Abstract
Background
Thyrotropin (TSH) can be increased in humans with primary hypoadrenocorticism (HA) before glucocorticoid treatment. Increase in TSH is a typical finding of primary hypothyroidism and both diseases can occur concurrently (Schmidt's syndrome); therefore, care must be taken in assessing thyroid function in untreated human patients with HA.
Objective
Evaluate whether alterations in cTSH can be observed in dogs with HA in absence of primary hypothyroidism.
Animals
Thirty dogs with newly diagnosed HA, and 30 dogs in which HA was suspected but excluded based on a normal ACTH stimulation test (controls) were prospectively enrolled.
Methods
cTSH and T4 concentrations were determined in all dogs and at selected time points during treatment (prednisolone, fludrocortisone, or DOCP) in dogs with HA.
Results
cTSH concentrations ranged from 0.01 to 2.6 ng/mL (median 0.29) and were increased in 11/30 dogs with HA; values in controls were all within the reference interval (range: 0.01–0.2 ng/dL; median 0.06). There was no difference in T4 between dogs with increased cTSH (T4 range 1.0‐2.1; median 1.3 μg/dL) compared to those with normal cTSH (T4 range 0.5‐3.4, median 1.4 μg/dL; P=0.69) and controls (T4 range 0.3‐3.8, median 1.8 μg/dL; P=0.35). After starting treatment, cTSH normalized after 2–4 weeks in 9 dogs and after 3 and 4 months in 2 without thyroxine supplementation.
Conclusions and Clinical Relevance
Evaluation of thyroid function in untreated dogs with HA can lead to misdiagnosis of hypothyroidism; treatment with glucocorticoids for up to 4 months can be necessary to normalize cTSH.
Keywords: ACTH, Addison, Cortisol, cTSH, Hypothyroidism
Abbreviations
- ACTH
adrenocorticotropic hormone
- BID
twice daily, q12 hours
- cTSH
canine thyroidstimulating hormone; canine thyrotropin
- DOCP
desoxycorticosterone pivalate
- FC
fludrocortisone acetate
- HA
hypoadrenocorticism
- NTI
nonthyroidal illness
- SID
once daily, q 24 hours
- TSH
thyroid‐stimulating hormone; thyrotropin
Hypoadrenocorticism (HA) is a disease in which adrenocortical steroid hormone secretion falls below the physiologic requirement of the animal. The vast majority of affected dogs have primary HA, which usually results from immune‐mediated destruction of the adrenal cortex, terminating in an absolute deficiency of glucocorticoids and mineralocorticoids.1, 2 Due to the lack of the negative feedback on the pituitary gland, an increase in plasma concentrations of endogenous ACTH is typically observed.3, 4, 5
Human patients with primary HA can have thyroid‐stimulating hormone (TSH; thyrotropin) concentrations that are increased despite normal T4 concentrations before initiating glucocorticoid treatment.6 As TSH will normalize after glucocorticoid supplementation, the increase has been explained by a lack of the inhibitory effect of cortisol on the secretion of TSH.7, 8, 9 Increased TSH concentrations in patients with HA are also observed in those patients that suffer from concomitant primary hypothyroidism, known as type II autoimmune polyendocrine syndrome or Schmidt's syndrome. To avoid a misdiagnosis of hypothyroidism, it has been recommended that serum TSH and thyroid hormone concentrations should be interpreted in light of the patient's cortisol concentrations and that thyroid function is better assessed after glucocorticoid treatment has been started.6, 7
Although rare, polyglandular autoimmune syndromes do exist in veterinary medicine also, and concomitant primary hypothyroidism and HA occur in dogs.10, 11, 12 To the authors’ knowledge, cTSH concentrations have not yet been systematically evaluated in dogs with HA. However, it would be important to know whether increased cTSH concentrations can be observed before HA treatment, as has been described in human patients. An increased cTSH is considered to be rather rare in euthyroid dogs, except in the recovery phase from a nonthyroidal illness (NTI),13, 14 meaning that a high TSH is rather specific for the diagnosis of primary hypothyroidism, especially in combination with a decreased T4. Therefore, this finding could easily lead to a misdiagnosis of hypothyroidism and an unnecessary, lifelong thyroxine treatment.
Thus, the aim of this study was to evaluate cTSH concentrations in dogs with HA before starting glucocorticoid treatment. Further, these results will be compared to those of dogs in which HA has been excluded, based on a normal ACTH stimulation test. In addition, we wanted to evaluate cTSH concentrations in dogs with HA during treatment with gluco‐ and mineralocorticoids.
Materials and Methods
Animals
Thirty client‐owned dogs presented between December 2010 and May 2016 with newly diagnosed, naturally occurring HA were prospectively enrolled in the study. Work‐up included complete blood count, serum biochemical profile, urinalysis, ACTH stimulation test, measurement of plasma endogenous ACTH (eACTH), and abdominal ultrasonography. HA was confirmed by an insufficient ACTH‐stimulated serum cortisol concentration (<2 μg/dL).
Primary HA was diagnosed on the basis of abnormal serum sodium and potassium concentrations or increased plasma eACTH concentrations. Dogs with iatrogenic causes of HA (eg, previous steroid or trilostane treatment) were excluded from the study.
Thirty dogs with diseases mimicking HA, diagnosed between November 2013 and December 2015, were prospectively enrolled in the study. All dogs had initially been suspected of having HA but were finally determined to have a different disease; all had post‐ACTH serum cortisol concentrations ≥4.5 μg/dL. Diseases mimicking HA were associated with clinical signs and/or laboratory findings routinely seen in dogs with HA, for example, vomiting, diarrhea, weakness, lethargy, hyperkalemia, hyponatremia, or some combination of these.
All procedures were officially approved and conducted in accordance with guidelines established by the Animal Welfare Act of Switzerland (permission number: 133/2013). In addition, informed consent was obtained from the dog owners.
Analytical Procedures
For the ACTH stimulation test, blood samples were taken before and 60 min after intravenous or intramuscular injection of 5 μg/kg synthetic ACTH.a For the determination of plasma eACTH, blood was collected, before ACTH application, into chilled EDTA‐coated tubes placed on ice and centrifuged at 4°C within 30 min. For the determination of cortisol, T4, and cTSH concentration, serum was harvested by low‐speed centrifugation after clot retraction at room temperature. All samples were stored at −20°C until assayed. Plasma eACTH concentrations were determined by a 2‐site solid‐phase chemiluminescent immunometric assay,b previously validated for dogs.15, 16 Serum cortisol concentrations were measured by a competitive immunoassay.c The intra‐assay coefficients of variation were 10.0 and 6.3% at cortisol levels of 2.7 and 18.9 μg/dL, respectively. The sensitivity of the assay was 0.2 μg/dL. Serum cTSH concentrations were measured by use of a solid‐part, 2‐site chemiluminescent enzyme immunometric assay.d The intra‐assay coefficients of variation were 5.0, 4, and 3.8% at TSH levels of 0.20, 0.50, and 2.6 ng/mL, respectively. The interassay coefficients of variation were 6.3 and 8.2% at TSH levels of 0.16 and 2.8 ng/mL, respectively. The sensitivity of the assay was 0.03 ng/mL; upper limit of the reference range was 0.5 ng/mL. Serum T4 concentrations were determined with a homologous solid‐phase, chemiluminescent enzyme immunoassay.e The intra‐assay and interassay coefficients of variation (T4 concentrations between 0.65 and 11.9 μg/dL; each concentration tested in duplicate twice daily over the course of 20 days) were 3.9–10.8 and 5.2–13.8%, respectively, reference range, 1.0–2.9 μg/dL.
Statistical Analyses
Statistical analysis was performed by commercial softwaref, g using nonparametric tests. Data are expressed as median and range. Differences between groups were tested by the use of the Kruskal‐Wallis H test and Mann‐Whitney U‐test with a Dunn's post‐test. Friedman's repeated‐measures test and Dunn's multiple comparisons test were used for evaluating cTSH and T4 concentrations at the different time points. Correlation was determined by Spearman rank correlation coefficient.
For cTSH values below the detection limit, the mean between 0 and the detection limit of 0.03 ng/mL (corresponding to 0.015 ng/mL) was entered for statistical analysis, and for plasma eACTH concentrations >1,250, 1,251 pg/mL was used. Values of P < 0.05 were considered significant.
Results
Dogs
In the 30 dogs with HA, age ranged from 0.5 to 12 years (median, 5 years) and body weight from 1.8 to 75.2 kg (median, 13.2 kg). There were 10 males (4 castrated) and 20 females (19 spayed). There was no difference in age between male and female dogs. The HA group consisted of 23 purebred and 7 mixed‐breed dogs. Twenty‐seven of the 30 dogs had abnormal serum electrolyte concentrations, 2 of the 3 dogs with normal electrolytes had high plasma eACTH concentrations, and in 1 of those 3, eACTH had not been determined. From the 20 dogs, in which eACTH had been determined, 19 had a high eACTH. All 30 dogs were treated with prednisolone; starting dose in the hospital ranged between 0.5 and 1 mg/kg IV, q6 to q12 h, depending on the severity of the signs for a duration of 12–48 h, depending on the clinical condition of the dog. Three dogs received prednisolone only, due to normal serum electrolytes. Fourteen of them received DOCPj injection with a starting dose of 1.5–2 mg/kg SC every 28 days in addition to the prednisolone, and 13 dogs additionally to the prednisolone received fludrocortisonei at a starting dose of 0.01 mg/kg q 12 h. In all dogs, the prednisolone dose was reduced over several weeks after discharge to a final dose of 0.05–0.1 mg/kg per day. Median (range) follow‐up time of the dogs was 6.4 months (0.5–67.4). In dogs with a follow‐up of less than 3 months, either the referring practitioner or the owners were contacted to confirm good clinical control of the disease.
In the dogs with diseases mimicking HA, age ranged from 1 to 9 years (median, 5 years) and body weight from 1.7 to 44 kg (median, 11.3 kg). There were 14 males (7 castrated) and 16 females (9 spayed). This group consisted of 28 purebred dogs and 2 mixed‐breed dogs. There was no significant difference in age (P = 0.38), body weight (P = 0.76), or sex (P = 0.45) between dogs with HA and the non‐HA dogs. The final diagnoses reached were acute gastroenteritis (8), intoxication (5), chronic gastroenteritis (4), psychogenic polyuria/polydipsia (1), acute colitis (2), pancreatitis (2), idiopathic epilepsy (2), laryngeal paralysis (1), kidney injury (1), insulinoma (1), idiopathic megaesophagus (1), no final diagnosis reached (2).
Serum cTSH and Serum T4 Concentrations at the Time Point of Diagnosis
At the time of diagnosis of HA, cTSH ranged from 0.01 to 2.6 ng/mL (median 0.29) and was above the reference interval in 11 of 30 dogs. There was no difference in body weight (P = 0.8) and electrolyte concentrations (K; P = 0.052 and Na; P = 0.44) between dogs with increased and normal cTSH values. However, increased cTSH concentrations were only observed in female dogs (1 intact).
T4 concentrations at the time of diagnosis of HA ranged from <0.5 to 3.8 (median 1.8) and was below the reference interval in only 2 dogs, both of which had cTSH within the reference interval. Dogs with cTSH concentrations above the reference interval did not have lower T4 concentrations compared to those with normal cTSH (P = 0.69; Fig 1).
Figure 1.
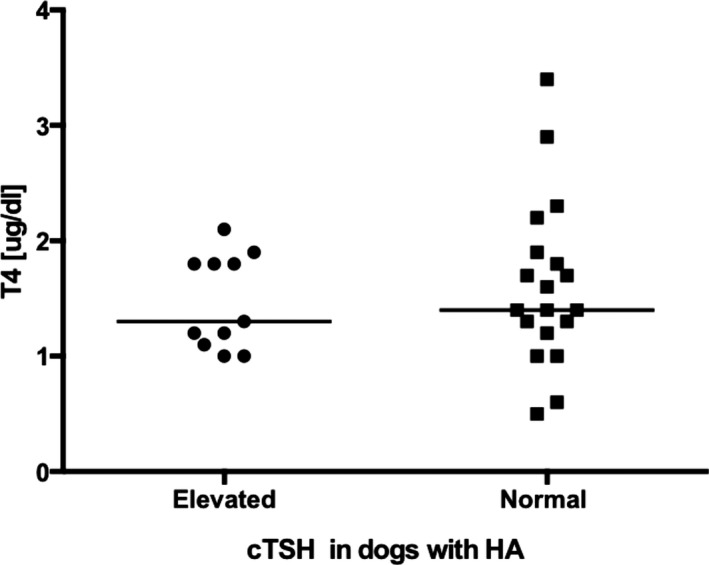
Scatter plot comparing serum T4 concentrations in dogs with hypoadrenocorticism (HA) and serum cTSH concentrations above the reference interval (n = 11) with those with normal serum cTSH concentrations (n = 19). The horizontal bars represent the median of each group. There was no significant difference between the 2 groups (P = 0.69).
Serum cTSH and Serum T4 Concentrations During Treatment of HA
Compared with the time of diagnosis, there was a significant (P = 0.0004) decrease in cTSH concentrations at the first recheck after 0.5–1 month of treatment, with a range (median) of 0.01–1.3 ng/mL (0.13 ng/mL) (Fig 2). In all but 2 dogs with cTSH, concentrations above the reference interval cTSH have been normalized at that time point. In 1 dog, cTSH had increased from 0.83 to 1.3 ng/mL at the first recheck. A TSH stimulation test was performed to exclude hypothyroidism, which turned out to be normal, and 4 months after diagnosis of HA, cTSH had decreased to 0.4 ng/mL (without thyroxine supplementation). In the other dog, cTSH had increased from 0.56 to 0.63 ng/mL at the first recheck. However in this dog also, cTSH decreased to 0.4 ng/mL after 3 months of treatment. Neither of the 2 dogs had clinical signs suggestive of hypothyroidism and in both clinical signs were well controlled with HA treatment alone.
Figure 2.
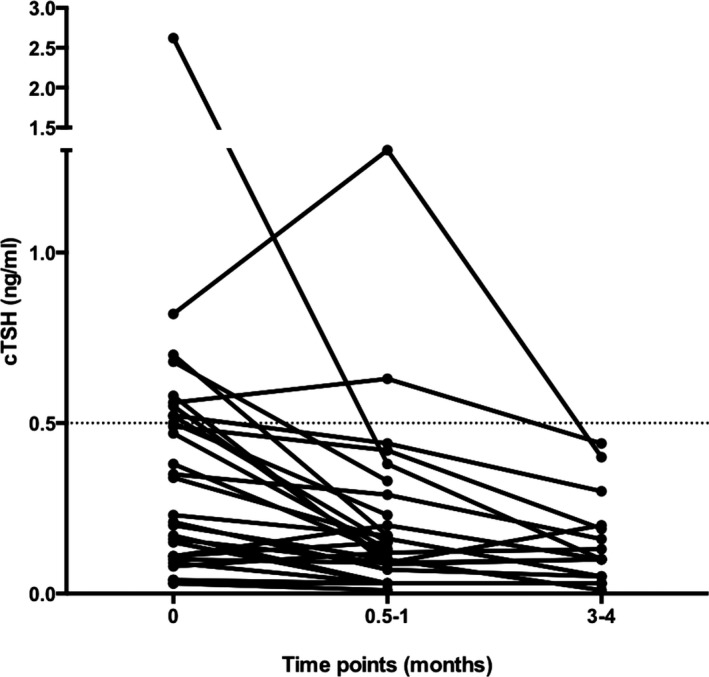
Change in serum cTSH concentrations of the 30 dogs with HA at the time point of diagnosis (0), 0.5–1 month, and 3–4 months after starting HA treatment. Dotted line represents the upper limit of the laboratory reference interval for serum cTSH concentration (0.5 ng/mL).
T4 concentrations at all time points after the start of treatment were not significantly different from before treatment (P = 0.56).
Serum cTSH of the Dogs with Disease Mimicking HA
Median (range) cTSH concentrations in dogs with disease mimicking HA were 0.06 ng/dL (0.01–0.2), which was significantly lower (<0.0001) than those of the dogs with HA at the time point of diagnosis. None of them showed values above the upper limit of the reference interval (Table 1).
Table 1.
Median, range, reference interval of serum cTSH, serum T4, plasma eACTH, and serum cortisol (before and after ACTH stimulation) concentrations of dogs with HA and with diseases mimicking HA and the P values of the differences between the 2 groups. For cTSH values below the detection limit, the mean between 0 and the detection limit of 0.03 ng/mL (corresponding to 0.015 ng/mL) was entered for statistical analysis, and for plasma eACTH concentrations >1,250, 1,251 pg/mL was used
| Reference Interval | Dogs with Hypoadrenocorticism | Dogs with Diseases Mimicking HA | P value | |||
|---|---|---|---|---|---|---|
| Range | Median | Range | Median | |||
| cTSH (ng/mL) | <0.5 | 0.015–2.62 | 0.22 | 0.015–0.2 | 0.06 | <0.0001 |
| T4 (μg/dL) | 1.3–2.9 | 0.5–4.4 | 1.4 | <0.5–3.8 | 1.8 | 0.35 |
| eACTH (pg/mL) | 18.3–1,251 | 1,250 | <10–45.8 | 18.3 | <0.0001 | |
| Baseline Cortisol (μg/dL) | >2.0 | <0.2–0.7 | <0.2 | <0.2–13.8 | 1.5 | <0.0001 |
| Post‐ACTH Cortisol (μg/dL) | <0.2–0.6 | <0.2 | 4.7–41.1 | 11.7 | <0.0001 | |
HA, hypoadrenocorticism
Serum T4 Concentrations of the Dogs with HA Compared with those of Dogs with Disease Mimicking HA
There was no statistically significant difference in T4 concentrations between dogs with disease mimicking HA and those with HA (P = 0.35). The results of both groups are summarized in Table 1.
Discussion
In the present study, we were able to show that 37% of dogs with HA had cTSH concentrations typically seen in cases of hypothyroidism. By comparison, none of the dogs with diseases mimicking HA had increased cTSH concentrations. To our knowledge, this phenomenon has not yet been described in dogs. Interestingly, 1 of our dogs with clinical signs of weakness had been treated with thyroxine for several weeks by a private veterinarian. Despite thyroxine supplementation (10 μg/kg q12 h), clinical signs did not improve and the owner stopped the treatment. Two months thereafter, the dog was referred to our clinic and at that time point showed an increased cTSH concentration of 1.06 ng/mL and a normal T4 of 2.3 μg/dL with no other abnormal findings in CBC and serum biochemistry. The dog was monitored during several months and cTSH remained increased with a T4 in the reference range. Clinical signs were unchanged and samples finally were sent to Michigan State University, Diagnostic Center for Population and Animal Health. Increased cTSH with T4 and fT4 within the reference range could be confirmed. Thyroglobulin, and T4 and T3 autoantibodies were negative. The owner decided not to proceed with any diagnostic work‐up and declined trial treatment with thyroxine at a dosage of 20 μg/kg q12 h. Not until 9 months after the first presentation, diagnosis of HA could be made as at that time point, potassium had increased to 6.0 (reference interval 4.3–5.3 mmol/L), with a normal Na 155 mmol/L (reference interval 152–159). An ACTH stimulation test confirmed the diagnosis (basal cortisol <0.2 μg/dL and post‐ACTH cortisol <0.2, eACTH 616 pg/mL). Treatment with prednisolone and fludrocortisone led to a normalization of the clinical signs and cTSH concentration within only 2 weeks. cTSH remained within the reference interval during a follow‐up period of 4 years, without thyroxine supplementation. This case is similar to what was reported in a human patient with untreated primary cortisol deficiency who had been supplemented with thyroxine for 6 years. Despite thyroxine supplementation, cTSH had remained increased.17 After cortisol replacement and withdrawal of the thyroxine, TSH normalized, confirming that hypothyroidism had been misdiagnosed. Based on these observations, it can be stated that hypothyroidism can easily be misdiagnosed in individuals with untreated HA and that care should be taken in assessing thyroid function not only in human patients but also in dogs with HA.
The influence of cortisol concentrations on the serum concentration of TSH has been intensively studied in humans, and a direct relationship between low concentrations of corticosteroid concentration and increased TSH secretion was demonstrated. In healthy humans given metyrapone to block cortisol synthesis, significantly higher TSH concentrations compared to baseline levels were observed.9 Further, patients with HA showed a gradual rise in TSH over a 48‐h hydrocortisone withdrawal period.18 Variable infusion rates of hydrocortisone led to higher TSH concentrations during low cortisol states and reestablishment of a physiologic cortisol pattern led to significant decreases in TSH concentrations during the day in these patients.8, 19 The authors concluded that mildly increased TSH concentrations are possible if patients have not taken their daily hydrocortisone dose. They speculated that a certain minimum or even a precise threshold of cortisol concentration at physiologic concentrations would be necessary for TSH suppression. In our study also, we demonstrated that higher corticoid concentrations lead to a suppression of cTSH secretion, as serum concentrations decreased to within the reference interval in all dogs after glucocorticoid supplementation. Therefore, we assume that the TSH excess before adrenal replacement can be attributed to an enhancement of TSH release due to the chronic cortisol deficiency.
In contrast to our study, in which only female dogs had increased cTSH concentrations, studies in human patients have found that both male and female patients with HA may have increased TSH concentrations. Based on the present data, we cannot explain our observation. All except 1 of the 11 female dogs were spayed, therefore it could also be hypothesized that cTSH is affected by ovariectomy as has been shown for FSH and LH: Both of these pituitary hormones have been shown to increase after gonadectomy in female and male dogs.20, 21 However, as cTSH concentrations remained unaffected in male dogs after gonadectomy,22 this hypothesis seems less likely. As case numbers were low, the dominance of female animals could merely be a statistical phenomenon. The latter assumption is confirmed by the following observation: a significant cTSH increase in male and female dogs has been described in dogs with hypercortisolism during cortisol‐lowering treatment with trilostane.23 This indicates that if the glucocorticoid effect on TSH is diminished, an increase in TSH can be observed, independent of the animal's sex. We did see a significant decrease in cTSH after starting treatment with prednisolone, not only in those dogs with increased cTSH concentrations, but also in the majority of our other dogs with cTSH within the reference interval, further confirming a sex‐independent influence of cortisol on TSH concentrations.
Taken together, glucocorticoids seem to play an important role in the hypothalamus‐pituitary‐thyroid axis, although to our knowledge, the exact molecular mechanisms involved in the regulation of TSH release by glucocorticoids are up to now unknown. Moreover, one interesting question still remains, namely, why only some of the dogs with HA have high cTSH concentrations while others do not. It could be a matter of duration of the cortisol deficiency or a matter of disease severity; however, this must remain speculative because a shortcoming of the present study is that disease severity and duration of clinical signs were not systematically assessed and documented. Clearly, what we also cannot exclude based on our data is that the one or the other of the dogs with high cTSH had subclinical hypothyroidism. This has been hypothesized in human medicine: some human patients with Addison's disease are assumed to have chronic autoimmune thyroiditis that is responsible for the increase in TSH.6, 24 This is based on the finding of serologic markers of thyroiditis in some of these patients. Subclinical hypothyroidism associated with immune‐mediated thyroiditis, high serum TSH, and normal thyroid hormone concentrations is well documented in people. And even a relatively low dose of glucocorticoid replacement can decrease the intensity of the antithyroid autoimmune response to the point that TSH will normalize.25 What speaks in favor of this aspect is that in most of our dogs with high cTSH, T4 was normal and not increased which one could expect due to the pituitary‐thyroid feedback physiology. Unfortunately, markers of autoimmune thyroiditis have not been determined in the present study but are underway.
As mentioned above, none of the dogs with diseases mimicking HA had increased cTSH concentrations, although almost 30% of them had T4 below the reference interval. Influence of NTI on T4 and cTSH concentrations has been studied by several authors14, 26, 27, 28 and frequency of low T4 concentrations is comparable between the different studies and ours. However, discrepancies exist concerning the cTSH concentrations with high cTSH values observed in 3–8% of the cases.14, 27 Interestingly, the combination of a low T4 and fT4 together with a high cTSH seems to be even less common in dogs with NTI.13, 14, 27, 28 From human medicine, there is evidence that nonthyroidal disease can decrease TSH secretion,29 and only in the recovery phase of NTI, temporary increases in TSH concentrations are observed. An increase in TSH has also been described in veterinary medicine.13, 14 In our study, as far as assessable, none of the dogs was in the recovery phase of a disease. Therefore, this might be one explanation for the differences in the prevalence of high cTSH observed between the different studies. However, also in dogs with hypothyroidism, occurrence of increased cTSH concentrations is highly variable between different studies, ranging from 25 to 40%. Several explanations in this setting, for example, suppression of pituitary TSH secretion by concurrent disease or drug administration, and inability of the current TSH assay to detect all isoforms of circulating TSH, might also explain different frequency of increased cTSH concentrations in dogs with NTIs.
In summary, because there were several dogs with cTSH concentrations typically seen in hypothyroidism, care must be taken in evaluating thyroid function in dogs with untreated HA. A minimum of 2 weeks of glucocorticoid treatment seems to be necessary to normalize cTSH concentrations. On the other hand, detection of an increased cTSH concentration in a dog with weakness should lead every veterinarian to consider HA as a differential diagnosis.
Acknowledgments
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
The study was performed at the Clinic for Small Animal Internal Medicine, Vetsuisse Faculty, University of Zurich, Switzerland, and at the Department of Veterinary Medical Sciences, University of Bologna, Italy.
The authors thank their colleagues at the Clinic for Small Animal Internal Medicine, Vetsuisse Faculty University of Zurich, and at the Department of Veterinary Medical Sciences, University of Bologna for contributing cases.
Footnotes
aSynacthen, Novartis Pharma Schweiz AG, Bern, Switzerland
bDPC Immulite 1000, Siemens Schweiz AG, Zurich, Switzerland, ACTH
cDPC Immulite 1000, Siemens Schweiz AG, Zurich, Switzerland, canine cortisol
dDPC Immulite 1000, Siemens Schweiz AG, Zurich, Switzerland, canine TSH
eDPC Immulite 1000, canine total T4
fSPSS, Statistical Package for the Social Science, Software Packets for Windows, version 21
gGraphPad Prism 6, GraphPad Software, San Diego, CA
hPercorten V, Novartis Animal Health US, Greensboro, NC
iFlorinef, Bristol‐Myers Squibb SA, 6340 Baar, Switzerland
References
- 1. Melian C, Peterson ME. Diagnosis and treatment of naturally occurring hypoadrenocorticism in 42 dogs. J Small Anim Pract 1996;37:268–275. [DOI] [PubMed] [Google Scholar]
- 2. Peterson ME, Kintzer PP, Kass PH. Pretreatment clinical and laboratory findings in dogs with hypoadrenocorticism: 225 cases (1979‐1993). J Am Vet Med Assoc 1996;208:85–91. [PubMed] [Google Scholar]
- 3. Javadi S, Galac S, Boer P, et al. Aldosterone‐to‐renin and cortisol‐to‐adrenocorticotropic hormone ratios in healthy dogs and dogs with primary hypoadrenocorticism. J Vet Intern Med 2006;20:556–561. [DOI] [PubMed] [Google Scholar]
- 4. Lathan P, Scott‐Moncrieff JC, Wills RW. Use of the cortisol‐to‐ACTH ratio for diagnosis of primary hypoadrenocorticism in dogs. J Vet Intern Med 2014;28:1546–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boretti FS, Meyer F, Burkhardt WA, et al. Evaluation of the Cortisol‐to‐ACTH ratio in dogs with Hypoadrenocorticism, dogs with Diseases Mimicking Hypoadrenocorticism and in healthy dogs. J Vet Intern Med 2015;29:1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Topliss DJ, White EL, Stockigt JR. Significance of thyrotropin excess in untreated primary adrenal insufficiency. J Clin Endocrinol Metab 1980;50:52–56. [DOI] [PubMed] [Google Scholar]
- 7. Husebye ES, Allolio B, Arlt W, et al. Consensus statement on the diagnosis, treatment and follow‐up of patients with primary adrenal insufficiency. J Intern Med 2014;275:104–115. [DOI] [PubMed] [Google Scholar]
- 8. Hangaard J, Andersen M, Grodum E, et al. Pulsatile thyrotropin secretion in patients with Addison's disease during variable glucocorticoid therapy. J Clin Endocrinol Metab 1996;81:2502–2507. [DOI] [PubMed] [Google Scholar]
- 9. Re RN, Kourides IA, Ridgway EC, et al. The effect of glucocorticoid administration on human pituitary secretion of thyrotropin and prolactin. J Clin Endocrinol Metab 1976;43:338–346. [DOI] [PubMed] [Google Scholar]
- 10. Cartwright JA, Stone J, Rick M, et al. Polyglandular endocrinopathy type II (Schmidt's syndrome) in a Dobermann pinscher. J Small Anim Pract 2016;57:491–494. [DOI] [PubMed] [Google Scholar]
- 11. Fritzen R, Bornstein SR, Scherbaum WA. Megaoesophagus in a patient with autoimmune polyglandular syndrome type II. Clin Endocrinol 1996;45:493–498. [DOI] [PubMed] [Google Scholar]
- 12. Kooistra HS, Rijnberk A, van den Ingh TS. Polyglandular deficiency syndrome in a boxer dog: Thyroid hormone and glucocorticoid deficiency. Vet Q 1995;17:59–63. [DOI] [PubMed] [Google Scholar]
- 13. Dixon RM, Mooney CT. Canine serum thyroglobulin autoantibodies in health, hypothyroidism and non‐thyroidal illness. Res Vet Sci 1999;66:243–246. [DOI] [PubMed] [Google Scholar]
- 14. Mooney CT, Shiel RE, Dixon RM. Thyroid hormone abnormalities and outcome in dogs with non‐thyroidal illness. J Small Anim Pract 2008;49:11–16. [DOI] [PubMed] [Google Scholar]
- 15. Rodriguez Pineiro MI, Benchekroun G, de Fornel‐Thibaud P, et al. Accuracy of an adrenocorticotropic hormone (ACTH) immunoluminometric assay for differentiating ACTH‐dependent from ACTH‐independent hyperadrenocorticism in dogs. J Vet Intern Med 2009;23:850–855. [DOI] [PubMed] [Google Scholar]
- 16. Scott‐Moncrieff JC, Koshko MA, Brown JA, et al. Validation of a chemiluminescent enzyme immunometric assay for plasma adrenocorticotropic hormone in the dog. Vet Clin Pathol 2003;32:180–187. [DOI] [PubMed] [Google Scholar]
- 17. Barnett AH, Donald RA, Espiner EA. High concentrations of thyroid‐stimulating hormone in untreated glucocorticoid deficiency: Indication of primary hypothyroidism? Br Med J (Clin Res Ed) 1982;285:172–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nicoloff JT, Fisher DA, Appleman MD Jr. The role of glucocorticoids in the regulation of thyroid function in man. J Clin Invest 1970;49:1922–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samuels MH. Effects of variations in physiological cortisol levels on thyrotropin secretion in subjects with adrenal insufficiency: A clinical research center study. J Clin Endocrinol Metab 2000;85:1388–1393. [DOI] [PubMed] [Google Scholar]
- 20. Beijerink NJ, Buijtels JJ, Okkens AC, et al. Basal and GnRH‐induced secretion of FSH and LH in anestrous versus ovariectomized bitches. Theriogenology 2007;67:1039–1045. [DOI] [PubMed] [Google Scholar]
- 21. de Gier J, Buijtels JJ, Albers‐Wolthers CH, et al. Effects of gonadotropin‐releasing hormone administration on the pituitary‐gonadal axis in male and female dogs before and after gonadectomy. Theriogenology 2012;77:967–978. [DOI] [PubMed] [Google Scholar]
- 22. Gunzel‐Apel AR, Seefeldt A, Eschricht FM, et al. Effects of gonadectomy on prolactin and LH secretion and the pituitary‐thyroid axis in male dogs. Theriogenology 2009;71:746–753. [DOI] [PubMed] [Google Scholar]
- 23. Kenefick SJ, Neiger R. The effect of trilostane treatment on circulating thyroid hormone concentrations in dogs with pituitary‐dependent hyperadrenocorticism. J Small Anim Pract 2008;49:139–143. [DOI] [PubMed] [Google Scholar]
- 24. Shigemasa C, Kouchi T, Ueta Y, et al. Evaluation of thyroid function in patients with isolated adrenocorticotropin deficiency. Am J Med Sci 1992;304:279–284. [DOI] [PubMed] [Google Scholar]
- 25. Yamada T, Ikejiri K, Kotani M, et al. An increase of plasma triiodothyronine and thyroxine after administration of dexamethasone to hypothyroid patients with Hashimoto's thyroiditis. J Clin Endocrinol Metab 1978;46:784–790. [DOI] [PubMed] [Google Scholar]
- 26. Nelson RW, Ihle SL, Feldman EC, et al. Serum free thyroxine concentration in healthy dogs, dogs with hypothyroidism, and euthyroid dogs with concurrent illness. J Am Vet Med Assoc 1991;198:1401–1407. [PubMed] [Google Scholar]
- 27. Kantrowitz LB, Peterson ME, Melian C, et al. Serum total thyroxine, total triiodothyronine, free thyroxine, and thyrotropin concentrations in dogs with nonthyroidal disease. J Am Vet Med Assoc 2001;219:765–769. [DOI] [PubMed] [Google Scholar]
- 28. Torres SM, Feeney DA, Lekcharoensuk C, et al. Comparison of colloid, thyroid follicular epithelium, and thyroid hormone concentrations in healthy and severely sick dogs. J Am Vet Med Assoc 2003;222:1079–1085. [DOI] [PubMed] [Google Scholar]
- 29. Warner MH, Beckett GJ. Mechanisms behind the non‐thyroidal illness syndrome: An update. J Endocrinol 2010;205:1–13. [DOI] [PubMed] [Google Scholar]


