Abstract
Background
Antifungal susceptibility testing is a subject of interest in the field of medical mycology. The aim of the present study were the distributions and antifungal susceptibility patterns of various Candida species isolated from colonized and infected immunocompromised patients admitted to ten university hospitals in Iran.
Methods
In totally, 846 Candida species were isolated from more than 4000 clinical samples and identified by the API 20 C AUX system. Antifungal susceptibility testing was performed by broth microdilution method according to CLSI.
Results
The most frequent Candida species isolated from all patients was Candida albicans (510/846). The epidemiological cutoff value and percentage of wild-type species for amphotericin B and fluconazole in Candida albicans, Candida tropicalis, Candida glabrata and Candida krusei were 0.5 μg/ml (95%) and 4 μg/ml (96%); 1 μg/ml (95%) and 8 μg/ml (95%); 0.5 μg/ml (99%) and 19 μg/ml (98%); and 4 μg/ml (95%) and 64 μg/ml (95%), respectively. The MIC90 and epidemiological cutoff values to posaconazole in Candida krusei were 0.5 μg/ml. There were significant differences between infecting and colonizing isolates of Candida tropicalis in MIC 90 values of amphotericin B, and isolates of Candida glabrata in values of amphotericin B, caspofungin, and voriconazole (P < 0.05).
Conclusions
Our findings suggest that the susceptibility patterns of Candida species (colonizing and infecting isolates) in immunocompromised patients are not the same and acquired resistance was seen in some species.
Keywords: Colonizing Candida, Candida infected patients, Candida wild-type, Candida susceptibility testing, Candida Albicans
Background
Antifungal susceptibility patterns of infectious fungi are a crucial determinant that contributes to the outcome of patients. While the incidence of Candida infections is increasing, the choice of suitable antifungal agents is limited due to the resistance of some species to several antifungals. Candida species can cause superficial to life-threatening candidemia and hospital-acquired infections in humans [1, 2]. Candida albicans remains the leading Candida species that causes infection, but the epidemiology of non-albicans Candida species has been on the rise [3, 4]. These species cause infections in patients, especially those with underlying diseases. The activities of antifungal agents are important therapeutic options to control infections caused by these yeasts. The appropriate treatments are dependent on the immune status and underlying diseases of patients, the specific Candida species involved and its susceptibility pattern to antifungal agents.
The Clinical and Laboratory Standards Institute (CLSI) developed new Candida species-specific clinical breakpoints for some antifungal agents, like fluconazole, voriconazole, and echinocandins [5, 6]. Use of such breakpoints can change the previously known Candida species sensitivity impact patterns and consequently the management of the patients.
A few multicenter surveillance studies have been conducted comparing antifungal susceptibility patterns of isolates obtains from the infected (INFECT) and colonized (COL) hospitalized patients. Therefore, in the present study, the distributions and antifungal susceptibility patterns of various Candida species isolated from infected and colonized immunocompromised patients admitted to 10 university hospitals in Iran were reported using CLSI species-specific clinical breakpoints and epidemiological cutoff values (ECV).
Methods
Study design and patients
The present study is a cross-sectional study carried out during 2014-2015 in patients admitted to 10 university hospitals in Iran. The participant university hospitals were as follows: Ahvaz, Isfahan, Kerman, Mashhad, Sanandaj, Sari, Shiraz, Tehran, Urmia, and Yasuj. Candida species isolated were divided into infecting and colonizing isolates. Infecting Candida species were isolated from various clinical samples, like blood, cerebrospinal fluid, bronchoalveolar lavage, and sputum of the infected patients according to European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group criteria [7]. Colonizing species were isolated from the oral cavity, urine, nose and swab rectum of immunocompromised hospitalized patients without any clinical signs and symptoms of Candida infections. The underlying diseases in patients were a solid organ and bone marrow transplantation, hematologic disorders including acute lymphoblastic leukemia, chronic lymphocytic leukemia, acute and chronic myeloid leukemia, aplastic anemia, pancytopenia, Burkitt lymphoma; Rhabdomyosarcoma and histiocytosis.
Species identification and antifungal susceptibility testing of the isolates were performed at Professor Alborzi Clinical Microbiology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. All samples were cultured on sabouraud dextrose agar (Merck, Germany) at room temperature and all isolates were subcultured on potato dextrose agar (OXOID LTD, Basingstoke, Hampshire, England) twice for 48 h at 35 °C to check the purity of the colonies. Species identification was confirmed by germ tube and chlamydospore production tests, and API 20 C AUX system (bioMerieux, Swiss), according to the manufacturer’s instructions.
Antifungal susceptibility studies
Susceptibility values to amphotericin B (AMB), fluconazole (FLU), voriconazole (VOR), itraconazole (ITR), and posaconazole (POS) were assessed by the CLSI broth microdilution methods M27-A3 and M27-S4 [5, 8]. Two reference strains, C. parapsilopsis ATCC 22019 and C. krusei ATCC 6258, were included in each test as quality control isolates.
Powders of AMB and POS (Sigma, Germany), FLU, ITR, VOR, and CAS (Sigma, USA) were obtained from the respective manufacturers. RPMI 1640 (Sigma, St. Louis, Missouri) was made according to the manufacturer’s protocol and buffered to pH 7.0 with 0.165 N-morpholino propanesulfonic acid (MOPS) buffer (Sigma, USA). Stock solutions with 10-fold concentration for each antifungal were prepared in dimethyl sulfoxide (DMSO). The final concentrations of the working solutions were obtained by using RPMI medium. The final concentrations of the antifungal agents were 0.032 to 16 μg/ml for AMB, ITR, POS and VOR 0.125 to 64 μg/ml for FLU, and 0.016-8 μg/ml for CAS. The inoculum suspensions (0.5 McFarland) were prepared by the spectrophotometric method (at 530 nm) (Pharmacia biotech Cambridge, England ultrospec 3000 UV/visible spectrophotometer), and diluted to 0.5 × 103 or 2.5 × 103 cells/ml using RPMI 1640 medium. A 100-μl volume of yeast inoculum and an equal volume of antifungal agents were added to each well. Drug-free and yeast-free wells were included as positive and negative controls. The MIC of AMB was reported as the lowest drug concentration that complete inhibition of any discernible growth (100%) and for FLU, ITR, VOR, POS, and CAS the lowest concentration that inhibits 50% of the growth, compared to positive controls was taken.
Data collection and statistical analysis
Data were collected in WHONET version 5.6 database and SPSS version 16 (SAS Institute, Cary, NC, USA). The comparison of antifungal susceptibility rates between INFECT and COL species was made using student T-test and Mann-Whitney U tests. P < 0.05 was considered significant.
Results
More than 4000 samples from different sites of patients were examined and 846 Candida species were isolated. Sample site and distribution of cultured Candida species isolated from the immunocompromised patients were presented in Table 1. The INFEC species were isolated from bronchoalveolar lavage, blood, fluid (joint, abdominal fluid, peritoneal fluid), abscess, sputum, and COL species from mouth, nose, rectum, urine, and vagina. The most frequent Candida species isolated from the patients was C. albicans (Table 2). The rates of the other Candida species were: C. tropicalis 74 (8.8), C. glabrata 71 (8.3%), Candida famata 48 (5.7%), C. parapsilopsis 47 (5.6%), Candida kefyr 38 (4.5%), Candida krusei 23 (2.7%), Candida dubliniensis 13 (1.5%) and C. intermedia, Candida lusitaniae and Candida guilliermondii 22 (2.6%).
Table 1.
Sample site and distribution of cultured Candida species isolated from the immunocompromised patients
| Sample Site | Distribution of Candida species |
|---|---|
| Bronchoalveolar lavage | Candida albicans, Candida tropicalis, Candida famata, Candida parapsilopsis, Candida kefyr |
| Blood | Candida albicans, Candida tropicalis, Candida glabrata, Candida famata, Candida parapsilopsis, Candida kefyr |
| Fluida | Candida albicans, Candida parapsilopsis |
| Abscess | Candida albicans |
| Sputum | Candida albicans, Candida glabrata, Candida famata, Candida kefyr, Candida dubliensis, other |
| Mouth | Candida albicans, Candida tropicalis, Candida glabrata, Candida famata, Candida parapsilopsis, Candida kefyr, Candida dubliensis, other |
| Nose | Candida albicans, Candida tropicalis, Candida famata, Candida parapsilopsis |
| Rectum | Candida albicans, Candida tropicalis, Candida glabrata, Candida famata, Candida kefyr, Candida dubliensis, others |
| Urine | Candida albicans, Candida tropicalis, Candida glabrata, Candida famata, Candida parapsilopsis, Candida kefyr |
| Vagina | Candida albicans, Candida tropicalis, Candida glabrata, Candida famata, Candida parapsilopsis, Candida kefyr, Candida dubliensis, other |
aFluid include: Joint, abdominal fluid, peritoneal fluid
Table 2.
Distribution of Candida species isolated from the colonized and infected patients
| Candida spp. | Colonized isolates Number/% | Invasive isolates Number/% | Total |
|---|---|---|---|
| Candida albicans | 237 (56.3%) | 273(64.2%) | 510 (60.3%) |
| Candida tropicalis | 36 (8.6%) | 38 (8.9%) | 74 (8.8%) |
| Candida glabrata | 53(12.6%) | 18 (4.7%) | 71(8.3%) |
| Candida famata | 28 (6.7%) | 20 (4.6%) | 48 (5.7%) |
| Candida parapsilosis | 12 (2.9%) | 35 (8.2%) | 47 (5.6%) |
| Candida kefyr | 18 (4.3%) | 20 (4.7%) | 38(4.5%) |
| Candida krusei | 13 (3%) | 10 (2.4%) | 23(2.7%) |
| Candida dubliniensis | 10 (2.3%) | 3 (0.7%) | 13(1.5%) |
| Othersa | 14 (3.3%) | 8 (1.9%) | 22 (2.6%) |
| Total | 421 | 425 | 846 |
aOthers included: Candida intermedia, Candida lusitaniae and Candida guilliermondii
The susceptibility patterns of COL and INFEC isolates to six antifungal agents are shown in Table 3. In INFEC and COL isolates, the MIC90 values for AMB in C. albicans (0.25 μg/ml and 0.25 μg/ml), C. parapsilosis (0.032 μg/ml, and 0.25 μg/ml), and C. famata (0. 25 μg/ml and 0.25 μg/ml) did not differ significantly (P > 0.05). The MIC90 values of AMB in C. tropicalis and C. glabrata in INFEC and COL isolates were 4 μg/ml and 0.125 μg/ml; and 8 μg/ml and 0.064 μg/ml, respectively (P < 0.05). The MIC90 values of C. krusei in INFECT and COL isolates to AMB were 8 μg/ml and 4 μg/ml, respectively, with an ECV of 4 μg/ml. The resistance rates for FLU in INFEC isolates of C. albicans, C. tropicalis, C. glabrata, and C. parapsilosis were 4.9% (12/273), 10.5% (4/38), 11.1% (2/18) and 2.9% (1/35), respectively. The resistance rates for INFECT and COL isolates in C. albicans and C. krusei to ITR were 12.7% (35/273) and 2.6% (7/273); and 33.3% (3/10) and 20% (2/10), respectively.
Table 3.
In vitro suseptibility patterns of Candida species isolates from Colonized (C) and Infected (I) patients
| Species Antifungal | I Ra |
I R% |
I GM |
I MIC90 (μg/ml) |
C Ra |
C R% |
C GM |
C MIC90 (μg/ml) |
Total ECV (μg/ml) |
Total Wild type (%) |
Total Non-wild type (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Candida albicans | |||||||||||
| AMB | 0.032-16 | 3.3 | 0.052 | 0.25 | 0.032-32 | 0.9 | 0.039 | 0.25 | 0.5 | ≤ 0.5(95%) | > 0.5(5%) |
| CAS | 0.032-1 | 0.5 | 0.03 | 0.25 | 0.032-1 | 0.4 | 0.041 | 0.125 | 0.25 | ≤ 0.25(98%) | > 0.25(2%) |
| VOR | 0.032-2 | 6.9 | 0.032 | 0.125 | 0.032-16 | 5.4 | 0.035 | 0.064 | 1 | ≤ 1(97%) | > 1(3%) |
| FLU | 0.032-64 | 4.9 | 0.254 | 2 | 0.032-64 | 0.5 | 0.254 | 2 | 4 | ≤ 4(96%) | > 4(4%) |
| POSa | 0.032-8 | – | 0.044 | 0.25 | 0.032-4 | – | 0.031 | 0.032 | 0.25 | ≤ 0.25(96%) | > 0.25(4%) |
| ITR | 0.032-16 | 12.7 | 0.104 | 1 | 0.032-16 | 2.6 | 0.049 | 0.125 | 1 | ≤ 1(98%) | > 1(2%) |
| Candida tropicalis | |||||||||||
| AMB | 0.032-8 | 18.4 | 0.115 | 4 | 0.032-0.5 | 0 | 0.033 | 0.125 | 1 | ≤ 1(95%) | > 1(5%) |
| CAS | 0.032-0.5 | 0 | 0.041 | 0.25 | 0.032-4 | 2.9 | 0.046 | 0.125 | 0.5 | ≤ 0.5(99%) | > 0.5(1%) |
| VOR | 0.032-16 | 14.3 | 0.056 | 1 | 0.032-16 | 8.3 | 0.033 | 0.125 | 1 | ≤ 1(96%) | > 1(4%) |
| FLU | 0.032-64 | 10.5 | 0.361 | 4 | 0.064-64 | 8.8 | 0.302 | 2 | 4 | ≤ 4(95%) | > 4(5%) |
| POS | 0.032-0.25 | – | 0.029 | 0.25 | 0.032-16 | – | 0.035 | 0. 125 | 0.25 | ≤ 0.25(95%) | > 0.25(5%) |
| ITR | 0.032-2 | 13.2 | 0.1 | 1 | 0.032-16 | 0 | 0.078 | 0.5 | 1 | ≤ 1(96%) | > 1(4%) |
| Candida glabrata | |||||||||||
| AMB | 0.032-16 | 11.1 | 0.101 | 8 | 0.032-0.5 | 0 | 0.031 | 0.064 | 0.5 | ≤ 0.5(99%) | > 0.5(1%) |
| CAS | 0.032-8 | 22.2 | 0.086 | 4 | 0.032-0.5 | 9.8 | 0.113 | 0.5 | 0.5 | ≤ 2 (96%) | > 2 (4%) |
| VOR | 0.16-4 | 0 | 0.088 | 2 | 0.032-0.5 | 0 | 0.05 | 0. 25 | 0.5 | ≤ 0.5 (96%) | > 0.5(4%) |
| FLU | 0.25 | 11.1 | 1.17 | 16 | 0.064-16 | 0 | 0.842 | 4 | 16 | ≤ 16 (98%) | > 16(2%) |
| POS | 0.032-16 | – | 0.179 | 8 | 0.032-16 | – | 0.082 | 0.5 | 4 | ≤ 4 (95%) | > 4(5%) |
| ITR | 0.032-16 | 77.8 | 0.739 | 4 | 0.032-16 | 15 | 0.233 | 1 | 2 | ≤ 2 (96%) | > 2(4%) |
| Candida famata | |||||||||||
| AMB | 0.032-2 | 5 | 0.038 | 0.25 | 0.032-1 | 0 | 0.037 | 0.25 | 0.25 | ≤ 0.25(95%) | > 0.25(5%) |
| CAS | 0.032-16 | 0 | 0.043 | 0.5 | 0.032-0.25 | 0 | 0.035 | 0.25 | 0.25 | ≤ 0.25(95%) | > 0.25(5%) |
| VOR | 0.032-0.5 | 0 | 0.024 | 0.5 | 0.032-1 | 0 | 0.034 | 0.125 | 0.25 | ≤ 0.5(98%) | > 0.5(2%) |
| FLU | 0.064-8 | 0 | 0.278 | 0.5 | 0.032-8 | 0 | 0.268 | 0.5 | 1 | ≤ 1(95%) | > 1(5%) |
| POS | 0.032-1 | – | 0.026 | 0.5 | 0.032-0.5 | 0 | 0.031 | 0.064 | 0.5 | ≤ 0.5(98%) | > 0.5(2%) |
| ITR | 0.032-1 | 10 | 0.081 | 0.5 | 0.032-1 | 4.3 | 0.062 | 0.5 | 0.5 | ≤ 0.5(98%) | > 0.5(2%) |
| Candida parapsilopsis | |||||||||||
| AMB | 0.032-0.5 | 0 | 0.023 | 0.032 | 0.032-0.25 | 0 | 0.027 | 0.032 | 0.25 | ≤ 0.25(95%) | > 0.25(5%) |
| CAS | 0.032-0.25 | 0 | 0.037 | 4 | 0.032-4 | 0 | 0.283 | 0.125 | 0.125 | ≤ 4(98%) | > 4(2%) |
| VOR | 0.032-0.032 | 0 | 0.017 | 0.032 | 0.032-0.25 | 0 | 0.025 | 0.032 | 0.032 | ≤ 0.032(96%) | > 0.032(4%) |
| FLU | 0.064-8 | 2.9 | 0.402 | 4 | 0.064-2 | 0 | 0.298 | 2 | 2 | ≤ 2(100%) | > 2(00%) |
| POS | 0.032-0.032 | – | 0.017 | 0.032 | 0.032-0.5 | – | 0.03 | 0.032 | 0.032 | ≤ 0.032(96%) | > 0.032(4%) |
| ITR | 0.032-0.5 | 0 | 0.0102 | 0.5 | 0.032-0.032 | 0 | 0.02 | 0.125 | 0.5 | ≤ 0.5(100%) | > 0.5(00%) |
| Candida kefyr | |||||||||||
| AMB | 0.032-1 | 0 | 0.045 | 1 | 0.032-1 | 0 | 0.03 | 0.064 | 1 | ≤ 1(99%) | > 1(1%) |
| CAS | 0.032-0.125 | 0 | 0.028 | 0.25 | 0.032-2 | 0 | 0.031 | 0.25 | 0.125 | ≤ 0.25(95%) | > 0.25(5%) |
| VOR | 0.032-0.032 | 0 | 0.018 | 0.032 | 0.032-0.125 | 0 | 0.021 | 0.032 | 0.032 | ≤ 0.032(96%) | > 0.032(4%) |
| FLU | 0.25-0.5 | 0 | 0.397 | 1 | 0.064-2 | 0 | 0.185 | 0.5 | 1 | ≤ 1(96%) | > 1(4%) |
| POS | 0.032-0.032 | – | 0.018 | 0.032 | 0.032-0.125 | – | 0.021 | 0.032 | 0.032 | ≤ 0.032(96%) | > 0.032(4%) |
| ITR | 0.032-0.125 | 0 | 0.032 | 0.125 | 0.032-0.25 | 0 | 0.037 | 0.125 | 0.125 | ≤ 0.125(96%) | > 0.125(96%) |
| Candida krusei b | |||||||||||
| AMB | 0.032-8 | 40 | 1.004 | 8 | 0.032-4 | 40 | 0.386 | 4 | 4 | ≤ 4(95%) | > 4(5%) |
| CAS | 0.032-2 | 30 | 0.2 | 2 | 0.032-0.5 | 0 | 0.092 | 0.25 | 2 | ≤ 2(99%) | > 2(1%) |
| VOR | 0.032-16 | 20 | 0.284 | 2 | 0.032-16 | 7.7 | 0.235 | 0.5 | 2 | ≤ 2(95%) | > 2(5%) |
| FLU | 2-64 | – | 17.9 | 64 | 0.25-64 | – | 6.817 | 64 | 64 | ≤ 64(95%) | > 64(5%) |
| POS | 0.032-0.5 | – | 0.126 | 0.5 | 0.032-16 | – | 0.214 | 0.5 | 0.5 | ≤ 0.5(95%) | > 0.5(5%) |
| ITR | 0.064-1 | 33.3 | 0.2 | 2 | 0.064-16 | 15.4 | 0.346 | 1 | 2 | ≤ 2(95%) | > 2(5%) |
| Other Candida spp.c | |||||||||||
| AMB | 0.032-0.032 | 0 | 0.032 | 0.032 | 0.032-1 | 0 | 0.032 | 0.064 | 0.064 | ≤ 0.064(95%) | > 0.064(95%) |
| CAS | 0.032-0.064 | 0 | 0.021 | 0.064 | 0.032-1 | 0 | 0.064 | 0.064 | 0.064 | ≤ 0.064(95%) | > 0.064(95%) |
| VOR | 0.032-0.032 | 0 | 0.032 | 0.032 | 0.032-0.125 | 0 | 0.026 | 0.032 | 0.125 | ≤ 0.125(100%) | > 0.125(100%) |
| FLU | 0.0125-4 | 0 | 0.33 | 4 | 0.064-8 | 0 | 0.227 | 0.5 | 4 | ≤ 4(95%) | > 4(95%) |
| POS | 0.032-0.032 | 0 | 0.032 | 0.032 | 0.032-0.064 | 0 | 0.023 | 0.032 | 0.032 | ≤ 0.032(95%) | > 0.032(95%) |
| ITR | 0.032-0.0125 | 0 | 0.024 | 0.125 | 0.032-0. 25 | 0 | 0.042 | 0.25 | 0.25 | ≤ 0.25(100%) | > 0.25(100%) |
Ra Range, MIC minimum inhibitory concentration, R resistant, MIC 90 Lowest concentration at which 90% of the isolates are inhibited
aPosaconazole has no breakpoint in new CLSI
bIsolates of C. krusei are considered resistant to fluconazole, irrespective of the MIC
cOthers: include; C. intermedia, C. dubliniensis, C. lusitaniae and M. guilliermondii (C. guilliermondii)
Resistance rates to VOR in the INFEC isolates of C. albicans, C. tropicalis, and C. krusei were 6.9% (19/273), 14.3% (5/38) and 20% (2/10), and in COL isolates 5.4% (13/237), 8.3% (3/36) and 7.7% (1/13), respectively, without significant differences in MIC values (P > 0.05). The MIC90 values of POS for all species were < 0.5 μg/ml, except in INFECT C. glabrata isolates that showed a MIC90 value of 8 μg/ml. The ECV and MIC 90 values for FLU in C. krusei were both 64 μg/ml in groups with GM 17.9 and 6.817 in INFEC and COL isolates, respectively. Susceptible dose dependence for ITR in C. albicans, C. krusei, and C. kefyr in INFECT and COL isolates were 35.3% and 24.2%; 33.3% and 69.2%; and 16.7% and 22.2%, respectively. Also, 72.9% of COL C. glabrata were susceptible dose dependent to ITR. The ECV for this antifungal agent for all Candida species was ≤ 1 μg/ml, except C. glabrata which were 2 μg/ml. The MIC90 values for CAS in all Candida isolates ranged between 0.25 μg/ml and 0.5 μg/ml, except in INFECT isolates of C. glabrata and C. parapsilosis (4 μg/ml), and for both group isolates of C. krusei a MIC90 of 2 μg/ml was observed.
The comparison of MIC90 values for antifungal agents in INFECT and COL isolates are shown in Fig. 1. There was no significant difference between COL and INFECT C. albicans, C. famata, C. kefyr, C. krusei, C. intermedia, C. dubliniensis, C. lusitaniae and C. guilliermondii isolates in all antifungal agents in this study (P > 0.05). However, a significant difference between INFECT and COL isolates of C. tropicalis in MIC90 values of AMB (P < 0.05) was observed. Candida krusei INFECT and COL isolates presented high ECV and MIC90 values for all antifungal agents, except POS. As for C. glabrata, there were significant differences between INFECT and COL isolates in AMB, CAS, and VOR (P < 0.05), and MIC90 values in isolates from both groups for FLU, POS, and ITR that were higher than those for other Candida species.
Fig. 1.
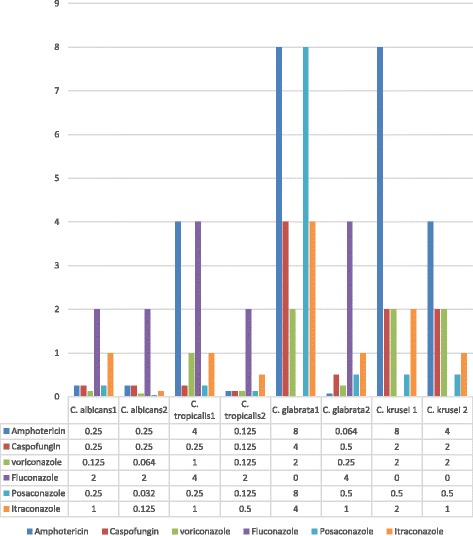
Comparison of MIC90 values in some Candida species in Infecting (1) and Colonizing (2) isolates
Discussion
The most frequent Candida species isolated from the patients was C. albicans which occurred more in INFEC isolates due to its pathogenic mechanism factors [9]. The rates of Candida species involved in infections differ in the literature, but in many studies, C. albicans is the most prevalent species [10–12]. The second most frequent Candida species in this study was C. tropicalis. In a study from one center in Iran, the second isolated species from immunocompromised patients was C. krusei, while in Indian and Korean populations was C. parapsilopsis [10–12]. Distribution of species was found to differ in each region and study population.
In this region, polyenes and azoles are the most common antifungal agents used in the treatment of patients with fungal infections. In this study, the MIC90 values of AMB were significantly different (P < 0.05) in C. tropicalis and C. glabrata INFEC and COL isolates. The highest MIC90 values to AMB belonged to C. krusei INFECT and COL isolates. In Castanheira et al. the MIC90 value for AMB was reported to be 1 μg/ml in all Candida isolates, except C. krusei which had a MIC of 2 μg/ml [13]. In the present study, resistance rates to AMB in INFECT isolates in C. albicans were (3.3%, 9/273), C. tropicalis (18.4%, 7/38), C. glabrata (11.1%, 2/18), and C. krusei (40%, 4/10), whereas in COL isolates resistant rates were only seen in C. albicans (0.9%, 3/273) and C. krusei (40%, 4/10) (Table 3). These rates were reported in INFECT C. albicans, C. tropicalis, C. glabrata and C. krusei isolate as 7% (12/172), 33.3% (2/6), 15% (6/40) and 10% (6/60), respectively [11]. There have been few studies about susceptibility patterns of Candida species in colonized patients. In Haddadi et al. the resistance rates of COL isolates to AMB in C. albicans, C. glabrata and C. krusei were reported to be 3% (4/117), 7.5% (1/14), and 27.7% (5/18), respectively [14]. The MIC 90 value for AMB in 1310 isolated C. albicans in Castanheira et al. was reported 1 μg/ml which is higher than of our study (0.25 μg/ml) [15].The results of the resistance rates and MIC90 value in the present study for INFECT and COL isolates were lower than that in other studies, due to their study population, and limited use of antifungal agents in some cities. Unfortunately, in our region, fungal infections are not clear for some clinicians and treatment and prophylaxis for it is not routine in some health care systems.
During the past few years, the CLSI have adjusted the breakpoints for FLU, VOR, and ITR and new breakpoints were reported [5]. In the present study, the most resistant species to FLU in INFEC isolates of the Candida species were C. glabrata and C. tropicalis. Compared with other Candida species, C. krusei and C. glabrata are generally documented as the causes of invasive candidiasis with reduced susceptibility to FLU. The MIC 90 values for FLU in C. albicans and C. glabrata in our study were 2 μg/ml and 16 μg/ml in the two groups, respectively. The MIC90 values for FLU in Castanheira et al. in clinical C. albicans and C. glabrata isolates were reported as 0.25 μg/ml and 32 μg/ml with resistance rates of 0.3 and 7.9%, respectively [13]. The resistance rate to FLU was reported 2.6% in infected patients in Korea [10]. In Pfaller et al. “resistance to fluconazole was seen in 0.5% of C. albicans isolates, 11.1% of C. glabrata isolates, 2.5% of C. parapsilosis isolates, 4.5% of C. tropicalis isolates, and 20.0% of C. guilliermondii isolates” [16]. In the present study, the resistance rates to ITR in C. glabrata INFEC and COL isolates were 77.8% (14/18) and 15% (8/53), respectively (P < 0.05). This resistance rate in INFECT was similar to the results obtained in immunocompromised patients in our previous study (i.e. 85.5%) [11]. Resistance to ITR was reported in many studies [11, 14, 17]. In Haddadi et al., this rate was 22.5% (49/217) in all COL isolates [14]. In Cuenca-Estrella et al. resistance of all Candida species to ITR was seen, especially in C. glabrata [18]. Strains susceptible dose dependent to ITR were reported in many studies [11, 14, 17]. Susceptible dose dependence in the present study for ITR was seen in many Candida species, especially COL C. glabrata isolates. Itraconazole and FLU are the most commonly prescribed azole agents in our region, which may explain the increased resistance rates and susceptible dose dependence observed for these antifungal agents in our study. The presence of many susceptible dose dependent isolates may suggest the future emergence of these species with resistant isolates.
Cross-resistance of VOR and other azoles, such as FLU and ITR, can occur due to previous exposure. Resistance rates to VOR in the INFEC and COL isolates of Candida species were observed without significant differences in MIC values. The ECV of VOR for C. glabrata according to the new CLSI breakpoint is 0.5 μg/ml. The MIC90 for C. glabrata INFECT isolates in the present study was 2 μg/ml, indicating that most of the isolates were non-WT. Voriconazole MICs of > 0.12 μg/ml were reported among C. glabrata and C. krusei isolates in Cuenca-Estrella M et al. [18].
According to the newly defined CLSI breakpoints, there is no breakpoint defined for POS. In the present study, the MIC90 of POS for INFECT C. glabrata was 8 μg/ml and other Candida species had < 0.5 μg/ml MIC90 value. A MIC value above 8 μg/ml for POS was reported in Soczo et al. for C. albicans and C. glabrata isolates that were resistant to FLU [19]. Also, a POS MIC90 value of 0.06 μg/ml was reported in C. albicans and 2 μg/ml for C. glabrata isolates in Castanheira et al. [13]. In Mahmoudabadi et al. 94% of the isolates were inhibited by POS at < 2 μg/mL after 24 h, whereas 6% of isolates had MICs of > 4 μg/mL [20]. According to the present study, the ECV and MIC90 values of POS in C. krusei were 0.5 μg/ml, and POS is the best antifungal agent for the treatment of infections due to this species. POS is an expensive antifungal in Iran and has a restricted use, which may contribute to the observed low MIC90 value.
In Iran, caspofungin has more usage than other echinocandin antifungal agents. In the current study, MIC90 values for CAS in INFECT C. glabrata and C. parapsilosis and for both groups of isolates of C. krusei were 4 μg/ml and 2 μg/ml, respectively. The MICs 90 of CAS for C. krusei were reported as 4 μg/mL [21, 22]. In the present study, significant differences occurred between the MIC 90 values of CAS in C. glabrata COL (0.5 μg/ml) and INFECT (4 μg/ml) isolates and C. parapsilosis COL (0.125 μg/ml) and INFECT (4 μg/ml) isolates (P < 0.05). Espinel-Ingrof et al. reported that CAS MICs value evaluation for some Candida species (such as C. glabrata and C. krusei according to CLSI breakpoints is not suitable and could lead to reporting an excessive number of wild-type or non-wild type isolates [23]. In Mahmoudabadi et al. the MIC of CAS in 90% of the total INFECT Candida isolates was lower than 2 μg/mL [20]. In Abad et al. MIC values for CAS in C. albicans, C. parapsilosis complex, C. tropicalis, C. glabrata complex, C. guilliermondii and C. krusei were reported as 0.008 μg/ml, 2 μg/ml, 0.12 μg/ml, 0.12 μg/ml, 2 μg/ml, and 0.5 μg/ml, respectively [24].
The infections by the other non-albicans Candida species (C. famata, C. parapsilosis, C. kefyr, C. dubliniensis, C. lusitaniae, C. guilliermondii, and C. intermedia) have been increasing during the past decades. Their susceptibility patterns to antifungal drugs and knowledge of their resistance rates are helpful to the patient management in each region. These non-albicans Candida isolated species in this study were susceptible to most antifungal agents. In INFECT isolates, 5 and 10% resistance rates to AMB and ITR were seen in C. famata, respectively. Candida parapsilosis INFECT isolates were found to have 2.9% resistance to FLU.
According to the ECVs observed in this study, many isolated species in both groups were WT and present an ECV lower than the CLSI breakpoint. Non-WT species were seen most in INFECT isolates. As for C. glabrata, there were significant differences between the MIC90 values of INFECT and COL isolates in AMB, CAS, and VOR. Jensen et al. reported treatment ≥ 7 days with azoles following fungal infection can produce resistant species, especially C. glabrata that colonizes mucosa [25]. In this study, the population study comprised of immunocompromised humans and all had a history of use of antifungal agents as prophylaxis or treatment while COL isolates were WT without increased MIC values.
Conclusion
Our work represents the first Iranian multicenter study demonstrating antifungal susceptibility patterns and ECV among INFECT and COL Candida species isolates among immunocompromised patients. Our findings suggest that the susceptibility patterns of Candida species (COL and INFECT isolates) in patients are not the same. The COL species may be recognized as a reservoir and are important for the management of the patients. Increasing use of antifungal agents needs to be monitored by ongoing national surveillance program.
Acknowledgements
The authors wish to thank Dr. Hassan Khajehei for editing the manuscript and Zahra Jafarpour for help with laboratory procedures. This study was supported by Prof. Alborzi Clinical Microbiology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
Funding
-No funding was received for this study.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AMB
Amphotericin B
- CLSI
The Clinical and laboratory standards institute
- COL
Colonized
- DMSO
Dimethyl sulfoxide
- ECV
Epidemiological cutoff values
- FLU
Fluconazole
- GM
Geometric mean
- INFECT
Infected
- ITR
Itraconazole
- MOPS
N-morpholino propanesulfonic acid
- POS
Posaconazole
- Ra
Range
- VOR
Voriconazole
- WT
Wild-type
Authors’ contributions
PB conception and design of the study and drafted the manuscript; TB analysis and interpretation of data, revising the manuscript critically; HJ performed the experiments and analyzed the data; PB, HB, KD, AGM, AHN, RM, HM, MJN, AS and JS contributed to design of the study and acquisition of data in each city. All authors have been involved in drafting the manuscript and approved the final manuscript.
Ethics approval and consent to participate
The study protocol was approved by the ethics committee at Clinical Microbiology Research Center, Shiraz University of Medical Sciences. The study protocol conformed to the ethical guidelines of the 1975 Helsinki Declaration. For colonized patients, written informed consents were obtained before sampling, but in infected patients, because the sampling was a part of their clinical process, consents were obtained verbally.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Parisa Badiee, Email: badieep@sums.ac.ir.
Hamid Badali, Email: badalii@yahoo.com.
Teun Boekhout, Email: t.boekhout@cbs.knaw.nl.
Kambiz Diba, Email: kambiz37diba@gmail.com.
Abdolkarim Ghadimi Moghadam, Email: dr_karim56@yahoo.com.
Ali Hossaini Nasab, Email: Ali4221@gmail.com.
Hadis Jafarian, Email: hdsjafarian@yahoo.com.
Rasoul Mohammadi, Email: Dr.rasoul_mohammadi@yahoo.com.
Hossein Mirhendi, Email: mirhendi@tums.ac.ir.
Mohammad Javad Najafzadeh, Email: najafzadehmj@mums.ac.ir.
Ahmad Shamsizadeh, Email: shamsizadeh.a@gmail.com.
Jafar Soltani, Email: soltanjaf@hotmail.com.
References
- 1.Quindós G. Epidemiology of candidaemia and invasive candidiasis. A changing face. Rev Iberoam Micol. 2014;31:42–48. doi: 10.1016/j.riam.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Badiee P, Alborzi A. Assessment of real-time PCR method to detect human non-Cryptococcal fungal meningitis. Arch Iran Med. 2011;14:381–384. [PubMed] [Google Scholar]
- 3.Adhikary R, Joshi S. Species distribution and anti-fungal susceptibility of Candidaemia at a multi super-specialty center in southern India. Indian J Med Microbiol. 2011;29:309. doi: 10.4103/0255-0857.83920. [DOI] [PubMed] [Google Scholar]
- 4.Colombo AL, Nucci M, Park BJ, Nouér SA, Arthington-Skaggs B, da Matta DA, et al. Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. J Clin Microbiol. 2006;44:2816–2823. doi: 10.1128/JCM.00773-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wayne P. Clinical and Laboratory Standards Institute: Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. Fourth Informational Supplement. CLSI document. 2012; M27-S4.
- 6.Pfaller MA, Diekema DJ. Progress in antifungal susceptibility testing of Candida species. By use of clinical and laboratory standards institute broth microdilution methods, 2010 to 2012. J Clin Microbiol. 2012;50:2846–2856. doi: 10.1128/JCM.00937-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of cancer/invasive fungal infections cooperative group and the National Institute of Allergy and Infectious Diseases mycoses study group EORTC/MSG consensus group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wayne P. Clinical and Laboratory Standards Institute: Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. CLSI document 2008; M27-A3.
- 9.Wächtler B, Citiulo F, Jablonowski N, Förster S, Dalle F, Schaller M, et al. Candida albicans epithelial interactions: dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS One. 2012;7:e36952. doi: 10.1371/journal.pone.0036952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Won EJ, Shin JH, Choi MJ, Lee WG, Park YJ, Uh Y, et al. Antifungal susceptibilities of bloodstream isolates of Candida species from nine hospitals in Korea: application of new antifungal breakpoints and relationship to antifungal usage. PLoS One. 2015;10:e0118770. doi: 10.1371/journal.pone.0118770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badiee P, Alborzi A, Shakiba E, Farshad S, Japoni A. Susceptibility of Candida species isolated from immunocompromised patients to antifungal agents. East Mediterr Health J. 2011;17:425. [PubMed] [Google Scholar]
- 12.Khan PA, Fatima N, Nabeela S, Khan HM, Malik A. Antifungal susceptibility pattern of Candida isolates from a tertiary care hospital in north India: a five-year study. Int J Curr Microbiol App Sci. 2015;Special Issue 1:177–181. [Google Scholar]
- 13.Castanheira M, Messer SA, Rhomberg PR, Dietrich RR, Jones RN, Pfaller MA. Isavuconazole and nine comparator antifungal susceptibility profiles for common and uncommon Candida species collected in 2012: application of new CLSI clinical breakpoints and epidemiological cutoff values. Mycopathologia. 2012;178:1–9. doi: 10.1007/s11046-014-9772-2. [DOI] [PubMed] [Google Scholar]
- 14.Haddadi P, Zareifar S, Badiee P, Alborzi A, Mokhtari M, Zomorodian K, et al. Yeast colonization and drug susceptibility pattern in the pediatric patients with neutropenia. Jundishapur J Microbiol. 2014;7:e11858. doi: 10.5812/jjm.11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castanheira M, Deshpande LM, Davis AP, Rhomberg PR, Pfaller MA. Monitoring Antifungal Resistance in a Global Collection of Invasive Yeasts and Moulds: Application of CLSI Epidemiological Cutoff Values and Whole Genome Sequencing Analysis for Detection of Azole Resistance in Candida albicans. Antimicrob Agents Chemother. 2017:AAC-00906. [DOI] [PMC free article] [PubMed]
- 16.Pfaller MA, Rhomberg PR, Messer SA, Jones RN, Castanheira M. Isavuconazole, micafungin, and 8 comparator antifungal agents' susceptibility profiles for common and uncommon opportunistic fungi collected in 2013: temporal analysis of antifungal drug resistance using CLSI species-specific clinical breakpoints and proposed epidemiological cutoff values. Diagn Microbiol Infect Dis. 2015;82(4):303–313. doi: 10.1016/j.diagmicrobio.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Badiee P, Alborzi A, Shakiba E, Ziyaeyan M, Rasuli M. Molecular identification and in-vitro susceptibility of Candida albicans and Candida dubliniensis isolated from immunocompromised patients. Iran Red Crescent Med J. 2009;11(4):391–397. [Google Scholar]
- 18.Cuenca-Estrella M, Gomez-Lopez A, Cuesta I, Zaragoza O, Mellado E, Rodriguez-Tudela JL. Frequency of voriconazole resistance in vitro among Spanish clinical isolates of Candida species. According to breakpoints established by the antifungal Subcommittee of the European Committee on antimicrobial susceptibility testing. Antimicrob Agents Chemother. 2011;55:1794–1797. doi: 10.1128/AAC.01757-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soczo G, Kardos G, McNicholas PM, Falusi E, Gergely L, Majoros L. Posaconazole susceptibility testing against Candida species: comparison of broth microdilution and E-test methods. Mycoses. 2007;50:178–182. doi: 10.1111/j.1439-0507.2007.01356.x. [DOI] [PubMed] [Google Scholar]
- 20.Mahmoudabadi AZ, Rezaei-Matehkolaei A, Ghanavati F. The susceptibility patterns of Candida species isolated from urine samples to posaconazole and caspofungin. Jundishapur J Microbiol. 2015;8:e24298. doi: 10.5812/jjm.24298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakki M, Staab JF, Marr KA. Emergence of a Candida krusei isolates with reduced susceptibility to caspofungin during therapy. Antimicrob Agents Chemother. 2006;50:2522–2524. doi: 10.1128/AAC.00148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montagna MT, Lovero G, Coretti C, Martinelli D, De Giglio O, Iatta R, et al. Susceptibility to echinocandins of Candida species strains isolated in Italy assessed by European Committee for Antimicrobial Susceptibility Testing and Clinical Laboratory Standards Institute broth microdilution methods. BMC Microbiol. 2015;15:1. doi: 10.1186/s12866-015-0442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, et al. Inter laboratory variability of caspofungin MICs for Candida spp. using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother. 2013;57:5836–5842. doi: 10.1128/AAC.01519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shekari Ebrahim Abad H, Zaini F, Kordbacheh P, Mahmoudi M, Safara M, Mortezaee V. In vitro activity of caspofungin against fluconazole-resistant Candida species isolated from clinical samples in Iran. Jundishapur J Microbio. 2015;l8:e18353. doi: 10.5812/jjm.18353v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen RH, Johansen HK, Søes LM, Lemming LE, Rosenvinge FS, Nielsen L, et al. Post treatment antifungal Resistance among colonizing Candida isolates in candidemia patients: Results from a systematic multicenter study. Antimicrob Agents Chemother. 2015; 28; 60(3):1500-8. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


