Abstract
Background
Oral administration of glucocorticoid alters serum cystatin C (sCysC) concentration in humans.
Objective
To determine if oral administration of prednisone alters sCysC in dogs without pre‐existing renal disease.
Animals
Forty six dogs were included: 10 dogs diagnosed with steroid responsive meningitis arteritis (SRMA; group A), 20 dogs diagnosed of pituitary‐dependent hyperadrenocorticism (PDH; group B), and 16 healthy control dogs (group C).
Methods
Retrospective observational study. SRMA diagnosed dogs were administered prednisone 4 mg/kg/24 h PO 7 days, reducing the dose to 2 mg/kg/24 h 7 days before medication withdrawal. In group A, sampling was performed at days 0, 7, 14 and a final control at day 21. Blood and urine samples were collected in the 3 groups, and in group A, sampling was performed at all time points (days 1, 7, 14, and 21).
Results
In group A, sCysC was significantly higher at day 7 compared to the control group (0.4 ± 0.04 mg/L vs. 0.18 ± 0.03 mg/L mean ± SEM respectively P < 0.01); sCysC values decreased to basal at day 14 when the dose was decreased and after 1 week of withdrawal of prednisone (0.27 ± 0.03 mg/L for group A at day 14 and 0.15 ± 0.02 mg/L at day 21; P > 0.05). Dogs with PDH included in group B did not have significant differences in sCysC (0.22 ± 0.03 mg/L) compared to control (P > 0.05).
Conclusions and Clinical Importance
Oral administration of prednisone unlike altered endogenous glucocorticoid production, increases sCysC in dogs in a dose‐dependent fashion.
Keywords: Cystatin C, Dog, Glucocorticoid, Meningitis
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- CL
critical limit
- CSF
cerebrospinal fluid
- CV
coefficient of variation
- GFR
glomerular filtration rate
- LOD
limit of detection
- LOQ
limit of quantification
- PDH
pituitary‐dependent hyperadrenocorticism
- sCysC
serum cystatin C
- SRMA
steroid responsive meningitis arteritis
- UP/C
urinary protein/creatinine ratio
- USG
urinary specific gravity
Serum urea and creatinine levels are commonly used in veterinary medicine as indirect markers of glomerular filtration rate (GFR) to estimate renal function in dogs. However, these are delayed markers of renal failure as substantial variations in these parameters are only observed when approximately 75% of the functional renal mass is lost1 and can be influenced by nonrenal factors. For example, urea can vary due to a high protein diet and both markers (urea and creatinine) are modified by age, hydration status, and muscle mass.1 Therefore, when using serum urea or creatinine levels to estimate renal function, the results need to be carefully evaluated in light of the previously mentioned factors.
Cystatin C (Cys‐C) is a 120 amino acid polypeptide constantly produced by most nucleated cells in the body2; this molecule exhibits no tubular reabsorption, secretion, or metabolism and is freely filtered through the glomerulus.3 Thus, serum cystatin C (sCysC) levels can be considered as another renal marker with superior reliability compared to creatinine.4 In addition, sCysC shows lower individual variability than creatinine and has been reported not to be influenced by sex, age, or muscle mass in human medicine.5, 6 With these physiologic characteristics, sCysC has a great potential to be an excellent surrogate marker of GFR.7, 8, 9
Nevertheless, as its clinical application was initiated in human medicine, it has been reported that several conditions unrelated to renal failure such as thyroid dysfunction, chronic liver disease, malignancies, or asthma among others can alter sCysC.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 However, it has to be noted that the treatment of choice for all these conditions include exogenous glucocorticoid administration and that methylprednisolone or prednisone administration deeply influence sCysC in humans.10, 15, 22
In veterinary medicine, reference values for sCysC canvary with age and body weight (<15 kg),23 although these results are controversial.24, 25 Other conditions such as fasting23 or leishmaniasis26, 27 influence sCysC in dogs.
Until now, no reports have been published regarding the effect of glucocorticoid supplementation on sCysC in dogs. We therefore hypothesized that, as reported in humans, oral administration of prednisone would increase sCysC in dogs in the absence of a pre‐existing renal condition. To achieve this goal, a cohort of 10 dogs affected with steroid responsive meningitis arteritis (SRMA) was selected and the levels of sCysC before and after prednisone administration were evaluated.
Material and Methods
Animals
This study has not been subjected to any animal ethics committee as all the animals enrolled in this study were dogs referred to the Veterinary Hospital of the University of Extremadura. The excess of blood and urine samples were used for this study with the owner consent.
This study includes 46 dogs seen at the Veterinary Hospital of the University of Extremadura. The dogs were divided into the following groups: 10 dogs diagnosed with SRMA (group A), 20 dogs diagnosed of pituitary‐dependent hyperadrenocorticism (PDH; group B), and 16 healthy control dogs (group C).
Experimental Groups
The animals included in group A were sampled from January of 2015 to February of 2016) had a body weight ≥15 kg, different ages (range: 1–2 years), sex (6 males and 4 females), and breeds. They were selected based on the following inclusion criteria: having SRMA, absence of clinical and laboratorial signs of kidney disease, and proper state of hydration. All the dogs received a similar treatment with prednisone (Prednisona Alonga1 ) and none of them had previously been treated with glucocorticoids.2 Corticosteroid therapy consisted of oral administration of prednisone alone at 4 mg/kg/24 h for 7 days, reducing the dose to 2 mg/kg/24 h for further 7 days; after 14 days, prednisone was removed. Blood samples were collected in prednisone‐treated animals on days 1, 7, 14, and 21 after the onset of the treatment and were immediately processed; for all the rest of the groups (control and PDH), blood was obtained once in the absence of any treatment.
SRMA was diagnosed on the basis of: (1) characteristic clinical signs (reluctance to move, kyphosis, stiff gait, cervical and/or thoracolumbar pain, muscle rigidity, or apparent pain on opening the mouth), (2) hematology (WBC higher than 14.00 cells ×109/L due to neutrophilia) and normal biochemistry profile, (3) normal urianalysis, and (4) modifications in the cerebrospinal fluid or CSF (increased WBCs >10 cells/μL, with predominantly mature neutrophils, increased protein concentration in the CSF > 20 mg/dL, and IgA concentrations ≥0.2 mg/mL); failure to isolate an infectious agent from the CSF and positive response to therapy with corticosteroids were considered as diagnostic of SRMA.
A retrospective study (from June 2014 to February 2016) was performed to test the influence of an increase of endogenous steroids on the concentration of sCysC (positive control; group B). Twenty nonhemolyzed sera samples stored at −80°C were used as it has been demonstrated that sCysC remains unchanged for years.28 The cohort of dogs with untreated pituitary‐dependent hyperadrenocorticism (PDH) included in group B were of different breeds and sexes (10 males and 10 females), ages (4–12 years), and weighted over 15 kg. PDH diagnosis was based on the clinical condition of the dogs (polydipsia/polyuria, polyphagia, alopecia, pendulous abdomen, and/or hepatomegaly) and some of the following laboratorial findings: lymphopenia, hypercholesterolemia, high serum alkalinephosphatase (ALP), and alanineaminotransferase (ALT). In addition, an ACTH stimulation test was performed. A serum cortisol above 22 mg/dL was considered as abnormal in a sample obtained 1 hour after a single dose of 0.25 mg/dog IM of synthetic ACTH (Nuvacthen Depot). Adrenal ultrasound examination was performed with an 8 MHz curved array transducer. Dogs were positioned in lateral recumbency, the images in longitudinal planes were obtained, and the largest transverse diameter was recorded. Bilateral adrenal gland enlargement was considered as indicative of PDH; the upper limit of the normal adrenal gland width was 7.5 mm for dogs weighing over 10 kg and 6 mm for dogs weighing ≤10 kg.29
Finally, a group of healthy dogs (group C) was studied and used as negative control. All the dogs included were clinically healthy dogs presented for elective surgery or annual review. The animals included weighted over 15 kg and had different age (1–9 years old), sex (7 males and 9 females) and breeds. They were considered healthy on the basis of a normal physical examination, complete blood count determination, serum biochemical analysis, urinalysis, and fecal examination for parasites.
Clinical Pathology Testing
Blood samples were collected from the cephalic vein after a 12‐hour fasting and placed in tubes containing EDTA for the hematologic examination or with a clotting activator for the serum biochemistry. Sera were prepared by centrifuging blood samples at 200 × g for 10 min. The hematologic analyses were performed with an automated analyser (Mindray BC‐5300; Vet Spinreact), and blood smears were stained with Diff‐Quick. The biochemical variables determined included urea, creatinine, ALT, ALP, total protein, albumin, cholesterol, calcium, and phosphorus by a commercial kit (Spinreact, Barcelona, Spain) and a clinical chemistry analyser (Saturno 100 Vet Crony Instruments, Rome, Italy).
sCysC concentration was determined by a latex turbidimetric commercial kit (Cystatin C turbilatex; Spinreact, Barcelona, Spain) as previously validated by Almy et al.30 As an indication of assay precision, the intraday coefficient of variation (CV) was calculated from 10 samples assayed on the same day, and the interday CV was calculated from 10 samples assayed on separate days. The accuracy of the assay was investigated by linearity during dilution using the mean of three calibration curves of four standards with known cystatin C concentrations (Cystatin C Calibrator; Spinreact, Barcelona, Spain). The CL (critical limit), LOD (limit of detection), and LOQ (limit of quantification) were calculated as follows31: CL = standard deviation (SD) × t (0.05,∞); LOD = SD × 2 t (0.05,∞); LOQ = 10 × SD, where the parameter t represents Student's t‐test. The repeatability and reproducibility of the cystatin C turbidimetric assay had satisfactory variability with a within‐day CV = 5.4% and a between‐day CV = 7.0%, both less than 10%. Regression analysis showed a linear relationship (R = 0.9997) between the real and theoretical values of the cystatin C concentration. All dogs were tested for the absence of canine heartworm disease, Anaplasma phagocytophylum, Borrelia burgdorferi, Ehrlichia canis antibodies (Canine SNAP 4Dx, IDEXX Laboratories, USA), and leishmaniasis (direct visualization of Leishmania infantum amastigotes in ganglia or bone marrow smears and/or a positive immunoassay commercial kit; kit Q letitest ELISA leishmania; Laboratorios Leti, Spain).
Urine was obtained by ultrasound‐guided aseptic cystocentesis. Three microliter of urine was used for routine urinalysis (Multistix Reagent Strips, Bayer Corporation, Madrid, Spain) according to the manufacturer's instructions using an Urispin reader (Spinreact). The strips were used to determine the presence of glucose, ketones, bilirubin, urobilinogen, blood, and protein in the urine, as well as urinary pH. The remaining sample was centrifuged for 5 min at 200 × g. The sediment was evaluated, and a part of the supernatant was used to measure urinary specific gravity (USG) by manual refractometry (ZUZI 300). The urinary protein/creatinine ratio (UP/C) was calculated by measuring the urinary protein concentration (pirogallol red and molybdate technique; RAL Laboratory, Chillton,U.K.) and the creatinine concentration in the urine (Jaffe method's; RAL Laboratory).
Statistical Analysis
Data were tested for normality by a Shapiro‐Wilk test; results are reported as mean ± standard error of the mean (SEM). Groups were compared using ANOVA on ranks due to their non‐Gaussian distribution. When statistically significant differences were found, a Dunn's posthoc test was used. All statistical analyses were performed by Sigma Plot software version 11.0 for Windows (Systat Software, Chicago, IL, USA). Differences among values were considered as statistically significant when P < 0.05 or P < 0.01.
Results
Hematology
The hematologic, biochemical, and urinalysis values of control group (Group C) were within the normal reference range established by the Clinical Pathology Service of the Clinical Veterinary Hospital of the UEx. WBC (Table 1) was significantly enhanced in groups A (over 18.00 × 109/L cells) and B (11.78 ± 1.14 cells × 109/L) compared to group C (9.47 ± 0.78 cells × 109/L; P < 0.001). Lymphopenia was observed in some dogs affected with PDH (11 out of 20) and leukocytosis (9 out of 20) due to neutrophilia; neutrophilia and monocytosis were detected in all SRMA cases throughout the study (data not shown). Platelet count (Table 1) differed statistically in Group A (day 14) and Group B compared to control (Table 1; P < 0.05).
Table 1.
Hematologic findings in the three groups of dogs included in the study
| Group | A | B | C | |||
|---|---|---|---|---|---|---|
| Day | 1 | 7 | 14 | 21 | ||
| PCV (%) | 45.64 ± 3.11 | 42.07 ± 1.88 | 43.20 ± 1.71 | 43.55 ± 1.55 | 46.72 ± 1.61 | 45.89 ± 1.09 |
| WBC (×109/L) | 20.21 ± 2.46** | 26.93 ± 2.56** | 24.57 ± 2.88** | 18.36 ± 1.87** | 11.78 ± 1.14** | 9.47 ± 0.79 |
| PLT (×109/L) | 334.60 ± 54.56 | 351.20 ± 45.44 | 367.00 ± 25.17* | 303.20 ± 27.16 | 434.25 ± 44.35* | 246.80 ± 26.55 |
PCV, white blood cell (WBC) and platelet (PLT) counts in dogs affected with steroid responsive meningitis arteritis (SRMA; group A, n = 10 at days 1, 7, 14, and 21 after treatment onset), dogs with hyperadrenocorticism (PDH; group B, n = 20), and control dogs (group C, n = 16). Values are presented as mean ± SEM. Values marked with * differ statistically from the control group: *P < 0.05 and **P < 0.01.
Serum Biochemistry and Urinalysis
Serum concentrations of total proteins, albumin, calcium, and phosphorus remained within the reference values in all dogs in Group A (Table 2). A significant raise in serum cholesterol (Table 2) was observed after prednisone administration at day 7 (280.04 ± 48.23 mg/dL; mean ± SEM) and 14 (324.43 ± 45.43 mg/dL) compared to the control group (186.19 ± 60.35 mg/dL group C; P < 0.01); ALP values were significantly enhanced in all groups (A and B) compared to control (group C; Table 2, P < 0.01). The dogs included in Group A (day 14) and B showed an increased ALT (Table 2; P < 0.01). In dogs diagnosed with PDH, endogenous corticosteroid production was altered (mean pre‐ACTH cortisol of 8.7 ± 1.2 μg/dL; n = 20), while in the day 0 of the SRMA‐affected group, cortisol values remained in the reference range (2.2 ± 0.6 μg/dL; n = 16), as 8 μg/dL is the threshold value of the laboratory below which serum cortisol is considered as normal. The other biochemical determinations were within the normal intervals. No changes were observed in urinalysis in either group of dogs studied. The UP/C was lower than 0.4 in all groups (Table 2) although the higher value was observed in group B (0.35 ± 0.02; P < 0.01 vs. control), which is commonly found in dogs affected with PDH.32
Table 2.
Biochemic and urinary findings in the three groups of dogs included in the study
| Group | A | B | C | |||
|---|---|---|---|---|---|---|
| Day | 1 | 7 | 14 | 21 | ||
| Urea (mg/dL) | 22.69 ± 7.67 | 28.26 ± 4.96 | 26.75 ± 8.49 | 24.55 ± 4.68* | 45.66 ± 34.93 | 34.31 ± 8.35 |
| ALT (UI/L) | 31.8 ± 28.8 | 43.7 ± 4.03 | 104.4 ± 27.77** | 59.3 ± 9.85 | 95.95 ± 16.19** | 33.68 ± 2.86 |
| Creatinine (mg/dL) | 0.82 ± 0.07 | 0.69 ± 0.07* | 0.83 ± 0.08 | 0.86 ± 0.14 | 0.97 ± 0.71 | 0.96 ± 0.12 |
| TP (g/dL) | 6.2 ± 0.1 | 6.4 ± 0.2 | 6.2 ± 0.1 | 6.4 ± 0.1 | 6.9 ± 0.1** | 6.4 ± 0.1 |
| Albumin (g/L) | 0.37 ± 0.01 | 0.38 ± 0.02 | 0.38 ± 0.02 | 0.37 ± 0.02 | 0.39 ± 0.01 | 0.36 ± 0.01 |
| Cholesterol (mg/dL) | 210.65 ± 27.08 | 280.04 ± 48.23** | 324.43 ± 45.43 | 237.50 ± 39.48 | 315.53 ± 131.52** | 186.19 ± 60.35 |
| ALP (UI/L) | 227.3 ± 27.97** | 360 ± 46.14** | 456 ± 61.8** | 306.7 ± 36.6** | 494.35 ± 79.15** | 63.68 ± 5.40 |
| Calcium (mg/dL) | 11.07 ± 0.78* | 11.18 ± 0.66* | 11.09 ± 0.71* | 11.14 ± 0.60* | 9.49 ± 0.44 | 9.95 ± 1.29 |
| Phosphorus (mmol/L) | 3.96 ± 0.19 | 4.00 ± 0.19 | 4.00 ± 0.25 | 3.93 ± 0.22 | 4.31 ± 0.12 | 3.78 ± 0.15 |
| UP/C | 0.24 ± 0.02 | 0.26 ± 0.02 | 0.22 ± 0.02 | 0.25 ± 0.02 | 0.35 ± 0.02** | 0.2 ± 0.02 |
Serum urea, ALT, total protein (TP), albumin, cholesterol, ALP, calcium, phosphorus, and UP/C (urinary protein/creatinine ratio) values in dogs affected with steroid responsive meningitis arteritis (SRMA; group A, at days 1, 7, 14, and 21 after treatment onset, n = 10), dogs with hyperadrenocorticism (PDH; group B, n = 20), and control dogs (group C, n = 16). Values are presented as mean ± SEM. Values marked with * differ statistically from the control group: *P < 0.05 and **P < 0.01.
Serum Cystatin C determinations
In group A, sCysC concentration was significantly higher at day 7 (0.4 ± 0.04 mg/L; mean ± SEM) compared to the control group (0.18 ± 0.03 mg/L; Graph 1); in addition, sCysC values decreased to basal values when the prednisone dose was reduced to 2 mg/kg/d (0.27 ± 0.03 mg/L; Group A, day 14) and further decreased after 1 week of cortisone withdrawal (0.15 ± 0.02 mg/L in group A, day 21; P > 0.05 compared to control). Furthermore, dogs with PDH included in group B did not show significant differences in sCysC (0.21 ± 0.03 mg/L) compared control (P > 0.05; Fig. 1).
Figure 1.
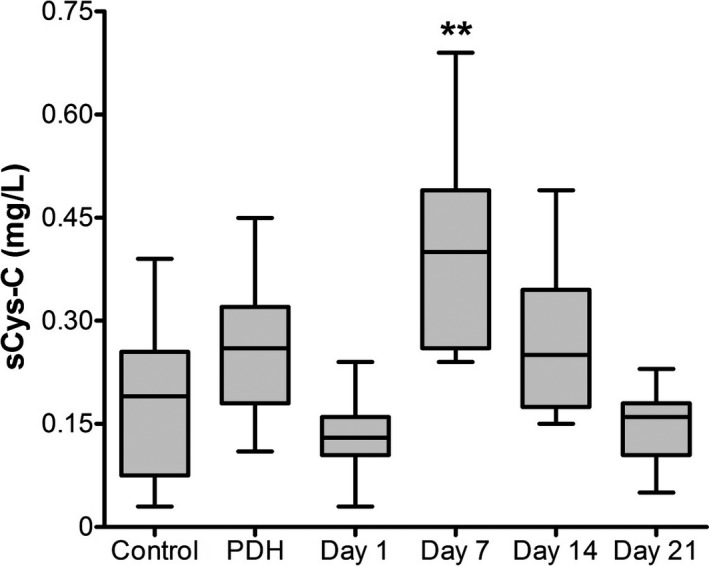
Serum cystatin C values in the three groups of dogs included in the study. sCysC values were determined after a 12‐hour fasting in dogs affected with steroid responsive meningitis arteritis (SRMA; group A at days 1, 7, 14, and 21 after prednisone treatment onset, n = 10), dogs with hyperadrenocorticism (PDH; group B, n = 20), and control dogs (group C, n = 16). Bars represent the mean and standard error of the mean. Values bearing ** differ statistically from the control group (P < 0.01).
Discussion
The aim of the present work was to elucidate if exogenous administration of corticosteroids in dogs without renal failure influence sCys‐C. These data demonstrate that PO administered prednisone at 4 mg/kg enhances sCys‐C and that dogs affected with PDH did not exhibit altered sCys‐C values. These results are clinically relevant if sCys‐C needs to be evaluated in any setting in which dogs are administered PO with high doses of glucocorticoids (4 mg/kg daily). In the first part of this study, the hematologic findings revealed an increase in the total WBC in SRMA‐affected dogs at day 0 compared to control (Table 1; P < 0.01); this finding is related to neutrophilia, often seen with the onset of the SRMA clinical signs. In addition, enhanced platelet counts were found in group B and group A at day 14 compared to control (P < 0.05), but they were not clinically relevant.
Corticoids are widely used in veterinary and human medicine due to their potent anti‐inflammatory and immunosuppressive effects. Several studies have demonstrated that cortisone administration induces a rise in sCysC in humans.12, 13, 14, 33 These results parallel the ones above described as a similar increase in sCysC takes place in dogs after oral prednisone administration for 7 days at 4 mg/kg/24 hours; this finding is not related to impaired glomerular filtration as serum creatinine, urea and UP/C remain within reference ranges in all groups (Table 2). As previously mentioned, sCysC has been demonstrated to be an earlier indicator of decreased glomerular function compared to creatinine.34, 35 However, exogenous corticoid administration does not seem to induce a raise in sCysC by decreasing the GFR. Instead, other mechanisms have been proposed, for example Bjarnadóttir et al., in 199515 demonstrated that in vitro addition of dexamethasone to HeLa cells induced a dose‐dependent increase Cys‐C secretion in culture after 40 hours. The authors suggested that the increase observed in Cys‐C was due to a corticoid‐related stimulatory effect on the Cys‐C gene promoter, thus increasing the transcription of the Cys‐C gene. In methylprednisolone‐treated asthma patients, the raise observed in sCysC has also been related to the pathogenesis of the process, as it is actively secreted by macrophagues in the alveolus.10 Although the pathologic causes, dosages, and administration schedule vary between veterinary and human medicine, and with the ones used in the present study, these results show that sCysC significantly increases after exogenous glucocorticoid administration in dogs. These results showed that sCysC significantly increased at day 7 (4 mg/kg/d prednisone) and decreased to basal values at day 14 when the dose was reduced to half (2 mg/kg/d prednisone) and further decreased after treatment withdrawal (Fig. 1). These results parallel those of Risch et al.16 who demonstrated that in humans subjected to kidney transplantation sCysC was higher in patients treated with corticosteroids and raised with increasing glucocorticoid doses. Similar results were reported by Pöge et al.36 who described that, in patients subjected to kidney transplantation treated with 500 mg of methylprednisolone, sCysC peaked after 24 hours and that this rise was dose‐dependent. Interestingly, these results demonstrate that in dogs diagnosed with PDH in which endogenous corticosteroid production is altered (mean pre‐ACTH cortisol of 8.7 ± 1.2 μg/dL; n = 20), and sCysC values are not significantly different than those obtained in the control group (P > 0.05; Fig. 1). These results are in agreement with a recent report by Marynissen et al.37 who demonstrated that in dogs affected with hyperadrenocorticism followed for 12 months, sCysC values were not significantly different compared to healthy dogs. In view of these results and previous publications,35 it can be concluded that exogenous administration of corticosteroids increases sCysC in a dose‐dependent fashion, but impaired endogenous production of corticosteroids does not alter sCysC. Hence, these results suggest that in dogs affected with PDH, the endogenous corticosteroid production is not sufficiently high to induce a raise in sCysC and that there is a threshold below which glucocorticoids do not alter this parameter (Fig. 1). In view of these results, the high immunosuppressive corticosteroid doses used in dogs (4 mg/kg) induce significant sCysC rise, while low immunosuppresive doses (2 mg/kg) do not. However, it remains to be studied if corticosteroid influence over sCysC is transitory in dogs, as previously observed in human patients affected with lupus nephritis chronically treated with corticoids.38
In conclusion, oral prednisone administration increases sCysC in the canine species, and this rise seems to be dose‐dependent; however, altered sCysC was not observed in dogs showing impaired endogenous corticosteroid production due to PDH. Hence, these results need to be considered when interpreting sCysC values in dogs receiving corticosteroid therapy.
Acknowledgments
Beatriz Macías‐García holds a postdoctoral grant Juan de la Cierva Incorporación IJCI‐2014‐19428 from the Spanish Ministry of Economy and Competitiveness.
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
The study was conducted at the Animal Medicine Department, Faculty of Veterinary Sciences, University of Extremadura, 10071 Cáceres, Spain.
The study was presented in abstract form at the 25th ECVIM‐CA Congress, Lisbon, Portugal, September 2015.
Footnotes
Prednisona Alonga; sanofi‐aventis, S.A., Barcelona, Spain
Nuvacthen Depot; Sigma‐Tau Laboratory, Madrid, Spain
References
- 1. Braum JP, Lefebvre HP. Kidney function and damage In: Kaneko JJ, Harvey JW, Bruss ML, eds. Clinical Biochemistry of Domestic Animals, 6th ed London: Elsevier; 2008:485–528. [Google Scholar]
- 2. Abrahamson M, Olafsson I, Palsdottir A, et al. Structure and expression of the human cystatin C gene. Biochem J 1990;268:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Royakkers AA, Korevaar JC, Van Suijlen JD, et al. Serum and urine cystatin C are poor biomarkers for acute kidney injury and renal replacement therapy. Intensive Care Med 2011;37:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 2004;65:1416–1421. [DOI] [PubMed] [Google Scholar]
- 5. Finney H, Newman DJ, Price CP. Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Ann Clin Biochem 2000;37:49–59. [DOI] [PubMed] [Google Scholar]
- 6. Vinge E, Lindergard B, Nilsson‐Ehle P, Grubb A. Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest 1999;59:587–592. [DOI] [PubMed] [Google Scholar]
- 7. Newman JD, Cystatin C. Ann Clin Biochem 2002;39:89–104. [DOI] [PubMed] [Google Scholar]
- 8. Simonsen O, Grubb A, Thysell H. The blood serum concentration of cystatin C (gamma‐trace) as a measure of the glomerular filtration rate. J Clin Lab Invest 1985;45:97–101. [DOI] [PubMed] [Google Scholar]
- 9. Lamb EJ, ORiordan SE, Webb MC, Newman DJ. Serum cystatin C may be a better marker of renal impairment than creatinine. J Am Geriatr Soc 2003;51:1674–1675. [DOI] [PubMed] [Google Scholar]
- 10. Cimerman N, Brguljan PM, Krasovec M, et al. Serum cystatin C, a potent inhibitor of cysteine proteinases, is elevated in asthmatic patients. Clin Chim Acta 2000;300:83–95. [DOI] [PubMed] [Google Scholar]
- 11. Bökenkamp A, van Wijk JA, Lentze MJ, Stoffel‐Wagner B. Effect of corticosteroidtherapy on serumcystat in C and beta2‐microglobulin concentrations. Clin Chem 2002;48:1123–1126. [PubMed] [Google Scholar]
- 12. Risch L, Saely C, Reist U, et al. Course of glomerular filtration rate markers in patients receiving high‐dose glucocorticoids following subarachnoidal hemorrhage. Clin Chim Acta 2005;360:205–207. [DOI] [PubMed] [Google Scholar]
- 13. Manetti L, Genovesi M, Pardini E, et al. Early effects of methylprednisolone infusion on serum cystatin C in patients with severe Graves’ ophthalmopathy. Clin Chim Acta 2005;356:227–228. [DOI] [PubMed] [Google Scholar]
- 14. Zhai JL, Ge N, Zhen Y, et al. Corticosteroids significantly increase serum Cystatin C concentration without affecting renal function in symptomatic heart failure. Clin Lab 2016;62:203–207. [DOI] [PubMed] [Google Scholar]
- 15. Bjarnadóttir M, Grubb A, Olafsson I. Promoter‐mediated, dexamethasone‐induced increase in cystatin C production by HeLa cells. Scand J Clin Lab Invest 1995;55:617–623. [DOI] [PubMed] [Google Scholar]
- 16. Risch L, Herklotz R, Blumberg A, Huber A. Effects of glucorticoid immunosuppression on serum cystatin concentrations in renal transplant patients. Clin Chem 2001;47:2055–2059. [PubMed] [Google Scholar]
- 17. Laterza O, Price C, Scott M. Cystatin C: An improved estimator of glomerular filtration rate? Clin Chem 2002;48:699–707. [PubMed] [Google Scholar]
- 18. Wesli P, Schwegler B, Spinas G, Schmid C. Serum cystatin C is sensitive to small changes in thyroid function. Clin Chim Acta 2003;338:87–90. [DOI] [PubMed] [Google Scholar]
- 19. Demirtas S, Akan O, Can M, et al. Cystatin C can be affected by non renal factors: A preliminary study on leukemia. Clin Biochem 2006;39:115–118. [DOI] [PubMed] [Google Scholar]
- 20. Rule A, Bergstralh E, Slezak J, et al. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int 2006;69:399–405. [DOI] [PubMed] [Google Scholar]
- 21. Madero M, Wassel C, Peralta C, et al. Cystatin C associates with arterial stiffness in older adults. J Am Soc Nephrol 2009;20:1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Filler G, Priem F, Lepage N, et al. Beta‐trace protein, cystatin C, beta(2)‐microglobulin, and creatinine compared for detecting impaired glomerular filtration rates in children. Clin Chem 2002;48:729–736. [PubMed] [Google Scholar]
- 23. Braun JP, Perxachs A, Pechereau D, De La Farge F. Plasma cystatin C in the dog: Reference values and variations with renal failure. Comp Clin Pathol 2002;11:44–49. [Google Scholar]
- 24. Wehner A, Hartmann K, Hirschberger J. Utility of serum cystatin C as a clinical measure of renal function in dogs. J Am Anim Hosp Assoc 2008;44:131–138. [DOI] [PubMed] [Google Scholar]
- 25. Pagitz M, Frommlet F, Schwendenwein I. Evaluation of biological variance of cystatin C in comparison with other endogenous markers of glomerular filtration rate in healthy dogs. J Vet Intern Med 2007;21:936–942. [DOI] [PubMed] [Google Scholar]
- 26. Antognoni MT, Siepi D, Porciello F, et al. Serum cystatin‐C evaluation in dogs affected by different diseases associated or not with renal insufficiency. Vet Res Commun 2007;31:269–271. [DOI] [PubMed] [Google Scholar]
- 27. Pasa S, Bayramli G, Atasoy A, et al. Evaluation of serum cystatin‐C in dogs with visceral leishmaniasis. Vet Res Commun 2009;33:529–534. [DOI] [PubMed] [Google Scholar]
- 28. Séronie‐Vivien S, Delanaye P, Piéroni L, et al. Cystatin C: Current position and future prospects. Clin Chem Lab Med 2008;46:1664–1686. [DOI] [PubMed] [Google Scholar]
- 29. Choi J, Kim H, Yoon J. Ultrasonographic adrenal gland measurements in clinically normal small breed dogs and comparison with pituitary‐dependent hyperadrenocorticism. J Vet Med Sci 2011;73:985–989. [DOI] [PubMed] [Google Scholar]
- 30. Almy FS, Christopher MM, King DP, Brown SA. Evaluation of cystatin C as an endogenous marker of glomerular filtration rate in dogs. J Vet Intern Med 2002;16:45–51. [DOI] [PubMed] [Google Scholar]
- 31. Duffau B, Rojas F, Guerrero I, Roa L, Rodríguez L, Soto M, Aguilera M, Sandoval S. Eds. Validación de métodos y determinación de la incertidumbre de la medición: aspectos generales sobre la validación de métodos. Guía técnica editada por el Instituto de Salud Púbica. Ministerio de Salud. Gobierno de Chile. http://www.ispch.cl/content/guia-tecnica-de-validacion-de-metodos-y-determinacion-de-la-incertidumbre-de-la-medicion.
- 32. Smets PM, Lefebvre HP, Kooistra HS, et al. Hypercortisolism affects glomerular and tubular function in dogs. Vet J 2012;192:532–534. [DOI] [PubMed] [Google Scholar]
- 33. Risch L, Huber AR. Glucocorticoids and increased serum cystatin C concentrations. Clin Chim Acta 2002;320:133–134. [DOI] [PubMed] [Google Scholar]
- 34. Roos JF, Doust J, Tett SE, Kirkpatrick CM. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children–a meta‐analysis. Clin Biochem 2007;40:383–391. [DOI] [PubMed] [Google Scholar]
- 35. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta‐analysis. Am J Kidney Dis 2002;40:221–226. [DOI] [PubMed] [Google Scholar]
- 36. Pöge U, Gerhardt T, Bökenkamp A, et al. Time course of low molecular weight proteins in the early kidney transplantation period–influence of corticosteroids. Nephrol Dial Transplant 2004;19:2858–2863. [DOI] [PubMed] [Google Scholar]
- 37. Marynissen SJJ, Smets PMY, Ghys LFR, et al. Long‐term follow‐up of renal function assessing serum cystatin C in dogs with diabetes mellitus or hyperadrenocorticism. Vet Clin Pathol 2016;45:320–329. [DOI] [PubMed] [Google Scholar]
- 38. Vinicius M, Moscoso G, Kiyomi S, Mastroianni G. Are serum cystatin C levels influenced by steroid doses in lupus nephritis patients? J Bras Nefrol 2011;33:306–312. [DOI] [PubMed] [Google Scholar]


