbial diversity in FCG is narrow, comprised mainly by Gram‐negative microorganisms.26 Microbiota of the healthy proximal human esophagus is expected to be similar to the oral microbiota, whereas the microbiota of the distal esophagus is intermediate between the oral‐like flora of the proximal esophagus and that of the stomach.27 Α shift of the microbiome has been identified in humans with esophagitis and Barrett's esophagus.17 Alterations of the oral microbiota in cats with FCG raise the issue of a possible role of dysbiosis in the pathogenesis of esophagitis.
Esophagitis secondary to GER13 and Barrett's esophagus28 has been reported previously in cats. Gastroesophageal reflux can be seen in animals without GT disease, so the clinical relevance of not seeing GER during endoscopy should be considered in the context of clinical signs and pathological findings. Lack of fluoro endoscopy and sphincter pressure measurements poses limitations to the study, although dysmotility was not suspected clinically. Metaplastic esophageal epithelium was found in the middle and distal part of the esophagus, although the number of evaluated biopsies was relatively small because of technical difficulties. Metaplasia should not be ruled out in the proximal part of the esophagus. Such epithelium may be protective against inflammatory insults of various origins. Barrett's esophagus results from re‐epithelization by pluripotent, undifferentiated stem cells, and has been defined as a premalignant condition.29 Making similar assumptions in the cat may not be warranted, given the shortlife expectancy of the species, but this possibility also should not be overlooked.
One limitation of our study was the small number of endoscopic re‐examinations because owners of clinically healthy animals refused general anesthesia. However, the endoscopic re‐examination of 2 cats that no longer exhibited FCG signs also showed macroscopic healing of the esophagus. On the other hand, re‐examination of a cat with FCGrelapse showed deterioration of the esophagitis despite appropriate treatment for esophagitis. Even so, in light of the above findings, FCG seems to be present concurrently with esophagitis in the majority of cases.
Based on the results of our study, cats with FCG have a high incidence of asymptomatic esophagitis, especially in the proximal and distal parts (P < 0.001) of the esophagus. Thus, cats with FCG should undergo endoscopy and appropriate treatment depending on the extent of inflammation, despite lack of typical signs of esophagitis. Biopsies should be obtained whenever possible because grossly normal‐appearing epithelium may be deceiving concerning the magnitude of histological changes.
Esophagitis should be addressed as a separate entity in cats with FCG because it contributes to morbidity and, although not yet reported, it potentially could predispose a cat to neoplasia of the esophagus.
Acknowledgments
The authors thank Alex. Konstadinidis DVM, MSc, PhD candidate the Unit of Internal Medicine for his help during endoscopies.
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
The study was carried out at the Companion Animal Clinic, Surgery and Obstetrics Unit, School of Veterinary Medicine, Aristotle University Thessaloniki, Greece.
The study was self‐funded.
Preliminary results were presented at the 25th European Congress of Veterinary Dentistry, 19‐22 May 2016, Dublin, Ireland.
Footnotes
Olympus type XP‐20
Acepromazine, alfasan, NederlandB.V.
Dolorex, Intervet International, Holland
Propofol MCT/LCT/Fresenius 1%Fresenius Kabi Hellas
IsoFlo, Zoetis UK Limited
pH‐day2, Medica S.p.A., Modena‐Italy
Olympus FB‐241D, Needle fenestrated biopsy forceps
SDAI available at http://www.dentalvets.co.uk/files/Docs/Common%20Case%20Types/Initialevaluationform2010.pdf
Stata 13, Stata Corp., TX USA
References
- 1. Dolieslager SMJ, Lappin DF, Bennett D, et al. The influence of oral bacteria on tissue levels of Toll‐like receptor and cytokine mRNAs in feline chronic gingivostomatitis and oral health. Vet Immunol Immunopathol 2013;151:263–274. [DOI] [PubMed] [Google Scholar]
- 2. Harley R, Helps CR, Harbour DA, et al. Cytokine mRNA expression in lesions in cats with chronic gingivostomatitis. Clin Diagn Lab Immunol 1999;6:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harley R, Gruffydd‐Jones TJ, Day MJ. Salivary and serum immunoglobulin levels in cats with chronic gingivostomatitis. Vet Rec 2003;152:125–129. [DOI] [PubMed] [Google Scholar]
- 4. Harley R, Gruffydd‐Jones TJ, Day MJ. Characterization of immune cell populations in oral mucosa tissue of healthy adult cats. J Comp Pathol 2003;128:146–155. [DOI] [PubMed] [Google Scholar]
- 5. Hornickel IN, Kacza J, Schnapper A, et al. Demonstration of substances of innate immunity in the esophageal epithelium of domesticated mammals: Part II‐Defence mechanisms, including species comparison. Acta Histochem 2011;113:175–188. [DOI] [PubMed] [Google Scholar]
- 6. Frowde PE, Battersby IA, Whitley NT, Elwood CM. Oesophageal disease in 33 cats. JFMS 2011;13:564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Washabau RJ. Diseases of the gastrointestinal tract. Esophagus In: Washabau RJ, Day MJ, eds. Canine & Feline Gastroenterology, 1st ed Missouri: Saunders, Elsevier; 2013:570–595. [Google Scholar]
- 8. Chedgy FJQ, Kandiah K, Thayalasekaran S, et al. Advances in the endoscopic diagnosis and treatment of Barrett's neoplasia [version 1; referees: 2 approved]. F1000Research 2016;5(F1000 Faculty Rev):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strombeck DR. Pathophysiology of esophageal motility disorders in the dog and cat. Application to management and prognosis. Vet Clin North Am Small Anim Pract 1978;8:229–244. [DOI] [PubMed] [Google Scholar]
- 10. Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008;134:577–594. [DOI] [PubMed] [Google Scholar]
- 11. Rieder F, Biancani P, Harnett K, et al. Inflammatory mediators in gastroesophageal reflux disease: Impact on esophageal motility, fibrosis, and carcinogenesis. Am J Physiol Gastrointest Liver Physiol 2010;298:G571–G581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cao W, Cheng L, Behar J, et al. Proinflammatory cytokines alter/reduce esophageal circular muscle contraction in experimental cat esophagitis. Am J Physiol Gastrointest Liver Physiol 2004;287:G1131–G1139. [DOI] [PubMed] [Google Scholar]
- 13. Han E, Broussard J, Baer KE. Feline esophagitis secondary to gastroesophageal reflux disease: Clinical signs and radiographic, endoscopic, and histopathological findings. J Am Anim Hosp Assoc 2003;39:161–167. [DOI] [PubMed] [Google Scholar]
- 14. Tams TR. Esophagoscopy. Esophagitis In: Tams TR, ed. Small Animal Endoscopy, 2nd ed St. Louis: Mosby; 1999:70–74. [Google Scholar]
- 15. Altschuler SM, Boyle JT, Nixon TE, et al. Simultaneous reflex inhibition of lower esophageal sphincter and crural diaphragm in cats. Am J Physiol 1985;249:G586–G591. [DOI] [PubMed] [Google Scholar]
- 16. Samadi F, Levine M, Rubesin S, et al. Feline esophagus and gastroesophageal reflux. AJR Am J Roentgenol 2010;194:972–976. [DOI] [PubMed] [Google Scholar]
- 17. Yang L, Nossa CW, Francois F, et al. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology 2009;137:588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mittal RK, Goyal RK. Sphincter mechanisms at the lower end of the esophagus. GI Motility online 2006. https://doi.org/10.1038/gimo14. [Google Scholar]
- 19. Liu B, Liu X, Tang C, et al. Esophageal dysmotility and the change of synthesis of nitric oxide in a feline esophagitis model. Dis Esophagus 2002;15:193–198. [DOI] [PubMed] [Google Scholar]
- 20. Kiljander TO, Laitinen JO. The prevalence of gastroesophageal reflux disease in adult asthmatics. Chest 2004;126:1490–1494. [DOI] [PubMed] [Google Scholar]
- 21. Hashim MA, Waterman AE. Effects of acepromazine, pethidine and atropine premedication on lower esophageal sphincter pressure and barrier pressure in anesthetized cats. Vet rec 1993;133:158–160. [DOI] [PubMed] [Google Scholar]
- 22. Garcia RS, Belafsky PC, Della Maggiore A, et al. Prevalence of gastroesophageal reflux in cats during anesthesia and effect of omeprazole on gastric pH. J Vet Intern Med 2017;31:734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Sousa‐Pereira P, Cova M, Abrantes J, et al. Cross‐species comparison of mammalian saliva using an LC‐MALDI based proteomic approach. Proteomics 2015;15:1598–1607. [DOI] [PubMed] [Google Scholar]
- 24. Sharmila B, Sangeeta M, Rahul K. Salivary pH: A diagnostic biomarker. J Indian Soc Periodontol 2013;17:461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sarosiek J, Scheurich CJ, Marcinkiewicz M, McCallum RW. Enhancement of salivary esophagoprotection: Rationale for a physiological approach to gastroesophageal reflux disease. Gastroenterology 1996;110:675–681. [DOI] [PubMed] [Google Scholar]
- 26. Dolieslager SMJ, Riggio MP, Lennon A, et al. Identification of bacteria associated with feline chronic gingivostomatitis using culture‐dependent and culture‐independent methods. Vet Microbiol 2011;148:93–98. [DOI] [PubMed] [Google Scholar]
- 27. Pilato VD, Freschi G, Ringressi MN, et.al. The esophageal microbiota in health and disease. Ann N Y Acad Sci 2016;1381:21–33. [DOI] [PubMed] [Google Scholar]
- 28. Gualtiery M, Olivero D. Reflux esophagitis in three cats associated with metaplastic columnar esophageal epithelium. J Am Anim Hosp Assoc 2006;42:65–70. [DOI] [PubMed] [Google Scholar]
- 29. Spechler SJ, Goyal RK. Barrett's esophagus. N Engl J Med 1986;15:362–371. [DOI] [PubMed] [Google Scholar]


