Abstract
Background
Genetic and epidemiologic evidence suggests that in horses, as in other species, different manifestations of hypersensitivity may occur together.
Hypothesis
Horses affected with insect bite hypersensitivity (IBH) show airway hyperreactivity (AH) to inhaled histamine, even in the absence of overt clinical signs of equine asthma (EA).
Animals
Twenty‐two healthy controls (group C), 24 horses suffering from IBH alone (group IBH), and 23 horses suffering from IBH and EA (group IBH/EA).
Methods
The clinical histories were assessed using 2 standardized questionnaires, the Horse Owner Assessed Respiratory Signs Index (HOARSI), and IBH scoring. Horses were classified as EA‐affected if their HOARSI was >1 and as IBH‐affected if IBH score was >0. Confounding disorders were excluded by clinical examination. The arterial partial pressure of oxygen (PaO2) was measured and flowmetric plethysmography used to assess airway reactivity to increasing doses of inhaled histamine.
Results
The median histamine provocation concentration (PC) when ∆flow values increased by 35% (PC35) was significantly higher in group C (5.94 [1.11–26.33] mg/mL) compared to group IBH (2.95 [0.23–10.13] mg/mL) and group IBH/EA (2.03 [0.43–10.94] mg/mL; P < 0.01). The PC50 and PC75 showed very similar differences between groups. Furthermore, PaO2 was significantly lower in group IBH (84 ± 8 mmHg) and group IBH/EA (78 ± 11 mmHg) compared to group C (89 ± 6 mmHg; P < 0.01).
Conclusions and Clinical Importance
IBH is associated with AH and decreased PaO2, even in the absence of overt respiratory clinical signs.
Keywords: Equine asthma, Horse, Insect bite hypersensitivity, Multiple equine allergies
Abbreviations
- AH
airway hyperreactivity
- EA
equine asthma
- HCS
histamine challenge score
- HOARS
Horse Owner Assessed Respiratory Sings Index
- IAD
inflammatory airway disease
- IBH
insect bite hypersensitivity
- IgE
serum immunoglobulin E
- IL4RA
interleukin‐4 receptor α chain
- IL13
interleukin 13
- MHS
multiple hypersensitivities
- PaO2
partial pressure of arterial oxygen
- PC
provocation concentration
- RAO
recurrent airway obstruction
- Th2
T‐helper‐2
- Th1
T‐helper‐1
Recent genetic and epidemiologic evidence suggests that different manifestations of hypersensitivity may occur together in horses. Specifically, horses with insect bite hypersensitivity (IBH) have been shown to have a higher risk of being concurrently affected by recurrent airway obstruction (RAO), and vice versa.1 Although IBH is an allergic dermatologic disorder triggered by allergens from Culicoides biting midges,2 RAO is thought to be the result of a hypersensitivity to inhaled allergens and represents the severe form of equine asthma (EA). Equine asthma, a recently introduced term, comprises the clinical severity spectrum from mild (inflammatory airway disease, IAD) to severe forms (RAO).3, 4 In horses that are predisposed to EA, inhaled allergens and irritants play a major role in the development of cough, decreased performance, impaired gas exchange, bronchoconstriction, and airway hyperreactivity (AH).4
Human patients with asthma often also suffer from dermatologic allergic hypersensitivities, such as atopic dermatitis.5 These cutaneous manifestations of atopy can mark the start of the so‐called “atopic march” with approximately two‐thirds of patients with atopic dermatitis later developing allergic rhinitis and half of them progressing to asthma.5 Similar concurrences of different hypersensitivity manifestations in the same individuals also have been observed in cats with pruritic dermatosis and feline asthma,6 in dogs with allergic dermatitis, conjunctivitis, rhinitis, and asthma‐like clinical signs7 and recently in horses with RAO, IBH, and chronic recurrent urticaria.1 These findings suggest a common immunogenetic background, route of sensitization, or both leading to these multiple hypersensitivities (MHS).
The outcome measures in the epidemiologic study of horses described above1 were solely based on owner‐reported medical history. No further examinations regarding the potential presence of respiratory abnormalities had been performed. Histamine bronchoprovocation testing using flowmetric plethysmography is an objective tool, available in equine medicine, that can be used to detect AH even in clinically normal horses.8, 9 We, therefore, aimed to specifically examine the respiratory status of IBH‐affected horses with and without a history of EA. Respiratory status was evaluated using plethysmography with a bronchial histamine provocation test and arterial partial pressure of oxygen (PaO2). Specifically, we hypothesized that IBH is accompanied by increased AH, even in the absence of overt respiratory clinical signs.
Materials and Methods
Study Design and Horses
This study was approved by the ethical committees of the Cantons of Berne, Freiburg, Solothurn and Geneva, Switzerland (BE10/13), and examinations were performed between March 2015 and July 2015.
The sample size calculation was based on raw data from research in our group.10 To detect a decrease in provocation concentration (PC) when ▵flow values increased by 35% compared to saline (PC35) from 7.1 mg/mL (healthy horses) to 3.6 ± 3.4 mg/mL (affected horses) at a 5% significance level and power of 80%, a sample size calculation1 indicated that a minimal sample size of 15 per group would be required. The aim was therefore to recruit at least 20 horses per group to account for potential dropouts. Horses were recruited by way of an advertisement published in the “Bulletin,” the official monthly publication of the Swiss equine sport and breeding associations. Additionally, the databases of the Swiss Institute of Equine Medicine (ISME) and the Department of Clinical Research of the University of Bern, Switzerland were screened for IBH‐affected horses.
Potential candidates were examined either at their home stables or at the clinic of the ISME in Berne. Horses were included if they had a history compatible with IBH alone or IBH and EA (see below). Healthy individuals of similar breed and sex distribution also were recruited. Any animals showing signs of confounding disorders (see below) were excluded. It was required that at the time of examination, all horses were free of any medication that might influence the outcome measures. Specifically, a washout period of at least 3 weeks was required for any previously administered corticosteroids. Horses were divided into 3 subgroups: healthy controls (group C), IBH‐affected animals (group IBH), and IBH and EA‐affected animals (group IBH/EA) using a questionnaire‐based classification (see below).
Questionnaires and Classification Criteria
For every horse, IBH severity and lung health status were determined using standardized questionnaires.
The IBH score was based on a previously described questionnaire11 completed by the owner and based on the most severe clinical signs the horse had experienced. Briefly, cutaneous lesions observed on the mane, tail head, ventral midline, or some combination of these were classified in 5 severity grades, each with an additional characteristic compared to the preceding grade with (0) no signs of IBH; (1) increased skin flaking with visible epithelial debris; (2) areas of broken hairs or alopecia from scratching; (3) indurated skin folds; (4) obvious crusts from serous exudate, but without bleeding; and (5) bleeding from self‐inflicted abrasions. Owners also were asked if a seasonal pattern (consistent with exposure to insects, that is, spring to fall exacerbation) regarding the clinical signs was observed. Horses were classified as IBH positive, if the IBH score was >0 and if they were seasonally affected. Because clinical signs of IBH are typical, 1 season of observed clinical signs was sufficient to qualify.
The Horse Owner Assessed Respiratory Signs Index (HOARSI) was used to grade EA severity, as described in detail in a previous study.12 The HOARSI also referred to the most severe clinical signs in the horse's history. Horses graded HOARSI 1 were classified as healthy, whereas HOARSI 2 (mild), HOARSI 3 (moderate), and HOARSI 4 (severe) were consistent with EA.
To avoid false negative control animals as far as possible, all horses had to have spent at least 1 warm season (spring to fall) in Switzerland exposed to Culicoides and also, by definition, all horses that were classified according to the HOARSI had been exposed previously to hay for at least 2 months.
Clinical Examination
Horses underwent a thorough clinical examination that included respiratory tract and cardiac auscultation, rectal temperature, mucous membrane color, capillary refill time, submandibular lymph node palpation, and lung auscultation at rest and during rebreathing to exclude confounding disorders. Furthermore, blood was collected from the common carotid artery before the rebreathing examination for determination of PaO2 (i‐STAT® Portable Clinical Analyze2 ).
Histamine Bronchoprovocation with Plethysmography
To characterize airway reactivity, a pulmonary function test, with flowmetric plethysmography (Open Pleth System3 )9 with histamine bronchoprovocation13 was used. The horses were sedated with detomidine4 IV (Equisedan, 0.01–0.02 mg/kg) and fitted with an airtight mask and a pneumotachograph. In addition, 2 respiratory inductance bands were placed at the 11th intercostal space and just behind the last rib, respectively. Subsequently, calibration of the system was performed, after the manufacturer's instructions. Baseline measurements were recorded for 2 minutes, and then sterile 0.9% saline solution was nebulized (negative control) with the compressor and nebulizer provided with the system to generate a particle size <4 μm, followed by another 3‐minute recording. If baseline ∆flow exceeded 3.5 L/s (∆flow is the maximum difference between the flows measured by the pneumotachograph compared to the calibrated flow from the bands) or the ∆flow increased more than 50% compared to baseline after saline administration, measurements were stopped, and no histamine was inhaled. Otherwise, histamine (histamine diphosphate monohydrate)5 in saline then was nebulized in doubling concentrations (2, 4, 8, 16, 32 mg/mL). After each dose of histamine, measurements were recorded for 3 minutes. This procedure was continued until 1 of the following conditions was achieved: (1) the highest concentration (32 mg/mL) of histamine was reached; (2) an increase of > 50% in ∆flow values from saline measurement was registered; (3) the horse showed markedly increased abdominal breathing; or (4) the horse started to cough repeatedly. The histamine challenge score (HCS) referred to the concentration of histamine when the inhalation was stopped with (1) =32 or 16 mg/mL, (2) =8 mg/mL, (3) =4 mg/mL, (4) =2 mg/mL, and (5) =no histamine inhalation possible. No histamine inhalation was possible when airway obstruction with increased breathing effort, excessive coughing, or both were observed at baseline measurements or after saline inhalation. When the inhalation protocol was completed, the software of the Open Pleth System also provided the PC35, PC50, and PC75 using log‐linear interpolation of the dose–response curve.
Statistics
Analyses were performed with the statistical package NCSS 2007.6 Descriptive statistics are reported as mean ± standard deviation (SD) or median (range) as appropriate. Normality was assessed with the Skewness and Kurtosis (Omnibus) test. For comparisons among the 3 groups, one‐way ANOVA was used for normally distributed data (PaO2) or Kruskal–Wallis one‐way ANOVA on ranks for non‐normally distributed and ranked data (PC35, PC50, PC75; HCS). The α‐level of statistical significance was set at P < 0.05. For post hoc testing, Kruskal–Wallis multiple‐comparison z‐value test (Dunn's test) with z‐value > 1.96 indicating a P‐value < 0.05 (Bonferroni test) was used. To assess group differences regarding the categoric values sex and breed (Icelandic versus non‐Icelandic), Fisher's exact test was used.
Results
Signalment and Clinical Examination
A total of 69 horses (41 geldings, 25 mares, 3 stallions) was recruited. There were 22 horses assigned to group C, 24 to group IBH, and 23 to group IBH/EA. Sex distribution (male [stallions and geldings combined] versus female; P = 0.98) and age of group IBH (11 [4–24] years), group IBH/EA (14 [3–27] years), and group C (13 [5–25] years; P = 0.36) did not significantly differ between groups. There were no differences between the groups regarding the distribution of Icelandic horses versus other breeds (P = 0.36). A total of 29 Icelandic horses was included. The other breeds included Shetland Ponies (n = 7), Swiss Warmbloods (n = 6), Freibergers (n = 6), Arabians (n = 3), Friesians (n = 2), Polo Ponies (n = 2), Tinkers (n = 2), a Connemara Pony, a Pinto, a Purebred Spanish Horse, an American Quarter Horse, a Westphalian horse, and mixed breeds (n = 7). Group C and group IBH contained 11 Icelandic horses each, whereas group IBH/EA included of seven Icelandic horses. No horses were excluded based on clinical signs suggestive of confounding disorders. Overall, the severity grades of the cutaneous lesions of IBH‐affected horses (distribution was similar between IBH alone and IBH/EA) were as follows: 1 (n = 2); 2 (n = 18); 3 (n = 3); 4 (n = 4); and, 5 (n = 20).
Histamine Bronchoprovocation with Plethysmography
Baseline ∆flow measurements did not differ among group C (n = 21; 1.01 [−0.25–3.29] L/s), group IBH (n = 17; 0.77 [−1.26–7.61] L/s), and group IBH/EA (n = 12; 1.15 [−0.45–3.90] L/s; P = 0.43). However, no histamine provocation was performed in 3 horses in group IBH and another 8 in IBH/EA, because the maximal ∆flow values at baseline exceeded 3.5 L/s or because ∆flow increased >50% compared to baseline after administration of saline. In 1 additional horse in group C, 4 horses in IBH, and 3 in IBH/EA, bronchoprovocation measurements were not possible because of technical problems (eg, software failure to calculate PC values), repeated coughing or both.
The PC35 values were higher in group C (n = 21; 5.94 [1.11–26.33] mg/mL) compared to group IBH (n = 17; 2.95 [0.23–10.13] mg/mL) and group IBH/EA (n = 12; 2.03 [0.43–10.94] mg/mL; P < 0.01). Comparison among the 3 groups showed that group C was different from group IBH (z‐value: 2.10) and group IBH/EA (z‐value: 2.74). Group IBH and group IBH/EA did not differ (z‐value: 0.81). However, group size for IBH/EA in this comparison was below the calculated n = 15 necessary to detect a difference between the IBH group and the IBH/EA group with a power of 80%.
Figure 1 illustrates the PC35 values of the different groups. The PC50 and PC75 values showed results similar to those of PC35 (see Figures S1 and S2).
Figure 1.
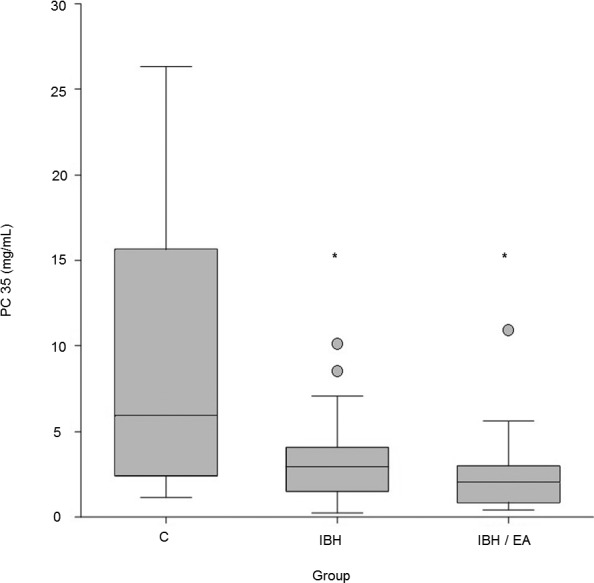
Box plot illustrating the provocation concentrations (PC) when ∆flow values increased by 35% (PC35) compared to baseline of healthy controls (C) (n = 21; 5.94 [1.11–26.33] mg/mL), horses suffering from insect bite hypersensitivity (IBH) (n = 17; 2.95 [0.23–10.13] mg/mL], and horses diagnosed with both IBH and equine asthma (IBH/EA) (n = 12; 2.03 [0.43–10.94]). *Indicates significant difference compared to C.
Distribution of Maximal Doses Reached
The median histamine challenge score (HCS) of group C was significantly lower (n = 22; 1 [1–3]) than that of group IBH (n = 24; 2.5 [1–5]) and group IBH/EA (n = 23; 4 [1–5]). Comparison among the three groups (z‐value > 1.96 = P < 0.05) indicated that group C was different from group IBH (z‐value: 3.24) and group IBH/EA (z‐value: 5.06). Group IBH and group IBH/EA did not show a difference (z‐value: 1.89). The numbers of horses achieving the different HCS in each group are illustrated in Figure 2.
Figure 2.
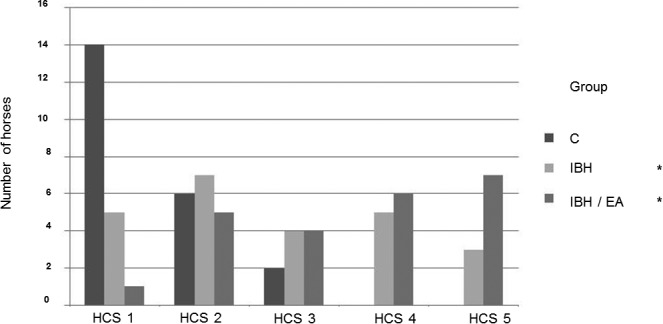
Bar graph illustrating the number of horses assigned a particular histamine challenge score (HCS). The histamine challenge score (HCS) refers to the dose of histamine when the inhalation was stopped with HCS1 = 32 or 16 mg/mL, HCS2 = 8 mg/mL, HCS3 = 4 mg/mL, HCS4 = 2 mg/mL, and HCS5 = no histamine inhalation possible. *indicates significant difference compared to group C.
Partial Pressure of Arterial Oxygen
Group C had a higher resting PaO2 (89 ± 6 mmHg) than both group IBH (84 ± 8 mmHg) and group IBH/EA (78 ± 11 mmHg). Comparison among the 3 groups (z‐value > 1.96 = P < 0.05) indicated that group C was different from group IBH (z‐value: 2.05) and group IBH/EA (z‐value: 4.16). Group IBH and group IBH/EA also were different (z‐value: 2.16, Fig 3).
Figure 3.
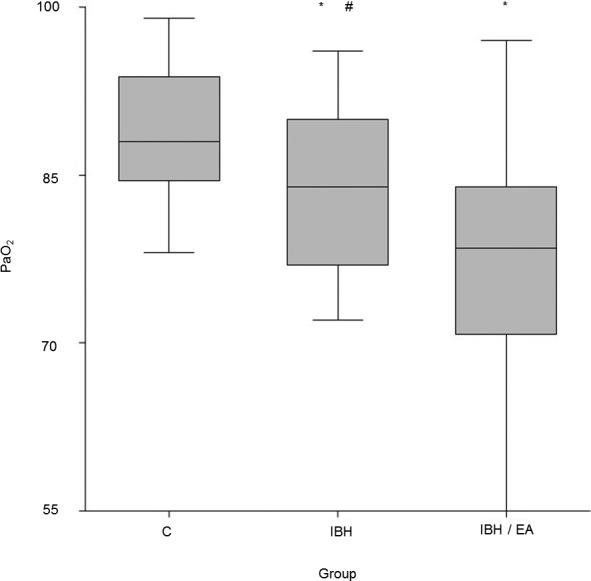
Box plots revealing the partial pressure of arterial oxygen (PaO2) of healthy horses (C) (n = 22; 89 ± 6 mmHg), horses suffering from insect bite hypersensitivity (IBH) (n = 24, 84 ± 8 mmHg), and horses suffering from both IBH and equine asthma (IBH/EA) (n = 23, 78 ± 11 mmHg). *Indicates a significant difference compared to group C. # indicates a significant difference between group IBH and IBH/EA.
Discussion
Our results indicate that IBH is associated with AH. Although this finding confirms our hypothesis, it is surprising, because these 2 hypersensitivity disorders affect separate organ systems. However, previous epidemiologic investigations in 2 distinct populations showed that horses suffering from the dermatologic disorders IBH or urticaria had an increased risk of also being affected by RAO, and vice versa.1
Histamine provocation clearly separated the IBH/EA group from the control group. Most interestingly, group IBH, the horses of which did not show overt clinical signs of EA, also displayed significantly decreased PC35, 50, and 75 as well as lower PaO2 values than the control group. Our study thus demonstrates that an allergic skin disorder can be accompanied by AH indicative of a subclinical effect on the lower respiratory tract.
However, PC values varied considerably within the groups and overlapped among groups as illustrated in Figure 1. Therefore, isolated PC values from single horses cannot accurately predict the EA or the IBH status of an individual animal.
Equine asthma had not been identified by the owners in this group because the inclusion criteria required that the owners had not noted any coughing, nasal discharge, or increased respiratory effort (HOARSI 1). The HCS, which was possible to assess in all horses, confirmed the calculated PC values, where a number of measurements had to be discarded. From our data, it was not possible to draw sound conclusions on the effect of IBH severity on airway reactivity, because of the small numbers of horses in some of the IBH score groups.
In humans, investigations into the immunogenetic background of these MHS disorders showed evidence for specific genetic determinants as a potential underlying reason for a general allergic phenotype.14 One candidate gene involved in different allergic diseases, specifically asthma, atopic dermatitis, and rhinitis, is interleukin‐4 receptor a‐chain (IL4RA). In EA, a genetic association between a region on equine chromosome (ECA) 13, containing the gene for IL4RA, and RAO was described in a high‐prevalence RAO family.15, 16 A linkage between RAO and a single nucleotide polymorphism (SNP BIEC2‐224511) on ECA 13 near IL4RA was confirmed in unrelated horses.17 However, this polymorphism was not associated with MHS in horses in a recent study.1
Additional genetic variants that might be associated with both airway hypersensitivities and skin allergies are polymorphisms in IL13, a gene playing the same role in the T‐helper‐2 (Th2)‐type response as IL4RA, and a mutation in a profilaggrin/filaggrin gene.18, 19 The IL13 polymorphisms have been associated with both asthma and rhinitis, suggesting a common etiologic pathway for their development.18, 20 Furthermore, these studies also showed an interaction between IL13 and IL4R polymorphisms in asthma. However, even though the RAO‐associated quantitative trait locus on equine chromosome 13 has triggered these investigations into MHS in horses, RAO and IBH exhibit important immunopathologic differences that must be considered in the context of genes such as IL13 or IL4RA. Importantly, IBH is characterized as a type I allergy with IgE‐ and Th2‐type driven immunopathogenesis, and lesional eosinophil and mast cell involvement.2
The immunopathology of RAO, in contrast, is still unclear. Many contradictory results regarding a Th1‐ Th2‐ Th17 or mixed immune response have been reported.3
A further interesting candidate gene in humans with MHS is filaggrin,21 which is not directly involved in immune function, but rather in integrity of epithelial barrier function.5 Cutaneous manifestations of atopy often represent the beginning of the atopic march in humans,21 and data from experimental animals as well as from clinical studies in humans suggest a role for the skin as an important route of sensitization to asthma‐inducing allergens.22 These findings support the concept that skin barrier dysfunction increases the risk of sensitization to allergens by facilitating crossing of the epidermal barrier by environmental allergens and subsequent induction of sensitization. In horses, there is presently no published data on a possible involvement of filaggrin mutations in IBH or RAO.
However, both RAO and IBH are genetically complex diseases. The heritability of IBH susceptibility has been reported in several breeds including Icelandic horses, Friesian horses, Shetland ponies, and Belgian Warmblood horses with a wide range from 0 to 0.36 on the observed scale.15, 23, 24, 25, 26, 27 Additional studies may explore some of the candidate genes such as filaggrin. It is very likely that multiple, even numerous genes are involved in these disorders and it must be determined which genes overlap and play a role in both hypersensitivity manifestations. Other potential causes for multiple allergies include epigenetic regulation as well as the gastrointestinal, respiratory tract and skin microbiome, which are postulated to play important roles in allergies in humans.28
Group classifications in our study were based on owner‐reported history using standardized scores. Previous studies have extensively described and validated the HOARSI scoring system in several populations with different degrees of EA.10, 12, 29 The results of these experiments suggest that horses with HOARSI 3 and 4 (regular to permanent coughing, increased breathing effort after work and, in HOARSI 4, also at rest) suffer from RAO, whereas HOARSI 2 corresponds best with IAD and HOARSI 1 indicates a clinically healthy lower respiratory tract. The latter 2 categories are less distinct however, making HOARSI a valuable, but imperfect surrogate marker for EA. Accordingly, in future studies, it might be interesting to investigate if the difference in hyperreactivity between IBH and IBH/EA is more marked if a larger group of severely EA (RAO)‐affected horses (ie, HOARSI 3 and 4) is studied.
It would have been useful to perform endoscopy to assess the mucus quantity in the trachea and collect bronchoalveolar fluid (BALF) for cytology. This is likely the main limitation of our study. Although the HOARSI provides a validated scoring system, it is still only a surrogate marker for EA. Current definitions of RAO and IAD are based on cytologic variable, and the relationship between hypersensitivity and cytology is still unclear.4, 30 Some studies have reported AH predominantly in mast cell‐type or eosinophilic IAD,4 which might fit with the atopic, Th2‐primed immune response observed in IBH,2, 31 but a recent report did not confirm these associations.32
Endoscopy and BALF cytology were not performed because owner compliance was particularly challenging, and the number of participating horses would have been insufficient if these slightly more invasive procedures would have been required. Furthermore, most horses were examined at their home stables, making additional examinations logistically challenging. However, we aimed to characterize horses as comprehensively as possible under the given conditions. The HOARSI scoring system was complemented by a thorough clinical examination, resting arterial oxygen tension measurements, baseline lung function measurements, and bronchoprovocation with histamine. The IBH scoring system used worked well in our hands and, as far as we are aware, there are no other published scoring systems for IBH. For an additional more in‐depth classification of IBH, future studies could use a validated cellular antigen stimulation test, and the period between the most severe historic clinical signs and time of examination could be more standardized.33 Finally, we only examined horses for allergic reactions involving the skin and the lungs (ie, IBH and EA). No other organ systems were evaluated for potential hypersensitivity disorders. Additional investigations concerning MHS in horses, therefore, are needed. For instance, it would be interesting to explore whether horses with urticaria, which also was shown to be associated with RAO,1 also suffer from hyperreactive airways or if food‐related allergies, such as the recently described gluten hypersensitivity in horses,34 play a role in MHS.
In conclusion, the airways of horses with IBH alone showed hyperreactivity similar to airways of horses with EA. Although future studies may identify the immunogenetic basis for this phenomenon, our results already suggest that horses suffering from IBH have a higher risk for AH and therefore might be predisposed to develop EA in the future. These findings should be taken into account when evaluating IBH‐horses presented for subtle respiratory signs.
Supporting information
Figure S1. Box plot illustrating the provocation concentrations (PC) when ∆flow values increased by 50% (PC50) compared to baseline of healthy controls (C) (n = 21; 6.71 [1.59–38.79] mg/mL), horses suffering from insect bite hypersensitivity (IBH) (n = 17; 3.11 [0.32–12.53] mg/mL) and horses diagnosed with both IBH and equine asthma (IBH/EA) (n = 12; 2.45 [0.61–11.53]). *Indicates significant difference compared to C.
Figure S2. Box plot illustrating the provocation concentrations (PC) when ∆flow values increased by 75% (PC75) compared to baseline of healthy controls (C) (n = 20; 8.77 [2.53–32.60] mg/mL), horses suffering from insect bite hypersensitivity (IBH) (n = 17; 4.28 [0.48–16.54] mg/mL) and horses diagnosed with both IBH and equine asthma (IBH/EA) (n = 12; 3.24 [0.0.91–12.50]). *Indicates significant difference compared to C.
Acknowledgment
The authors thank all participating horse owners and veterinarians for their support of this study. We thank all of the horse owners involved in the experimental part of the project and Dr. S. Axiak Flammer for excellent editorial support. The presented study was funded by the Swiss National Science Foundation: Grant No. 31003A‐162548/1; and Swiss Institute of Equine Medicine Research Funds (account 33‐890).
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
These authors contributed equally to the manuscript
Location where work was performed: Swiss Institute of Equine Medicine, University of Bern and Agroscope, Berne, Switzerland
Meetings where work was presented: Fourth International Havemeyer Workshop on Equine Skin Allergy, Agricultural University of Iceland, Hvanneyri, Iceland, June 22 – 25, 2016; oral presentation and abstract [<500 words without graph or tables].
Footnotes
Abbott Point of Care Inc., Princeton, New Jersey, USA.
Ambulatory Monitoring, Inc., Ardsley, New York, USA.
Dr E. Graeub AG, Berne, Switzerland.
NCSS Statistical Software, Kaysville, Utah, USA.
Christoffel‐Apotheke, Berne, Switzerland.
References
- 1. Kehrli D, Jandova V, Fey K, et al. Multiple hypersensitivities including recurrent airway obstruction, insect bite hypersensitivity, and urticaria in 2 warmblood horse populations. J Vet Intern Med 2015;29:320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schaffartzik A, Hamza E, Janda J, et al. Equine insect bite hypersensitivity: what do we know? Vet Immunol Immunopathol 2012;147:113–126. [DOI] [PubMed] [Google Scholar]
- 3. Bullone M, Lavoie JP. Asthma “of horses and men”–how can equine heaves help us better understand human asthma immunopathology and its functional consequences? Mol Immunol 2015;66:97–105. [DOI] [PubMed] [Google Scholar]
- 4. Couetil LL, Cardwell JM, Gerber V, et al. Inflammatory airway disease of horses‐revised consensus statement. J Vet Intern Med 2016;30:503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol 2003;112:S118–S127. [DOI] [PubMed] [Google Scholar]
- 6. Carlotti D, Prost C. L'atopie feline. Point Vét 1998;20:777–784. [Google Scholar]
- 7. Olivry T, Hill PB. The ACVD task force on canine atopic dermatitis (IX): the controversy surrounding the route of allergen challenge in canine atopic dermatitis. Vet Immunol Immunopathol 2001;81:219–225. [DOI] [PubMed] [Google Scholar]
- 8. Mazan MR, Hoffman AM, Manjerovic N. Comparison of forced oscillation with the conventional method for histamine bronchoprovocation testing in horses. Am J Vet Res 1999;60:174–180. [PubMed] [Google Scholar]
- 9. Hoffman A, Kuehn H, Riedelberger K, et al. Flowmetric comparison of respiratory inductance plethysmography and pneumotachography in horses. J Appl Physiol 1985;2001(91):2767–2775. [DOI] [PubMed] [Google Scholar]
- 10. Rettmer H, Hoffman AM, Lanz S, et al. Owner‐reported coughing and nasal discharge are associated with clinical findings, arterial oxygen tension, mucus score and bronchoprovocation in horses with recurrent airway obstruction in a field setting. Equine Vet J 2015;47:291–295. [DOI] [PubMed] [Google Scholar]
- 11. Babel C. Etude pilote sur l'incidence de l'hypersensibilité pulmonaire chez les chevaux ayant des allergies, 1ère partie: étude de l’échantillon basé sur des chevaux atteints de dermatite estivale. In: Vetsuisse Faculty. Berne: University of Bern; 2014. [Google Scholar]
- 12. Ramseyer A, Gaillard C, Burger D, et al. Effects of genetic and environmental factors on chronic lower airway disease in horses. J Vet Intern Med 2007;21:149–156. [DOI] [PubMed] [Google Scholar]
- 13. Nolen‐Walston RD, Kuehn H, Boston RC, et al. Reproducibility of airway responsiveness in horses using flowmetric plethysmography and histamine bronchoprovocation. J Vet Intern Med 2009;23:631–635. [DOI] [PubMed] [Google Scholar]
- 14. Kurt E, Demir AU, Cadirci O, et al. Immediate‐type drug hypersensitivity and associated factors in a general population. Allergol Immunopathol (Madr) 2011;39:27–31. [DOI] [PubMed] [Google Scholar]
- 15. Swinburne JE, Bogle H, Klukowska‐Rotzler J, et al. A whole‐genome scan for recurrent airway obstruction in Warmblood sport horses indicates two positional candidate regions. Mamm Genome 2009;20:504–515. [DOI] [PubMed] [Google Scholar]
- 16. Jost U, Klukowska‐Rotzler J, Dolf G, et al. A region on equine chromosome 13 is linked to recurrent airway obstruction in horses. Equine Vet J 2007;39:236–241. [DOI] [PubMed] [Google Scholar]
- 17. Shakhsi‐Niaei M, Klukowska‐Rotzler J, Drogemuller C, et al. Replication and fine‐mapping of a QTL for recurrent airway obstruction in European Warmblood horses. Anim Genet 2012;43:627–631. [DOI] [PubMed] [Google Scholar]
- 18. Bottema RW, Nolte IM, Howard TD, et al. Interleukin 13 and interleukin 4 receptor‐alpha polymorphisms in rhinitis and asthma. Int Arch Allergy Immunol 2010;153:259–267. [DOI] [PubMed] [Google Scholar]
- 19. Novak N, Bieber T. Allergic and nonallergic forms of atopic diseases. J Allergy Clin Immunol 2003;112:252–262. [DOI] [PubMed] [Google Scholar]
- 20. Bieber T. Atopic dermatitis. Ann Dermatol 2010;22:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marenholz I, Nickel R, Ruschendorf F, et al. Filaggrin loss‐of‐function mutations predispose to phenotypes involved in the atopic march. J Allergy Clin Immunol 2006;118:866–871. [DOI] [PubMed] [Google Scholar]
- 22. Redlich CA, Herrick CA. Lung/skin connections in occupational lung disease. Curr Opin Allergy Clin Immunol 2008;8:115–119. [DOI] [PubMed] [Google Scholar]
- 23. Peeters LM, Janssens S, Brebels M, et al. Genetic parameters and estimated breeding values of insect bite hypersensitivity in Belgian Warmblood horses. Vet J 2015;206:420–422. [DOI] [PubMed] [Google Scholar]
- 24. Lange S. Untersuchung zur Vererbung des Sommerekzems beim Islandpferd. Hannover, Germany: Tierärztliche Hochschule; 2004. [Google Scholar]
- 25. Eriksson S, Grandinson K, Fikse WF, et al. Genetic analysis of insect bite hypersensitivity (summer eczema) in Icelandic horses. Animal 2008;2:360–365. [DOI] [PubMed] [Google Scholar]
- 26. Schurink A, van Grevenhof EM, Ducro BJ, et al. Heritability and repeatability of insect bite hypersensitivity in Dutch Shetland breeding mares. J Anim Sci 2009;87:484–490. [DOI] [PubMed] [Google Scholar]
- 27. Schurink A, Ducro BJ, Heuven HC, et al. Genetic parameters of insect bite hypersensitivity in Dutch Friesian broodmares. J Anim Sci 2011;89:1286–1293. [DOI] [PubMed] [Google Scholar]
- 28. Claassen‐Weitz S, Wiysonge CS, Machingaidze S, et al. Current knowledge and future research directions on fecal bacterial patterns and their association with asthma. Front Microbiol 2016;7:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laumen E, Doherr MG, Gerber V. Relationship of horse owner assessed respiratory signs index to characteristics of recurrent airway obstruction in two Warmblood families. Equine Vet J 2010;42:142–148. [DOI] [PubMed] [Google Scholar]
- 30. Robinson NE. International workshop on equine chronic airway disease. Michigan State University 16‐18 June 2000. Equine Vet J 2001;33:5–19. [DOI] [PubMed] [Google Scholar]
- 31. Hamza E, Akdis CA, Wagner B, et al. In vitro induction of functional allergen‐specific CD4+ CD25high Treg cells in horses affected with insect bite hypersensitivity. Clin Exp Allergy 2013;43:889–901. [DOI] [PubMed] [Google Scholar]
- 32. Wichtel M, Gomez D, Burton S, et al. Relationships between equine airway reactivity measured by flowmetric plethysmography and specific indicators of airway inflammation in horses with suspected inflammatory airway disease. Equine Vet J 2016;48:466–471. [DOI] [PubMed] [Google Scholar]
- 33. Baselgia S, Doherr MG, Mellor P, et al. Evaluation of an in vitro sulphidoleukotriene release test for diagnosis of insect bite hypersensitivity in horses. Equine Vet J 2006;38:40–46. [DOI] [PubMed] [Google Scholar]
- 34. van der Kolk JH, van Putten LA, Mulder CJ, et al. Gluten‐dependent antibodies in horses with inflammatory small bowel disease (ISBD). Vet Q 2012;32:3–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Box plot illustrating the provocation concentrations (PC) when ∆flow values increased by 50% (PC50) compared to baseline of healthy controls (C) (n = 21; 6.71 [1.59–38.79] mg/mL), horses suffering from insect bite hypersensitivity (IBH) (n = 17; 3.11 [0.32–12.53] mg/mL) and horses diagnosed with both IBH and equine asthma (IBH/EA) (n = 12; 2.45 [0.61–11.53]). *Indicates significant difference compared to C.
Figure S2. Box plot illustrating the provocation concentrations (PC) when ∆flow values increased by 75% (PC75) compared to baseline of healthy controls (C) (n = 20; 8.77 [2.53–32.60] mg/mL), horses suffering from insect bite hypersensitivity (IBH) (n = 17; 4.28 [0.48–16.54] mg/mL) and horses diagnosed with both IBH and equine asthma (IBH/EA) (n = 12; 3.24 [0.0.91–12.50]). *Indicates significant difference compared to C.


