Abstract
Peste des petits ruminants virus (PPRV) causes an economically important disease that limits productivity in small domestic ruminants and often affects the livestock of the poorest populations in developing countries. Animals that survive PPRV develop strong cellular and humoral responses, which are probably necessary for protection. Vaccination should thus aim at mimicking these natural responses. Immunization strategies against this morbillivirus using recombinant adenoviruses expressing PPRV-F or -H proteins can protect PPRV-challenged animals and permit differentiation of infected from vaccinated animals. Little is known of the T cell repertoire these recombinant vaccines induce. In the present work, we identified several CD4+ and CD8+ T cell epitopes in sheep infected with PPRV. We also show that recombinant adenovirus vaccination induced T cell responses to the same epitopes, and led to memory T cell differentiation. T cells primed by these recombinant adenovirus vaccines expanded after PPRV challenge and probably contributed to protection. These data validate the use of recombinant adenovirus expressing PPRV genes as DIVA strategies to control this highly contagious disease.
Electronic supplementary material
The online version of this article (10.1186/s13567-017-0482-x) contains supplementary material, which is available to authorized users.
Introduction
Peste des petits ruminants virus (PPRV) causes an economically important disease that limits productivity in small domestic ruminants [1, 2]. Infection of naïve populations can be devastating, particularly in goats, leading to mortality rates of up to 90% [3–5]. PPRV is endemic in Central and East Africa, the Arabian Peninsula, Turkey, and India. Its prevalence in developing countries and its host spectrum often implies that the poorest populations within these countries are affected [6]. PPRV is highly contagious and in acute infections produces severe pyrexia, nasal and ocular discharges, pneumonia, enteritis and diarrhea [4, 5].
PPRV is a morbillivirus that belongs to the Paramyxoviridae family [7]. This genus of single-stranded negative sense enveloped RNA viruses causes relevant diseases (like measles or canine distemper) in human and animals. PPRV single-strand RNA genome encodes 6 structural and 2 non-structural proteins [1]. PPRV infection is immunosuppressive, which can lead to opportunistic pathogen infections that contribute to the high mortality and morbidity rates of infected animals [4, 8].
Current vaccines are based on live attenuated viruses that control the disease but cannot differentiate infected from vaccinated animals (the so-called DIVA approach) [9]. Traditional live attenuated vaccine can also produce immunosuppression, albeit to a lower extent than natural infections [10]. These drawbacks highlight the need for alternative vaccination strategies against this disease.
Most immunologically relevant determinants for protection in morbillivirus have been mapped to the surface fusion protein (F) and hemagglutinin (H) as well as to the nucleoprotein (NP) [11–15]. Recombinant vectors expressing these subunits thus represent attractive strategies for vaccination [16–22]. DIVA vaccines with recombinant adenovirus expressing the F or H protein can be protective in small ruminants [23–25], and potentially facilitate PPRV infection status monitoring.
Animals that survive PPRV infection develop a strong cellular and humoral response [11, 23, 26], which is probably essential for virus clearance and protection. In infection with the morbillivirus prototype measles virus (MeV), cellular and humoral immunity contribute to protection. Humoral immunity can protect against MeV re-infection, whereas cellular immunity controls virus clearance and dissemination [27–30]. Moreover, induction of neutralizing antibodies alone was also insufficient to protect cattle against rinderpest virus challenge, a virus closely related to PPRV [31]. It thus appears that protective natural immunity to morbilliviruses requires both humoral and cellular components of the adaptive immune system. Recombinant adenovirus vaccines should therefore aim at replicating the naturally occurring PPRV immunity. The immune responses that these vaccines elicit to the transgene are nonetheless not fully characterized. For instance determining whether the T cell repertoire they elicit is comparable to that of animals that recover from the disease could be indicative of vaccine efficacy.
In the present work, we set out to characterize T cell epitopes in mice and sheep from the main PPRV immunological determinants. We then assessed whether the responses to these immunogenic T cell epitopes overlapped in PPRV-infected and in recombinant adenovirus-vaccinated sheep. Finally, we measured the T cell functionality induced by these vaccines after heterologous PPRV challenge.
Materials and methods
Cells
Vero Dog-SLAM (VDS) and RMA/s cell lines were kindly provided by Dr Parida (IAH, Pirbright, UK) and Dr McArdle (The Nottingham Trent University, UK) respectively. HEK-293 (ATCC CRL-1573), VDS and RMA/s cell lines were maintained as described in [16, 32].
PPRV and replication-defective recombinant adenovirus 5 (Ad5) vaccines
PPRV vaccine strain Nigeria 1975/1 (PPRV Nig’75; lineage II) and PPRV infective strain Ivory Coast 1989 (PPRV IC’89; lineage I) were kindly provided by Dr Batten (IAH, Pirbright, UK). PPRV stocks were grown in VDS cells, purified as described in [16], and inactivated as described in [33]. Replication-defective recombinant adenovirus 5 construction expressing the F (Ad5-F) or H (Ad5-H) gene from PPRV Nig’75 vaccine strain is described [16]. Recombinant adenovirus stocks were grown in HEK-293 cells and purified as described [23].
PPRV peptide prediction and binding assays
Peptide binding to H-2b haplotype from PPRV Nig’75-F (GenBank #CAJ01699.1), -H (GenBank #CAJ01700.1) and -NP (GenBank #CAA52454.1) proteins was predicted using three algorithms available on the web [34–37]. Peptide F10 was selected as a PPRV-F homologue to a cross-reactive morbillivirus T cell epitope [38, 39]. Peptides were synthesized by AltaBioscience (UK) and H-2Db and H-2Kb binding assessed by flow cytometry using RMA/s cells and normalized to the lymphocytic choriomeningitic virus (LCMV) peptide gp (33–41) (KAVYNFATC) as described [32].
Animal experimentation
Six week old female C57BL/6 mice (H-2b) were purchased from Harlan. Two-month old naïve female “Colmenareña” breed sheep were purchased from a certified provider. Experiments were performed in a disease-secure isolation facility (BSL3) at the Centro de Investigación en Sanidad Animal (CISA), in strict accordance with the recommendations of the Code for Methods and Welfare Considerations in Behavioural Research with Animals (Directive 86/609EC; RD1201/2005). Experiments were approved by the Committee on the Ethics of Animal Experiments (CEEA) of the Spanish Instituto Nacional de Investigación y Tecnología Agraría y Alimentaria (INIA) and the “Comisión de ética estatal de bienestar animal”. A 2-week acclimatization period prior to experimentation was observed during which animals were monitored daily for general health status.
PPRV infection in mice and splenocyte preparation
Eight week-old C57BL/6 female were inoculated intraperitoneally with 1 × 105 plaque forming units (PFU) PPRV IC’89 three times at 2 week interval. Mice were sacrificed 3 days after the last inoculation and splenocytes prepared and cultured as described [32].
Sheep infection, peripheral blood mononuclear cell (PBMC) isolation and in vitro peptide restimulation
Sheep were randomly divided in 4 groups of 4 animals. PPRV IC’89 infection and recombinant adenovirus vaccination were performed as described in [23]. Control groups received two inoculations at 21 day interval of PBS or replication-defective recombinant empty Ad5 vaccine (Ad5-empty). Vaccinated groups received two immunizations at 21 day interval with Ad5-F or Ad5-H replication-defective recombinant vaccines [16]. All sheep were challenged intravenously with 1 × 106 PFU heterologous virulent PPRV IC’89 strain on day 42. Clinical details of vaccination results are reported in [23]. PBMC were prepared [33] and stored frozen until use. For in vitro peptide expansion, PBMC were thawed, rested for 2 h, stimulated with 10 µg/mL peptide for 6–7 days, washed and then used in functional assays.
ELISPOT and proliferation assays
Murine splenocytes or ovine PBMC (2 × 105) were plated with 10 µg/mL peptide overnight. As positive control, cells were activated with 0.5 µg/mL concanavalin-A (Con-A) or 20 ng/mL phorbol myristyl acetate (PMA) + 1 µg/mL ionomycin. PPRV responses were measured with inactivated virus. Murine IFN-γ ELISPOT assays were performed according to the manufacturer’s protocol (Diaclone, France). Ovine IFN-γ ELISPOT assays are described in [33]. ELISPOT assays were considered valid when control well spot counts were below 25; and positive counts were > 10 and at least 2 standard deviations above background [23, 32, 40]. Proliferation assays were performed as described [33].
Intracellular cytokine staining and flow cytometry
Ovine PBMC were stimulated overnight with 10 µg/mL PPRV peptide and brefeldin-A (5 µg/mL) added to the culture for the last 4 h incubation. As positive control, cells were stimulated with inactivated PPRV IC’89 or 20 ng/mL PMA + 1 µg/mL ionomycin. Cells were stained with anti-ovine CD45RO (ILA116A; KingFisher Biotech), anti-ovine CD4 (44.38; Biorad) and anti-ovine CD8 (38.65; Biorad), fixed in 4% paraformaldehyde, permeabilized with 0.2% saponin and stained with anti-bovine IFN-γ (CC302; Biorad). Appropriate isotype and fluorescence minus one-channel controls were used in these experiments for gate setting. Gating strategies are detailed in Additional file 1. FACScalibur was used for data acquisition and FlowJo software (Tree Star Inc.) for flow cytometry analysis.
Flow cytometry cytotoxicity assays
For cytotoxicity assays, in vitro peptide-stimulated ovine PBMC were used as effector cells and autologous LPS-blast cells (differentiated with 2 µg/mL LPS and 7 µg/mL dextran sulfate [32, 41]) were used as target cells. LPS-blast cells were labelled with PKH67 green fluorescent linker [42], and pulsed with relevant peptide or inactivated PPRV IC’89. Effector cells (E) and targets cells (T) were incubated for 4 h at 37 °C in 96 U-bottom well plates at different ratios (E:T). Cells were then transferred to FACS tubes; dead cells labelled with propidium iodide (PI) (2 µg/mL); and samples immediately analyzed by flow cytometry. Gating strategy is given in Additional file 1. Target cells were gated on bright FL1+ cells. Positive maximum cell death controls (target cells in PBS + 0.2% saponin) and spontaneous cell death controls were used in all experiments. The percentage of specific target cell lysis was calculated following the formula: % specific lysis = 100 × (% PI+ target − % spontaneous death)/(% maximum death − % spontaneous death).
Results
Identification of PPRV T cell epitopes in sheep
After characterizing PPPRV-F, -H and -NP binders to MHC class I molecules (Table 1) as well as their immunogenic potential in C57BL/6 mice (Additional file 2), the immunogenicity of these putative PPRV T cell epitopes was also explored in sheep. Peptide-specific IFN-γ production was measured in PBMC obtained 17–21 days after PPRV (IC’89) infection. Since previous work established that unvaccinated and Ad5-empty vaccinated sheep were naïve towards PPRV [23], sheep PBMC from both groups were used in these assays. As predicted, T cell responses to PPRV peptides depended on individual sheep as these animals are outbred. No differences in response frequency to peptides were observed between unvaccinated and Ad5-empty-vaccinated sheep and thus data are presented as one group. ELISPOT assays showed that peptides F3, F8, F9, F10, H4, H6, H9, H10, NP7, NP8 and NP9 induced significant IFN-γ production in at least 2 sheep (Figure 1). Since these peptides could contain epitopes that elicit T cell responses in several sheep, we focused our efforts on characterizing them further by intracellular IFN-γ staining (Figure 2). Peptides F8, F9, F10, H9, NP8 and NP9 induced IFN-γ production in CD4+ and CD8+ T cells. Peptides F3 and H6 induced specific IFN-γ production in CD4+ T cells, and peptides H4 and H10 induced IFN-γ production in CD8+ T cells. Peptide NP7 was not assessed in these assays due to limited PBMC availability. We also evaluated the cytotoxic T lymphocyte (CTL) response of CD8+ T cell epitopes against peptide-pulsed autologous target cells (Figure 3). Peptides F10, H4, H9, H10, NP8 and NP9 produced CTL responses (Figures 3A and B). Peptides F8 and F9 were also tested in these assays but no CTL activity was detected in the tested sheep (n = 2) (Table 2). F10, H4, H9, H10, NP8 and NP9 peptide-responding CTL could lyse target cells pulsed with inactivated PPRV IC’89 (Figure 3C). This indicates that these peptides are naturally processed and presented by target cells during PPRV infection.
Table 1.
PPRV-F, -H and -NP peptide prediction and binding assays to H-2D b and H-2 K b molecules
| PPRV protein (position) | Sequence | Predicted allele binding | Score SYFPEITHI | Score ProPred-I | Score NetMHC (predicted affinity nM) | Binding assay scorea | |
|---|---|---|---|---|---|---|---|
| F1 | F(53–61) | KLMPNITAI | Db | 23 | 138.312 | 365.28 | ++ |
| F2 | F(135–143) | QSLMNSQAI | Db | 25 | 3281.040 | 34.37 | ++ |
| F3 | F(369–377) | GTTSNRFIL | Db | 19 | 286.272 | 3413.74 | + |
| F4 | F(342–350) | NALYPMSPL | Db | 16 | 30.240 | 1927.14 | − |
| Kb | 18 | 0.330 | 791.56 | +++ | |||
| F5 | F(435–443) | SVYLHKIDL | Kb | 11 | 0.100 | 68.79 | + |
| F6 | F(278–286) | IAYPTLSEI | Db | 18 | 4.234 | 3403.64 | + |
| Kb | 9 | 1.650 | 168.59 | + | |||
| F7 | F(7–21) | LVFLFLFPNTVTCQI | I-Ab | NA | NA | 79.2 | NA |
| F8 | F(117–131) | VALGVATAAQITAGV | I-Ab | NA | NA | 192.1 | NA |
| F9 | F(341–355) | QNALYPMSPLLQECF | I-Ab | NA | NA | 351.5 | NA |
| F10 | F(284–298) | LSEIKGVIVHKIEAI | Homologue to morbillivirus epitope [38, 39] | NA | NA | 5014.3 (I-Ab) | NA |
| H1 | H(270–278) | FHMTNYLTV | Db | 21 | 17.280 | 43.88 | +++ |
| H2 | H(158–166) | AAVKSVEHI | Db | 21 | 50.168 | 593.13 | +++ |
| H3 | H(547–555) | RSSSYFYPV | Kb | 18 | 1.100 | 70.29 | ++ |
| H4 | H(549–557) | SSYFYPVRL | Kb | 18 | 5.500 | 8.75 | +++ |
| H5 | H(551–559) | YFYPVRLNF | Db | 7 | 0.000 | 42 098.88 | + |
| Kb | 7 | 0.120 | 1250.29 | − | |||
| H6 | H(44–52) | VMFLSLIGL | Db | 15 | 14.334 | 6563.96 | ++ |
| Kb | 11 | 2.000 | 44.36 | +++ | |||
| H7 | H(426–434) | VITSVFGPL | Kb | 21 | 12.000 | 96.10 | +++ |
| H8 | H(441–455) | MDLYNNPFSRAAWLA | I-Ab | NA | NA | 42.2 | NA |
| H9 | H(427–441) | ITSVFGPLIPHLSGM | I-Ab | NA | NA | 74.8 | NA |
| H10 | H(448–462) | FSRAAWLAVPPYEQS | I-Ab | NA | NA | 319.6 | NA |
| NP1 | NP(29–37) | RGIKNVIIV | Db | 19 | 38.880 | 265.97 | +++ |
| NP2 | NP(72–80) | VMISMLSLF | Db | 18 | 36.318 | 3827.32 | − |
| Kb | 11 | 0.030 | 97.12 | +++ | |||
| NP3 | NP(294–302) | STIESLMNL | Db | 18 | 12.773 | 7155.04 | − |
| Kb | 12 | 1.320 | 117.94 | ++ | |||
| NP4 | NP(335–343) | YAMGVGVEL | Db | 16 | 30.240 | 6163.56 | + |
| NP5 | NP(354–362) | RSYFDPAYF | Db | 11 | 0.091 | 4399.92 | − |
| Kb | 8 | 0.158 | 1703.38 | − | |||
| NP6 | NP(228–236) | SLRRFMVSL | Kb | 12 | 0.264 | 184.41 | ++ |
| NP7 | NP(298–306) | SLMNLYQQL | Kb | 22 | 26.400 | 85.56 | + |
| NP8 | NP(435–449) | REEVKAAIPNGSEGR | I-Ab | NA | NA | 95.2 | NA |
| NP9 | NP(327–341) | GAYPLLWSYAMGVGV | I-Ab | NA | NA | 290.8 | NA |
| NP10 | NP(176–190) | ILLAKAVTAPDTAAD | I-Ab | NA | NA | 355.6 | NA |
NA: not available, +++ strong binder (ratio > 0.9), ++ moderate binder (0.7 < ratio < 0.9), + weak binder (0.5 < ratio < 0.7), − no binder (ratio < 0.5).
aPeptide score was ranked relative to LCMV gp33-41 peptide binding (ratio PPRV peptide MFI vs gp 33–41 MFI) [32].
Figure 1.
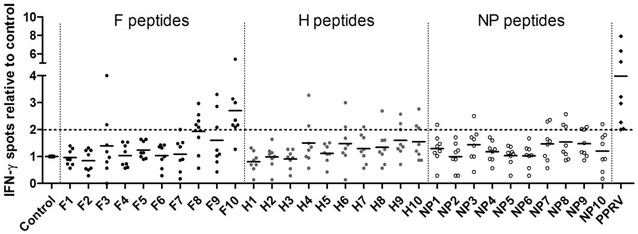
PPRV epitope screening in infected sheep. Sheep PBMC obtained 17–21 days post-PPRV (IC’89) infection were stimulated with predicted F, H or NP peptides and specific IFN-γ production assessed in ELISPOT assays. Data were normalized to spots detected in unstimulated cells. The dotted horizontal line represents the positive stimulation threshold (positive counts > 10 and at least 2 standard deviations above background; control well spot counts < 25).
Figure 2.
IFN-γ CD4 + and CD8 + T cell responses to PPRV peptides. Sheep PBMC obtained 17–21 days post-PPRV (IC’89) infection were expanded in vitro with peptide for 1 week and IFN-γ production was assessed by flow cytometry using intracellular staining. Representative dot-plots from 2 to 5 sheep are presented.
Figure 3.
Cytotoxic T lymphocyte responses to PPRV epitopes. In vitro peptide-stimulated PBMC obtained 17–21 days post-infection from PPRV (IC’89)-infected sheep were used as effector cells (E) in cytotoxicity assays. Autologous LPS-blast cells were used as target cells (T) and labeled with PKH67 cell linker dye. Cell cytotoxicity was measured by propidium iodide staining at different effector cells (E) to target cells (T) ratios (E:T). Gating on bright PKH67+ and propidium iodide+ events by flow cytometry was used to calculate the percentage of target cell lysis. A Specific lysis (mean ± SD) to F10, H4, H9, H10, NP8 and NP9 peptides in PPRV IC’89 infected sheep. Student’s t test (peptide vs no peptide); *p < 0.05. B Representative dot-plots showing dead target cells gating. C Specific lysis (mean ± SD) of unpulsed, peptide-, or PPRV IC’89-pulsed autologous target cells. One way ANOVA with Dunnett’s post-test (peptide or PPRV IC’89 vs no peptide); *p < 0.05.
Table 2.
Responses to F, H and NP peptides in PPRV IC’89-infected sheep
| Peptide | ELISPOT (positive/total sheep) | Intracellular IFN-γ staining | Cytotoxicity assay |
|---|---|---|---|
| F3 | 2/8 | CD4+ (n = 2) | ND |
| F7 | 1/8 | ND | ND |
| F8 | 5/8 | CD4+/CD8+ (n = 4) | Not detected (n = 2) |
| F9 | 3/8 | CD4+/CD8+ (n = 3) | Not detected (n = 2) |
| F10 | 7/8 | CD4+/CD8+ (n = 5) | Positive (n = 2) |
| H4 | 2/8 | CD8+ (n = 2) | Positive (n = 1) |
| H6 | 2/8 | CD4+ (n = 2) | ND |
| H7 | 1/8 | ND | ND |
| H8 | 1/8 | ND | ND |
| H9 | 3/8 | CD4+/CD8+ (n = 2) | Positive (n = 1) |
| H10 | 3/8 | CD8+ (n = 2) | Positive (n = 1) |
| NP1 | 1/8 | ND | ND |
| NP3 | 1/8 | ND | ND |
| NP7 | 2/8 | ND | ND |
| NP8 | 3/8 | CD4+/CD8+ (n = 2) | Positive (n = 1) |
| NP9 | 3/8 | CD4+/CD8+ (n = 2) | Positive (n = 2) |
| NP10 | 1/8 | ND | ND |
Due to limited PBMC numbers it was not possible to use all three techniques described on all sheep PBMC.
n: number of animal tested, ND: not done.
Our data thus show in sheep that peptides F3, F8, F9, F10, H6, H9, NP8 and NP9 contain CD4+ T cell PPRV epitopes and peptides F8, F9, F10, H4, H9, H10, NP8 and NP9 contain CD8+ T cell epitopes. Moreover, peptides F10, H4, H9, H10, NP8 and NP9 contain CTL epitopes. Based on the response frequency (Table 2), peptides F8, F9, F10, H9, H10, NP8 and NP9 are shared by several animals and could represent interesting targets for inclusion in PPRV vaccines.
Recombinant Ad5 vaccination primes T cell responses to PPRV epitopes that arise during infection
Since several T cell epitopes in PPRV-infected sheep were characterized, we wanted to determine whether vaccination with recombinant adenovirus expressing PPRV-F or -H proteins from the Nig’75 vaccine strain would trigger T cell responses to the F and H epitopes defined in animals infected with a heterologous strain (IC’89). In these experiments, we used PBMC from Ad5-F or Ad5-H immunized sheep obtained 21 days after booster vaccination and prior to PPRV challenge. PBMC from Ad5-F or Ad5-H-immunized sheep produced IFN-γ in response to peptides F3, F8, F9, F10 and H4, H6, H9 and H10 (Figures 4A and B), respectively. Vaccination therefore stimulated T cell responses towards F and H epitopes that coincide with some of those raised during PPRV infections. As observed in infected animals, peptides F3, F8, F9, F10, H6 and H9 induced CD4+ T cell IFN-γ production (Figures 4C and D) while peptides F8, F9, F10, H4, H9 and H10 induced CD8+ T cell IFN-γ production (Figures 4E and F) in recombinant Ad5-vaccinated animals. Thus Ad5-F or Ad5-H vaccination in sheep induced CD4+ and CD8+ T cell responses that are directed to several epitopes presented also during the course of a natural PPRV IC’89 infection.
Figure 4.
Ad5-F and Ad5-H vaccination activates T cell responses to epitopes induced in PPRV infections. The IFN-γ production to A F and B H epitopes was assessed in Ad5-F- and Ad5-H-vaccinated sheep, respectively by ELISPOT assays. The PBMC used in this experiment were from Ad5-F or Ad5-H immunized sheep obtained 21 days after booster vaccination and prior to PPRV challenge. Data are presented as mean IFN-γ spots normalized to control for each sheep. Inactivated PPRV IC’89 was used as positive control. The dotted horizontal line represents the positive stimulation threshold (positive counts > 10 and at least 2 standard deviations above background; control well spot counts < 25). C–F Intracellular IFN-γ staining and flow cytometry analysis used to identify T cell subsets responding to F and H epitopes in PBMC expanded with peptide for 1 week. Representative dot-plots of CD4+ T cell responses to C F epitopes and control (no peptide) in Ad5-F-vaccinated sheep and D H epitopes and control (no peptide) in Ad5-H-vaccinated sheep. Representative dot-plots of CD8+ T cell responses to E F epitopes and control (no peptide) in Ad5-F-vaccinated sheep and F H epitopes and control (no peptide) in Ad5-H-vaccinated sheep.
Recombinant Ad5 vaccines produce memory T cells that expand after PPRV challenge
We next evaluated whether Ad5-F or Ad5-H vaccination produced memory T cells by measuring the expression of the memory marker CD45RO [43, 44] on IFN-γ-producing cells that responded to PPRV immunogenic peptides. Anti-PPRV CD4+ (Figure 5A) and CD8+ (Figure 5B) IFN-γ-producing T cells predominantly expressed the memory marker CD45RO, which indicated that these cells are antigen-experienced. Ad5-F and Ad5-H vaccination therefore led to anti-PPRV memory T cell differentiation.
Figure 5.
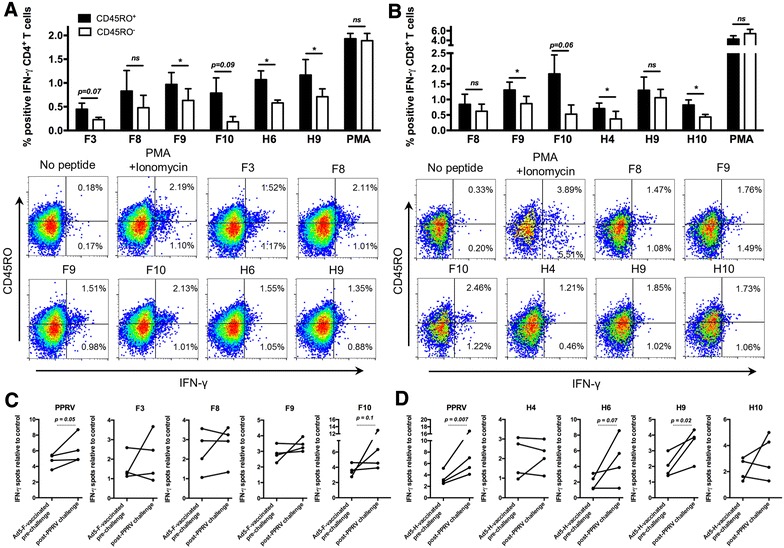
Ad5-F and Ad5-H vaccination produces functional memory T cells to PPRV immunogenic peptides. PBMC from Ad5-F- or Ad5-H-vaccinated sheep (21 days after booster and prior to PPRV challenge) were stimulated with PPRV immunogenic peptides and stained for CD4, CD8, the memory marker CD45RO and intracellular IFN-γ. A Mean (± SEM) IFN-γ production in CD45RO+ and CD45RO− A CD4+ or B CD8+ T cells in two to four Ad5-F- or Ad5-H-immunized sheep is plotted. PMA and ionomycin was used as positive control. Representative IFN-γ and CD45RO stainings in A CD4+ or B CD8+ T cells from Ad5-F- or Ad5-H-vaccinated sheep in response to PPRV immunogenic peptide stimulation. Student’s t test (CD45RO+ vs CD45RO−); *p < 0.05. IFN-γ production in PBMC from C Ad5-F- or D Ad5-H-vaccinated sheep was measured pre-challenge and after PPRV IC’89 challenge by ELISPOT assays. Data were normalized to spots detected in unstimulated cells. p ≤ 0.1 in paired Student’s t tests are shown.
To evaluate anti-PPRV memory T cell function, we measured the amplitude of the T cell responses by ELISPOT assays in Ad5-F- or Ad5-H-vaccinated sheep prior to PPRV challenge (21 days after booster vaccination) and 15 days after challenge. PPRV (IC’89) challenge increased T cell responses to the virus and to several F (Figure 5C) and H (Figure 5D) immunogenic peptides. These data confirm that Ad5-F and Ad5-H can induce a productive memory T cell response that can be reactivated after PPRV challenge.
Discussion
Recombinant adenovirus vector vaccines are highly immunogenic and induce innate and adaptive immunity. Adenoviruses are recognized through pattern recognition receptors in transduced cells [45–48] thus producing adjuvancy towards the transgene [49]. Adenoviruses induce B, CD4+ T, CD8+ T cell responses to the adenovirus [50–54] and to the inserted transgene [16, 17, 22–24, 55–57]. Recombinant Ad5 immunization therefore represents a promising tool for recombinant vaccine development.
Recombinant adenovirus expressing the protein F or H from the economically important morbillivirus PPRV are immunogenic [16, 17, 22], and can protect sheep and goats from virulent virus challenge [23–25]. These recombinant adenovirus vaccines can also overcome PPRV-induced immunosuppression [23], an aspect of PPRV infection that can result in infected animals succumbing to opportunistic infections. Adenoviral vectors are known to induce strong T cell responses to the transgene. However, the extent to which this T cell response mimics the protective repertoire induced by the pathogen is not fully understood. In the present work we detect a clear overlap between the CD4+ and CD8+ T cell responses triggered after recombinant Ad5 vaccination and PPRV experimental infections. Responses to the tested PPRV-F and -H epitopes elicited during PPRV infections were also induced by Ad5 vaccination.
CD8+ T cell responses to transgene expressed by adenovirus depend on dose and route of injection; with high antigen dose leading to CD8+ T cell activation but impaired memory development [55]. This impaired memory differentiation in CD8+ T cells after high dose adenoviral vaccine inoculations could be due to antigen persistence that exhausts CD8+ T cells, in a similar manner to that observed in LCMV chronic infections [58, 59]. An inverse correlation between vector dose and T cell response to the transgene has been observed in adenoviral vaccinations [60]. Repeated adenovirus vaccine administrations at moderate doses however do not affect CD8+ T cell memory development [61]. In the present work, sheep were immunized twice with 108 PFU of Ad5-F or Ad5-H vaccines, a low dose compared to murine T cell exhaustion studies that often used more than 109 PFU. This vaccine dose in sheep permitted CTL generation and memory differentiation of CD8+ T cells specific for PPRV-F and -H epitopes. Since CD8+ T cells limit the dissemination of the morbillivirus prototype MeV [27–30], the activation of these memory anti-PPRV CD8+ T cells could have thus contributed to sheep protection against virulent virus challenge [23]. Another study found that a single inoculation at similar concentrations of recombinant adenovirus vaccine expressing PPRV-F or -H could protect goats when challenged 12 weeks post-immunization with infectious PPRV [25]. This implies that even a moderate single dose of Ad5-F or Ad5-H is likely to induce memory T cell differentiation.
Recombinant adenovirus vaccines can also be manipulated to alter epitope hierarchy and favor the CD8+ T cell response to subdominant epitopes [62]. This strategy could be used in sheep to broaden the T cell repertoire in viral infections that narrowly focus the T cell response [63, 64]. Importantly, adenovirus vaccines can also produce inflationary CD8+ T cell memory to some epitopes [65–67]. This memory population is characterized by the enrichment in peripheral organs of functional antigen-specific CD8+ T cells at high frequency. These cells could thus constitute a first line of peripheral defense capable of swiftly responding to viral infections. We observed increased T cell responses to some PPRV-F and -H peptides after virus challenge, which could indicate that Ad5-F and Ad5-H vaccination induced inflationary memory responses to some PPRV epitopes, although further characterization of the responding T cells is necessary to confirm this observation.
Adenovirus vaccines also produce mucosal trafficking of antigen-specific CD4+ T cells [68–70]. In the present study, we detected anti-PPRV memory CD4+ T cells in PBMC after recombinant Ad5 vaccination. It would be interesting to determine in future work whether these antigen-specific experienced cells are detected in the mucosa of Ad5-F- or Ad5-H-vaccinated sheep, particularly in the oral mucosa that PPRV uses as a gateway for infection. Recombinant adenovirus vaccination has therefore the potential to produce inflationary CD8+ T cell responses to the transgene and recruit CD4+ T cells to the mucosa, both of which could contribute to Ad5-F and Ad5-H vaccine efficacy.
Overall, we identified several PPRV-F, -H and -NP T cell epitopes after PPRV infection in sheep and mice (Additional file 2). The immunogenic regions identified in the present work could be useful for monitoring T cell responses induced by novel recombinant vaccines, which potential efficacy is usually tested in murine models. This is particularly relevant in morbillivirus vaccines, in which induction of a strong T cell response is probably necessary for successful vaccination. Sheep immunization with recombinant Ad5 vaccines expressing PPRV-F or -H genes mimicked the analyzed T cell repertoire induced by PPRV infection. Ad5-F and Ad5-H vaccination induced CD4+ and CD8+ T cell memory differentiation that could be re-activated by virulent PPRV challenge. These anti-PPRV memory T cells probably contributed to Ad5-F and Ad5-H protective effects. These data validate the use of recombinant Ad5 vaccines for PPRV control. A better understanding of the T cell memory response induced by recombinant adenovirus vaccines could ultimately improve memory cell activation by these therapies.
Additional files
Additional file 1. Gating strategies for IFN-γ detection, cytotoxicity assays and CD45RO expression. (A) For IFN-γ detection, cells were selected by FSC/SSC discrimination. Gating for IFN-γ+ events was set using fluorescence minus one antibody (isotype) staining for CD4+ and CD8+ events. This gating was then maintained to measured IFN-γ+ events in stimulated cells. (B) In cytotoxicity assays, FSC/SSC discrimination was applied to gate putative live and dead cell events. Target cells labelled with the cell membrane marker PKH67 were first run on the cytometer to set up the target cell gate (PKH67+ events). Propidium iodide was used to discriminate live and dead cells. Bright PKH67+ and propidium iodide+ events were considered dead target cells. For each target cells, spontaneous and maximum cell death controls were acquired. In cytotoxicity co-culture assays, specific target cell lysis was assessed in the bright PKH67+gate. (C) For CD45RO expression, cells were first selected selected by FSC/SSC discrimination followed by CD4 or CD8 gating. Within these CD4+ or CD8+ gates, CD45RO+ gate was set using fluorescence minus one antibody (isotype) staining.
Additional file 2. PPRV T cell repertoire in mice: identification of immunoreactive PPRV-T cell epitopes in H-2 b context. To determine whether recombinant adenovirus vaccination elicits T cell responses to determinants that are also targeted during PPRV infection, we first set out to identify T cell epitopes in mice. Since few PPRV T cell epitopes have been reported [11–14], we attempted to describe new determinants in our experimental settings. We focused our approach on the F, H and NP proteins as T cell determinants involved in morbillivirus responses are usually mapped to these. Peptides predicted to bind to murine H-2b molecules (Db, Kb or I-Ab) were selected using algorithms available online (Table 1) [34–37] and synthesized. Using the TAP-deficient cell line RMA/s, we performed binding assays for MHC class I predicted binders. Most peptides bound their predicted MHC class I molecules. Only peptide NP5 did not bind to Db or Kb molecules. All 3 algorithms employed predicted Db binders quite accurately. The NetMHC prediction was nonetheless more accurate for Kb binding than ProPred-I or SYFPEITHI. PPRV-F, -H and -NP peptide immunogenicity data in C57BL/6 mice are presented in the figure of Additional file 2. PPRV peptide immunogenicity was tested on splenocytes from C57BL/6 PPRV-infected mice (IC’89; 1 × 106 PFU) using (A–C) IFN-γ ELISPOT and (D–F) proliferation assays. Responses to predicted peptides from PPRV (A and D) -F, (B and E) -H and (C and F) -NP proteins were measured in 8 mice per group. ELISPOT data are presented as average spots counted for 2 × 105 cells and proliferation as stimulation index (cpm ratio in test vs control). One-way ANOVA (Dunnett’s post-test: peptides vs control); *p < 0.05; **p < 0.01; ***p < 0.001. (A–C) Significant IFN-γ production was detected to peptides F2, F3, F7, F8, F9, F10, H2, H5, H6, H9, NP5, NP8, NP9 and NP10. (D–F) Significant splenocyte proliferation was detected to peptides F2, F7, H2, H5 and H9. Peptides F9 and F10 only tended to induce higher proliferation.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JMR, MA, EP and VM performed the experiments. JMR, NS and VM designed the study and wrote the manuscript. NS and VM directed the work. All authors read and approved the final manuscript.
Acknowledgements
The authors want to thank Lourdes Peña, Dr Héctor Moreno and Dr Félix Valcárcel for their help during sample collection. The authors wish to thank the members of the “new control strategies for relevant pathogens in animal health” laboratory for helpful discussion and comments.
Funding
MA is funded by an FPI Grant (BES-2013-066406) from the Spanish Ministerio de Economía y Competitividad. This work was funded by Grants RyC2010-06516, AGL2011-25025, AGL2012-33289, AGL2015-64290R and ADENONET-Redes de Excelencia from the Spanish Ministerio de Economía y Competitividad; and Grant S2013/ABI-2906-PLATESA from the Comunidad de Madrid and the European Union (Fondo Europeo de Desarrollo Regional, FEDER funds). Funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13567-017-0482-x) contains supplementary material, which is available to authorized users.
Contributor Information
José Manuel Rojas, Email: rojas.jose@inia.es.
Miguel Avia, Email: avia.jose@inia.es.
Elena Pascual, Email: elena.pascual@ufv.es.
Noemí Sevilla, Email: sevilla@inia.es.
Verónica Martín, Email: veronica.martin@inia.es.
References
- 1.Kumar N, Maherchandani S, Kashyap SK, Singh SV, Sharma S, Chaubey KK, Ly H. Peste des petits ruminants virus infection of small ruminants: a comprehensive review. Viruses. 2014;6:2287–2327. doi: 10.3390/v6062287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parida S, Muniraju M, Mahapatra M, Muthuchelvan D, Buczkowski H, Banyard AC. Peste des petits ruminants. Vet Microbiol. 2015;181:90–106. doi: 10.1016/j.vetmic.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh RK, Balamurugan V, Bhanuprakash V, Sen A, Saravanan P, Yadav MP. Possible control and eradication of peste des petits ruminants from India: technical aspects. Vet Ital. 2009;45:449–462. [PubMed] [Google Scholar]
- 4.Couacy-Hymann E, Bodjo C, Danho T, Libeau G, Diallo A. Evaluation of the virulence of some strains of peste-des-petits-ruminants virus (PPRV) in experimentally infected West African dwarf goats. Vet J. 2007;173:178–183. doi: 10.1016/j.tvjl.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Kumar P, Tripathi BN, Sharma AK, Kumar R, Sreenivasa BP, Singh RP, Dhar P, Bandyopadhyay SK. Pathological and immunohistochemical study of experimental peste des petits ruminants virus infection in goats. J Vet Med B Infect Dis Vet Public Health. 2004;51:153–159. doi: 10.1111/j.1439-0450.2004.00747.x. [DOI] [PubMed] [Google Scholar]
- 6.Diallo A. Control of peste des petits ruminants and poverty alleviation? J Vet Med. 2006;53:11–13. doi: 10.1111/j.1439-0450.2006.01012.x. [DOI] [Google Scholar]
- 7.Gibbs EP, Taylor WP, Lawman MJ, Bryant J. Classification of peste des petits ruminants virus as the fourth member of the genus Morbillivirus. Intervirology. 1979;11:268–274. doi: 10.1159/000149044. [DOI] [PubMed] [Google Scholar]
- 8.Rojas JM, Sevilla N, Martin V. PPRV-induced immunosuppression at the interface of virus–host interaction. Br J Virol. 2016;3:140–160. doi: 10.17582/journal.bjv/2016.3.5.140.160. [DOI] [Google Scholar]
- 9.Kumar N, Barua S, Riyesh T, Tripathi BN. Advances in peste des petits ruminants vaccines. Vet Microbiol. 2017;206:91–101. doi: 10.1016/j.vetmic.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heaney J, Barrett T, Cosby SL. Inhibition of in vitro leukocyte proliferation by morbilliviruses. J Virol. 2002;76:3579–3584. doi: 10.1128/JVI.76.7.3579-3584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinnathamby G, Renukaradhya GJ, Rajasekhar M, Nayak R, Shaila MS. Immune responses in goats to recombinant hemagglutinin-neuraminidase glycoprotein of peste des petits ruminants virus: identification of a T cell determinant. Vaccine. 2001;19:4816–4823. doi: 10.1016/S0264-410X(01)00210-9. [DOI] [PubMed] [Google Scholar]
- 12.Sinnathamby G, Seth S, Nayak R, Shaila MS. Cytotoxic T cell epitope in cattle from the attachment glycoproteins of rinderpest and peste des petits ruminants viruses. Viral Immunol. 2004;17:401–410. doi: 10.1089/vim.2004.17.401. [DOI] [PubMed] [Google Scholar]
- 13.Mitra-Kaushik S, Nayak R, Shaila MS. Identification of a cytotoxic T-cell epitope on the recombinant nucleocapsid proteins of Rinderpest and Peste des petits ruminants viruses presented as assembled nucleocapsids. Virology. 2001;279:210–220. doi: 10.1006/viro.2000.0698. [DOI] [PubMed] [Google Scholar]
- 14.Dechamma HJ, Dighe V, Kumar CA, Singh RP, Jagadish M, Kumar S. Identification of T-helper and linear B epitope in the hypervariable region of nucleocapsid protein of PPRV and its use in the development of specific antibodies to detect viral antigen. Vet Microbiol. 2006;118:201–211. doi: 10.1016/j.vetmic.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Rahman MM, Shaila MS, Gopinathan KP. Baculovirus display of fusion protein of peste des petits ruminants virus and hemagglutination protein of Rinderpest virus and immunogenicity of the displayed proteins in mouse model. Virology. 2003;317:36–49. doi: 10.1016/j.virol.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Rojas JM, Moreno H, Garcia A, Ramirez JC, Sevilla N, Martin V. Two replication-defective adenoviral vaccine vectors for the induction of immune responses to PPRV. Vaccine. 2014;32:393–400. doi: 10.1016/j.vaccine.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Liu G, Chen Z, Li C, Shi L, Li W, Huang H, Tao C, Cheng C, Xu B, Li G. Recombinant adenovirus expressing F and H fusion proteins of peste des petits ruminants virus induces both humoral and cell-mediated immune responses in goats. Vet Immunol Immunopathol. 2013;154:1–7. doi: 10.1016/j.vetimm.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Hu S, Qu L, Hu Q, Zhang Q, Zhi H, Huang K, Bu Z. A goat poxvirus-vectored peste-des-petits-ruminants vaccine induces long-lasting neutralization antibody to high levels in goats and sheep. Vaccine. 2010;28:4742–4750. doi: 10.1016/j.vaccine.2010.04.102. [DOI] [PubMed] [Google Scholar]
- 19.Caufour P, Rufael T, Lamien CE, Lancelot R, Kidane M, Awel D, Sertse T, Kwiatek O, Libeau G, Sahle M, Diallo A, Albina E. Protective efficacy of a single immunization with capripoxvirus-vectored recombinant peste des petits ruminants vaccines in presence of pre-existing immunity. Vaccine. 2014;32:3772–3779. doi: 10.1016/j.vaccine.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Diallo A, Minet C, Berhe G, Le Goff C, Black DN, Fleming M, Barrett T, Grillet C, Libeau G. Goat immune response to capripox vaccine expressing the hemagglutinin protein of peste des petits ruminants. Ann N Y Acad Sci. 2002;969:88–91. doi: 10.1111/j.1749-6632.2002.tb04356.x. [DOI] [PubMed] [Google Scholar]
- 21.Berhe G, Minet C, Le Goff C, Barrett T, Ngangnou A, Grillet C, Libeau G, Fleming M, Black DN, Diallo A. Development of a dual recombinant vaccine to protect small ruminants against peste-des-petits-ruminants virus and capripoxvirus infections. J Virol. 2003;77:1571–1577. doi: 10.1128/JVI.77.2.1571-1577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin J, Huang H, Ruan Y, Hou X, Yang S, Wang C, Huang G, Wang T, Feng N, Gao Y, Xia X. A novel recombinant peste des petits ruminants-canine adenovirus vaccine elicits long-lasting neutralizing antibody response against PPR in goats. PLoS One. 2012;7:e37170. doi: 10.1371/journal.pone.0037170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rojas JM, Moreno H, Valcarcel F, Pena L, Sevilla N, Martin V. Vaccination with recombinant adenoviruses expressing the peste des petits ruminants virus F or H proteins overcomes viral immunosuppression and induces protective immunity against PPRV challenge in sheep. PLoS One. 2014;9:e101226. doi: 10.1371/journal.pone.0101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbert R, Baron J, Batten C, Baron M, Taylor G. Recombinant adenovirus expressing the haemagglutinin of peste des petits ruminants virus (PPRV) protects goats against challenge with pathogenic virus; a DIVA vaccine for PPR. Vet Res. 2014;45:24. doi: 10.1186/1297-9716-45-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holzer B, Taylor G, Rajko-Nenow P, Hodgson S, Okoth E, Herbert R, Toye P, Baron MD. Determination of the minimum fully protective dose of adenovirus-based DIVA vaccine against peste des petits ruminants virus challenge in East African goats. Vet Res. 2016;47:20. doi: 10.1186/s13567-016-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diallo A, Minet C, Le Goff C, Berhe G, Albina E, Libeau G, Barrett T. The threat of peste des petits ruminants: progress in vaccine development for disease control. Vaccine. 2007;25:5591–5597. doi: 10.1016/j.vaccine.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 27.de Vries RD, Yuksel S, Osterhaus AD, de Swart RL. Specific CD8+ T-lymphocytes control dissemination of measles virus. Eur J Immunol. 2010;40:388–395. doi: 10.1002/eji.200939949. [DOI] [PubMed] [Google Scholar]
- 28.Mongkolsapaya J, Jaye A, Callan MF, Magnusen AF, McMichael AJ, Whittle HC. Antigen-specific expansion of cytotoxic T lymphocytes in acute measles virus infection. J Virol. 1999;73:67–71. doi: 10.1128/jvi.73.1.67-71.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Permar SR, Klumpp SA, Mansfield KG, Kim WK, Gorgone DA, Lifton MA, Williams KC, Schmitz JE, Reimann KA, Axthelm MK, Polack FP, Griffin DE, Letvin NL. Role of CD8+ lymphocytes in control and clearance of measles virus infection of rhesus monkeys. J Virol. 2003;77:4396–4400. doi: 10.1128/JVI.77.7.4396-4400.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Binnendijk RS, Poelen MC, Kuijpers KC, Osterhaus AD, Uytdehaag FG. The predominance of CD8+ T cells after infection with measles virus suggests a role for CD8+ class I MHC-restricted cytotoxic T lymphocytes (CTL) in recovery from measles. Clonal analyses of human CD8+ class I MHC-restricted CTL. J Immunol. 1990;144:2394–2399. [PubMed] [Google Scholar]
- 31.Bassiri M, Ahmad S, Giavedoni L, Jones L, Saliki JT, Mebus C, Yilma T. Immunological responses of mice and cattle to baculovirus-expressed F and H proteins of rinderpest virus: lack of protection in the presence of neutralizing antibody. J Virol. 1993;67:1255–1261. doi: 10.1128/jvi.67.3.1255-1261.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rojas JM, Rodriguez-Calvo T, Pena L, Sevilla N. T cell responses to bluetongue virus are directed against multiple and identical CD4+ and CD8+ T cell epitopes from the VP7 core protein in mouse and sheep. Vaccine. 2011;29:6848–6857. doi: 10.1016/j.vaccine.2011.07.061. [DOI] [PubMed] [Google Scholar]
- 33.Rojas JM, Pena L, Martin V, Sevilla N. Ovine and murine T cell epitopes from the non-structural protein 1 (NS1) of bluetongue virus serotype 8 (BTV-8) are shared among viral serotypes. Vet Res. 2014;45:30. doi: 10.1186/1297-9716-45-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 35.Singh H, Raghava GP. ProPred1: prediction of promiscuous MHC class-I binding sites. Bioinformatics. 2003;19:1009–1014. doi: 10.1093/bioinformatics/btg108. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen M, Lundegaard C, Worning P, Lauemoller SL, Lamberth K, Buus S, Brunak S, Lund O. Reliable prediction of T-cell epitopes using neural networks with novel sequence representations. Protein Sci. 2003;12:1007–1017. doi: 10.1110/ps.0239403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen M, Lund O. NN-align. An artificial neural network-based alignment algorithm for MHC class II peptide binding prediction. BMC Bioinform. 2009;10:296. doi: 10.1186/1471-2105-10-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Partidos CD, Steward MW. Prediction and identification of a T cell epitope in the fusion protein of measles virus immunodominant in mice and humans. J Gen Virol. 1990;71:2099–2105. doi: 10.1099/0022-1317-71-9-2099. [DOI] [PubMed] [Google Scholar]
- 39.Obeid OE, Partidos CD, Howard CR, Steward MW. Protection against morbillivirus-induced encephalitis by immunization with a rationally designed synthetic peptide vaccine containing B- and T-cell epitopes from the fusion protein of measles virus. J Virol. 1995;69:1420–1428. doi: 10.1128/jvi.69.3.1420-1428.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang W, Caspell R, Karulin AY, Ahmad M, Haicheur N, Abdelsalam A, Johannesen K, Vignard V, Dudzik P, Georgakopoulou K, Mihaylova A, Silina K, Aptsiauri N, Adams V, Lehmann PV, McArdle S. ELISPOT assays provide reproducible results among different laboratories for T-cell immune monitoring—even in hands of ELISPOT-inexperienced investigators. J Immunotoxicol. 2009;6:227–234. doi: 10.3109/15476910903317546. [DOI] [PubMed] [Google Scholar]
- 41.Firat H, Garcia-Pons F, Tourdot S, Pascolo S, Scardino A, Garcia Z, Michel ML, Jack RW, Jung G, Kosmatopoulos K, Mateo L, Suhrbier A, Lemonnier FA, Langlade-Demoyen P. H-2 class I knockout, HLA-A2.1-transgenic mice: a versatile animal model for preclinical evaluation of antitumor immunotherapeutic strategies. Eur J Immunol. 1999;29:3112–3121. doi: 10.1002/(SICI)1521-4141(199910)29:10<3112::AID-IMMU3112>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 42.Rojas JM, Spada R, Sanz-Ortega L, Morillas L, Mejias R, Mulens-Arias V, Perez-Yague S, Barber DF. PI3K p85 beta regulatory subunit deficiency does not affect NK cell differentiation and increases NKG2D-mediated activation. J Leukoc Biol. 2016;100:1285–1296. doi: 10.1189/jlb.1A1215-541RR. [DOI] [PubMed] [Google Scholar]
- 43.Blunt L, Hogarth PJ, Kaveh DA, Webb P, Villarreal-Ramos B, Vordermeier HM. Phenotypic characterization of bovine memory cells responding to mycobacteria in IFNgamma enzyme linked immunospot assays. Vaccine. 2015;33:7276–7282. doi: 10.1016/j.vaccine.2015.10.113. [DOI] [PubMed] [Google Scholar]
- 44.Hogg AE, Parsons K, Taylor G, Worth A, Beverley P, Howard CJ, Villarreal-Ramos B. Characterization of age-related changes in bovine CD8+ T-cells. Vet Immunol Immunopathol. 2011;140:47–54. doi: 10.1016/j.vetimm.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Iacobelli-Martinez M, Nemerow GR. Preferential activation of Toll-like receptor nine by CD46-utilizing adenoviruses. J Virol. 2007;81:1305–1312. doi: 10.1128/JVI.01926-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basner-Tschakarjan E, Gaffal E, O’Keeffe M, Tormo D, Limmer A, Wagner H, Hochrein H, Tuting T. Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-alpha production. J Gene Med. 2006;8:1300–1306. doi: 10.1002/jgm.964. [DOI] [PubMed] [Google Scholar]
- 47.Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam E, Stein S, Falck-Pedersen E. Adenovirus detection by the cGAS/STING/TBK1 DNA sensing cascade. J Virol. 2014;88:974–981. doi: 10.1128/JVI.02702-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geutskens SB, van der Eb MM, Plomp AC, Jonges LE, Cramer SJ, Ensink NG, Kuppen PJ, Hoeben RC. Recombinant adenoviral vectors have adjuvant activity and stimulate T cell responses against tumor cells. Gene Ther. 2000;7:1410–1416. doi: 10.1038/sj.gt.3301251. [DOI] [PubMed] [Google Scholar]
- 50.Olive M, Eisenlohr L, Flomenberg N, Hsu S, Flomenberg P. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum Gene Ther. 2002;13:1167–1178. doi: 10.1089/104303402320138952. [DOI] [PubMed] [Google Scholar]
- 51.Onion D, Crompton LJ, Milligan DW, Moss PA, Lee SP, Mautner V. The CD4+ T-cell response to adenovirus is focused against conserved residues within the hexon protein. J Gen Virol. 2007;88:2417–2425. doi: 10.1099/vir.0.82867-0. [DOI] [PubMed] [Google Scholar]
- 52.Yang TC, Dayball K, Wan YH, Bramson J. Detailed analysis of the CD8+ T-cell response following adenovirus vaccination. J Virol. 2003;77:13407–13411. doi: 10.1128/JVI.77.24.13407-13411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leen AM, Sili U, Vanin EF, Jewell AM, Xie W, Vignali D, Piedra PA, Brenner MK, Rooney CM. Conserved CTL epitopes on the adenovirus hexon protein expand subgroup cross-reactive and subgroup-specific CD8+ T cells. Blood. 2004;104:2432–2440. doi: 10.1182/blood-2004-02-0646. [DOI] [PubMed] [Google Scholar]
- 54.Bradley RR, Lynch DM, Iampietro MJ, Borducchi EN, Barouch DH. Adenovirus serotype 5 neutralizing antibodies target both hexon and fiber following vaccination and natural infection. J Virol. 2012;86:625–629. doi: 10.1128/JVI.06254-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holst PJ, Orskov C, Thomsen AR, Christensen JP. Quality of the transgene-specific CD8+ T cell response induced by adenoviral vector immunization is critically influenced by virus dose and route of vaccination. J Immunol. 2010;184:4431–4439. doi: 10.4049/jimmunol.0900537. [DOI] [PubMed] [Google Scholar]
- 56.Zhou D, Wu TL, Emmer KL, Kurupati R, Tuyishime S, Li Y, Giles-Davis W, Zhou X, Xiang Z, Liu Q, Ratcliffe SJ, Ertl HC. Hexon-modified recombinant E1-deleted adenovirus vectors as dual specificity vaccine carriers for influenza virus. Mol Ther. 2013;21:696–706. doi: 10.1038/mt.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suleman M, Galea S, Gavard F, Merillon N, Klonjkowski B, Tartour E, Richardson J. Antigen encoded by vaccine vectors derived from human adenovirus serotype 5 is preferentially presented to CD8+ T lymphocytes by the CD8α+ dendritic cell subset. Vaccine. 2011;29:5892–5903. doi: 10.1016/j.vaccine.2011.06.071. [DOI] [PubMed] [Google Scholar]
- 58.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 59.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 61.Steffensen MA, Holst PJ, Steengaard SS, Jensen BA, Bartholdy C, Stryhn A, Christensen JP, Thomsen AR. Qualitative and quantitative analysis of adenovirus type 5 vector-induced memory CD8 T cells: not as bad as their reputation. J Virol. 2013;87:6283–6295. doi: 10.1128/JVI.00465-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holst PJ, Jensen BA, Ragonnaud E, Thomsen AR, Christensen JP. Targeting of non-dominant antigens as a vaccine strategy to broaden T-cell responses during chronic viral infection. PLoS One. 2015;10:e0117242. doi: 10.1371/journal.pone.0117242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin V, Pascual E, Avia M, Pena L, Valcarcel F, Sevilla N. Protective efficacy in sheep of adenovirus-vectored vaccines against bluetongue virus is associated with specific T cell responses. PLoS One. 2015;10:e0143273. doi: 10.1371/journal.pone.0143273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rojas JM, Rodriguez-Calvo T, Sevilla N. Recall T cell responses to bluetongue virus produce a narrowing of the T cell repertoire. Vet Res. 2017;48:38. doi: 10.1186/s13567-017-0444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bolinger B, Sims S, O’Hara G, de Lara C, Tchilian E, Firner S, Engeler D, Ludewig B, Klenerman P. A new model for CD8+ T cell memory inflation based upon a recombinant adenoviral vector. J Immunol. 2013;190:4162–4174. doi: 10.4049/jimmunol.1202665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bolinger B, Sims S, Swadling L, O’Hara G, de Lara C, Baban D, Saghal N, Lee LN, Marchi E, Davis M, Newell E, Capone S, Folgori A, Barnes E, Klenerman P. Adenoviral vector vaccination induces a conserved program of CD8+ T cell memory differentiation in mouse and man. Cell Rep. 2015;13:1578–1588. doi: 10.1016/j.celrep.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colston JM, Bolinger B, Cottingham MG, Gilbert S, Klenerman P. Modification of antigen impacts on memory quality after adenovirus vaccination. J Immunol. 2016;196:3354–3363. doi: 10.4049/jimmunol.1502687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benlahrech A, Harris J, Meiser A, Papagatsias T, Hornig J, Hayes P, Lieber A, Athanasopoulos T, Bachy V, Csomor E, Daniels R, Fisher K, Gotch F, Seymour L, Logan K, Barbagallo R, Klavinskis L, Dickson G, Patterson S. Adenovirus vector vaccination induces expansion of memory CD4 T cells with a mucosal homing phenotype that are readily susceptible to HIV-1. Proc Natl Acad Sci USA. 2009;106:19940–19945. doi: 10.1073/pnas.0907898106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masek-Hammerman K, Li H, Liu J, Abbink P, La Porte A, O’Brien KL, Whitney JB, Carville A, Mansfield KG, Barouch DH. Mucosal trafficking of vector-specific CD4+ T lymphocytes following vaccination of rhesus monkeys with adenovirus serotype 5. J Virol. 2010;84:9810–9816. doi: 10.1128/JVI.01157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bukh I, Calcedo R, Roy S, Carnathan DG, Grant R, Qin Q, Boyd S, Ratcliffe SJ, Veeder CL, Bellamy SL, Betts MR, Wilson JM. Increased mucosal CD4+ T cell activation in rhesus macaques following vaccination with an adenoviral vector. J Virol. 2014;88:8468–8478. doi: 10.1128/JVI.03850-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Gating strategies for IFN-γ detection, cytotoxicity assays and CD45RO expression. (A) For IFN-γ detection, cells were selected by FSC/SSC discrimination. Gating for IFN-γ+ events was set using fluorescence minus one antibody (isotype) staining for CD4+ and CD8+ events. This gating was then maintained to measured IFN-γ+ events in stimulated cells. (B) In cytotoxicity assays, FSC/SSC discrimination was applied to gate putative live and dead cell events. Target cells labelled with the cell membrane marker PKH67 were first run on the cytometer to set up the target cell gate (PKH67+ events). Propidium iodide was used to discriminate live and dead cells. Bright PKH67+ and propidium iodide+ events were considered dead target cells. For each target cells, spontaneous and maximum cell death controls were acquired. In cytotoxicity co-culture assays, specific target cell lysis was assessed in the bright PKH67+gate. (C) For CD45RO expression, cells were first selected selected by FSC/SSC discrimination followed by CD4 or CD8 gating. Within these CD4+ or CD8+ gates, CD45RO+ gate was set using fluorescence minus one antibody (isotype) staining.
Additional file 2. PPRV T cell repertoire in mice: identification of immunoreactive PPRV-T cell epitopes in H-2 b context. To determine whether recombinant adenovirus vaccination elicits T cell responses to determinants that are also targeted during PPRV infection, we first set out to identify T cell epitopes in mice. Since few PPRV T cell epitopes have been reported [11–14], we attempted to describe new determinants in our experimental settings. We focused our approach on the F, H and NP proteins as T cell determinants involved in morbillivirus responses are usually mapped to these. Peptides predicted to bind to murine H-2b molecules (Db, Kb or I-Ab) were selected using algorithms available online (Table 1) [34–37] and synthesized. Using the TAP-deficient cell line RMA/s, we performed binding assays for MHC class I predicted binders. Most peptides bound their predicted MHC class I molecules. Only peptide NP5 did not bind to Db or Kb molecules. All 3 algorithms employed predicted Db binders quite accurately. The NetMHC prediction was nonetheless more accurate for Kb binding than ProPred-I or SYFPEITHI. PPRV-F, -H and -NP peptide immunogenicity data in C57BL/6 mice are presented in the figure of Additional file 2. PPRV peptide immunogenicity was tested on splenocytes from C57BL/6 PPRV-infected mice (IC’89; 1 × 106 PFU) using (A–C) IFN-γ ELISPOT and (D–F) proliferation assays. Responses to predicted peptides from PPRV (A and D) -F, (B and E) -H and (C and F) -NP proteins were measured in 8 mice per group. ELISPOT data are presented as average spots counted for 2 × 105 cells and proliferation as stimulation index (cpm ratio in test vs control). One-way ANOVA (Dunnett’s post-test: peptides vs control); *p < 0.05; **p < 0.01; ***p < 0.001. (A–C) Significant IFN-γ production was detected to peptides F2, F3, F7, F8, F9, F10, H2, H5, H6, H9, NP5, NP8, NP9 and NP10. (D–F) Significant splenocyte proliferation was detected to peptides F2, F7, H2, H5 and H9. Peptides F9 and F10 only tended to induce higher proliferation.


