Abstract
Introduction:
Incidence and mortality rate of cancer is increasing in all countries including low and middle-income countries. Hospital based cancer registry (HBCR) is an important tool for administration purpose and improvement of the quality of care. It is also important resource for population based cancer registries. In this study we reviewed HBCRs in different countries.
Methods:
We searched the published literature using the MEDLINE (PubMed), Google scholar, Scopus, ProQuest and Google. We also reviewed websites of the HBCRs in different countries. The search was carried out based on proper keywords in English for all motor engines including “hospital-based”, “clinical” and “data quality” combined with “registry”, “cancer” and “tumor” including all subheadings. We reviewed objectives, developer institutions, minimum datasets, data sources, quality control indicators and processes.
Results:
In total we found 163 papers in the first step. After screening of the titles, abstracts and the full texts, 14 papers remained for analysis. Analysis of the 14 papers showed that the improvement of the quality of the care were the most important objectives among the registries. HBCRs collect information about patients, tumor diagnosis, treatment and follow-up. Generally, indicators such as completeness and validity were used for quality control.
Conclusion:
Because of the increases in cancer burden in the world, more attention is needed to be paid on cancer surveillance systems, including HBCRs. We evaluated and highlighted the importance and characteristics HBCRs and believe that this paper would help the hospitals and policy makers for planning and establishment of new HBCRs. We suggest the establishment of a worldwide network for coordination and collaboration between HBCRs.
Keywords: Hospital-based registry, cancer, tumor, quality of care, registry
Introduction
Incidence and mortality rates of non-communicable diseases have increased remarkably in high and middle-income countries (Rouhollahi et al., 2014; Vineis and Wild, 2014). Cancer is a non- communicable disease, which has more dramatic prevalence rather than others (Torre et al., 2015; Siegel et al., 2016). Recent reports have indicated that global burden of cancers is increasing. According to the Golobocan (2012), there were 14.1 million new cancer cases, 8.2 million cancer deaths and five-year prevalence of 32.6 million patients living with cancer worldwide. Almost 57% (8 million) of new cancer cases, 65% (5.3 million) of the cancer deaths and 48% (15.6 million) of the 5-year prevalent cancer cases occurred in the less developed regions (Globocan, 2012).
Since mortality to incidence ratio is high for several cancer types, especially in the low and middle-income countries, early detection and improvement of cancer care are necessary to decrease the burden of cancer (Smith et al., 2001; Goss et al., 2013). Appropriate methods for diagnosis and treatment of cancer are one of the effective factors in cancer control (Organization, 2007). There are several guidelines about diagnosis and treatment of cancer including clinical practice guidelines provided by of the National Comprehensive Cancer Network (NCCN) (Mock et al., 2000), the National Institute for Health and Care Excellence (NICE) (Claxton et al., 2015), the European Society For Medical Oncology (ESMO) Guidelines (Stoffel et al., 2014), the American Society of Clinical Oncology (ASCO) (Somerfield et al., 2008) and etc. However different institutions may choose from these guidelines to provide diagnosis and treatment services for cancer patients (Kiel, 2011). In addition, they may also adapt them according to the local situation and evidences (Heins et al., 2016). HBCRs are important tool to evaluate the implementation of the guidelines and also study treatment outcome for the selected protocols and provide evidence for changes and updates of the treatment protocols (Ruiz and Facio, 2004).
Also success in cancer control programs requires strong surveillance and information systems (Mohammadzadeh et al., 2013; Ryerson and Massetti, 2017). Population based Cancer Registry (PBCR) collects cancer-related information in cancer patients within a target population for epidemiological goals (Bray et al., 2014). These help to estimate the worldwide surveillance of cancer survival and international comparison of cancer trends and as a result cancer control (Allemani et al., 2015). International Agency for Research on Cancer (IARC) supports establishment of the PBCRs in different countries and provide technical support. Data from high quality PBCRs are published in the IARC monograph titled “Cancer in Five Continents” and the 10th edition was published in 2013 (Ferlay et al., 2016). On the other hands, hospital-based cancer registries (HBCRs) that collect administration and clinical data are used for administration purpose and improvement of the quality of care. In addition, HBCRs addresses administrating challenges in the cancer hospitals (Young, 1991; Ekanem and Island, 2009). HBCRs also provides essential and effective information for tracking trends of cancer care plans over time and patterns of care in different institutions However, HBCRs are usually expensive and do not receive enough support from IARC or other international communities (SEER; Goldman et al., 2006; Shiki et al., 2008).
HBCR is an important tool for monitoring and improving the quality of cancer care, however, the experience and information on the methods and approach for establishment of HBCRs are limited. Therefore, we performed a systematic review of the literature to fill this gap and provide information on the characteristics, potential and weaknesses of HBCRs through.
Materials and Methods
Methods
Search strategy
This study is a systematic review; Authors searched the documents and reports of HBCRs using the MEDLINE (PubMed) during 1989 to 2017, Google scholar, Scopus, ProQuest and Google (the first 10 pages). The search was done based on keywords in English for all motor engines including “hospital-based”, “clinical” and “data quality” combined with “registry”, “cancer” and “tumor” including all subheadings. All keywords searched electronically by three Boolean operators with explained search strategy separately. After a complete search, all search results reviewed separately based on title or running title of studies and related documents were selected and then the duplicated documents were excluded. Finally, 14 documents were selected for survey.
Inclusion criteria
All documents and reports were included, if they were related to a hospital-based cancer registry and provided details about the characteristics of their program.
The limitation of our research was that number of publications about HBCRs was very low. Therefore, we tried to obtain our necessary information from various sources.
In addition, authors only studied the documents that were available in English language. Studies that published in other languages were not included in this study.
Evaluation
Two reviewers reviewed all documents separately. After excluding those reports that did not meet the inclusion criteria, the data from the review entered in to the data collection forms. Only documents related to hospital-based cancer registries were selected. The disagreements between reviewers were addressed by group discussions. Some data such as country names, name of registries, responsible institutes, objectives of registry, minimum dataset, data sources, quality indicator and quality control methods were determined.
Results
In this review, 653 documents were initially included. 542 documents were excluded after title and content reviewing and 89 documents were excluded after assessing for eligibility criteria. Finally, 14 documents were remained for analyses (Figure 1). Table 1 and 2 show the main characteristics of cancer registry systems obtained from reviewing all of the 14 documents.
Figure 1.
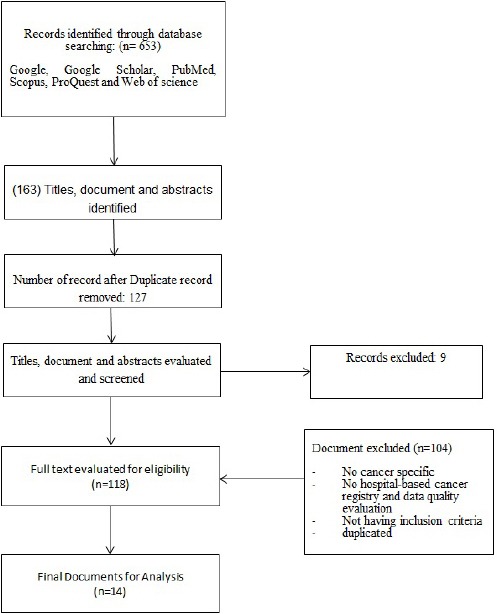
Screening Process of Documents That Included in This Study
Table 1.
Characteristics Of Hospital-Based Cancer Registries In Different Countries
| Registry, Country | Responsible institution | Objectives | Data sources | Software |
|---|---|---|---|---|
| National Cancer Database (NCDB) (1800 Hospitals), USA (Sergeons, 1996-2017) | American College of Surgeons and the American Cancer Society | Community Assessment, Quality Improvement, Cancer Program Administration | Data that abstracted from patient charts by Certified Tumor Registrars (CTR) | Abstract Plus |
| HBCRs in (397 hospital), Japan (Higashi et al., 2013; Anazawa et al., 2015) | National Cancer Center | Quality of care improvement, Monitoring Cancer Control program, Research | Medical records, Pathologic reports, Discharge summaries, Diagnostic codes on insurance claims, Chemotherapy records, Surgery records | HosCanR |
| Clinical Cancer Registry, Australia (Health, 2014) | Cancer Institute NSW | Quality of care improvement | Patient Administration System, Pathology, Scheduling, Medical Oncology Electronic Medical Records (EMR), Radiation Oncology EMR, Palliative Care EMR | NA |
| Khon Kaen HBCR (KKCR), Thailand (University, 2013) | Srinagarind Hospital, Khon Kaen University, Faculty of Medicine | Planning and monitoring of cancer control, Research | Medical records, Pathology laboratory records, Surgery and Radiology department. Death certificate | CanReg4 |
| Brazil SisRHC database (Pinheiro Zina Reis et al., 2008) | The National Cancer Institute (INCA) | Prevention, Screening, Treatment, Survival, and Palliation | Pathology reports, Hospital discharge summaries, Radiotherapy records and hematological reports. | SisRHC |
| Healthcare Quality Registries, Sweden (Emilsson et al., 2015) | The Swedish government and the Swedish Association of Local Authorities and Regions (SALAR) | Improving delivery of healthcare, Quality improvement, Monitoring of adherence to guidelines, Research. | Medical records, Laboratory test, Pathology report | NA |
| HBCRs (19 centres), Nigeria (Jedy-Agba Elima E. et al., 2012) | The Institute of Human Virology, Nigeria and the Nigerian Federal Ministry of Health | Improvement of cancer care delivery systems, Policy making, Planning | Medical record, Death certificates, Radiotherapy, Chemotherapy and Surgery reports | CanReg4 |
| Hacettepe HBCR, Turkey (Kutluk et al., 2013) | Hacettepe University | Planning, Monitoring, and measuring cancer-related services, Research, and Education. | Information that gathered from departments related to care of cancer patients | CanReg4 |
| HBCRs, India (Swaminathan Soumya, 2016) | Indian Council of Medical Research (ICMR). | Planning and evaluation of cancer control programs | Medical records | Web-based software “rccintranet.org”. |
| HBCR, Indonesia (Sibuea et al., 2000) | National Center General Hospital (RSUPNCM) | Provide statistical information for cancer control and management | Predefined form that filled by physician | NA |
| HBCRs (13 hospitals), Colombia (Cuervo Luis Gabriel et al., 1999) | National Cancer Institute of Colombia | NA | Collection of data from a large group of cancer diagnostic and treatment centers | Regiscan database software |
| Jinnah Hospital HBCR, Pakistan (Aziz et al., 2003) | Jinnah Hospital Lahore | NA | Medical records | Microsoft Access and Excel Database |
| HBCR, United States (Hendren et al., 2014) | University of Michigan | Comparison of processes and outcomes, identification of areas for Quality improvement. | Medical Records | NA |
| Fortis Memorial Research Institute (FMRI-HBCR), India (Institue, 2013) | Fortis Memorial Research Institute (FMRI) | Medical Researc | Information gathered through pre-devised questionnaire in hospital oncology departments | Microsoft Excel Datasheet |
Table 2.
Objectives of Hospital Based Cancer Registry (HBCR)
| Administration |
| 1. To improve hospital management (Base), 2017) |
| 2. Improving reimbursement plan (Base), 2017) |
| 3. To Identify areas to market programs through demographic variables that define gender, race/ethnicity, age group, and education level (Swan et al., 1998; Base), 2017) |
| Improving Quality Care |
| 4. To study survival rates by cancer types and stage (Base), 2017) |
| 5. To study short and long-term side effect of different treatment (Evelyn M. Shambaugh et al., 1999; Shiki et al., 2008) |
| 6. To evaluate prognostic factor (Shiki et al., 2008) |
| 7. To study implantation of clinical practice guideline (Ruiz and Facio, 2004) |
| 8. To enhance coordination of care and multidisciplinary tumor board (Swan et al., 1998) |
| 9. To demonstrate accountable, evidence based care at the local level (Higashi et al., 2013) |
| 10. To identify areas to focus quality improvement efforts (Shiki et al., 2008; Base), 2017) 11. To be the basis for clinical research (Ruiz and Facio, 2004) |
| 12. Education of residents and fellows (SEER) |
| Community Based objectives |
| 13. To evaluate delay in the diagnosis (Ruiz and Facio, 2004; Shiki et al., 2008) |
| 14. To study Screening program and evaluate proportion of the screened cases by cancer site, age group, ethnicity/race (Ruiz and Facio, 2004) |
| 15. To develop cancer prevention program if population based registry does not exist (Jedy-Agba Elima E. et al., 2012) |
| 16. Support population based cancer registry (PBCR) (Swan et al., 1998; Evelyn M. Shambaugh et al., 1999; Shiki et al., 2008) |
| 17. To provide some idea of cancer incidence and prevalence if PBCR is lacking in the region (Aziz et al., 2003) |
| 18. Provide information for cancer control program and cancer prevention in particular (Young, 1991) |
| 19. To study potential area of outreach and patient navigation (Base), 2017) |
| 20. To develop treatment practice based on the population need (Evelyn M. Shambaugh et al., 1999) |
| 21. To study pattern of care and evaluate variation of access to care (Ruiz and Facio, 2004; Base), 2017) |
| 22. Performing etiologic/ epidemiological research (Ruiz and Facio, 2004) |
Characteristics of HBCRs
HBCRs are applied for different purposes. Results of this study showed that objectives of most studied HBCRs were management of cancer control programs and improving quality of care. However, other purposes of HBCRs include epidemiological and clinical research, education, policy making, evaluation of implantation of clinical practice guidelines, planning and monitoring of cancer control programs, including prevention, screening, treatment and palliative care (Table 1). Table 2 provides an extensive list of objectives for HBCR in three categories of administration, improving quality of care and community based objectives.
Quality control methods of HBCRs
Although the details of quality control process and methods were not defined, level and methods of quality control were varied in different registry programs. HBCRs used both automated and manual methods for quality control (Table 3). Validity and completeness were the main quality indicators that surveyed by this registries. Validity stands for valid value of the data items and consistency checks, including comparing the values of certain variables against others. Completeness was registration of all cases referred to the hospital for diagnosis and treatment.
Table 3.
Quality Control Methods in Hospital-Based Cancer Registries
| Name of registry | Quality control methods |
|---|---|
| NCDB, USA (Sergeons, 1996-2017; Bilimoria et al., 2008) | 1) NCDB GenEDITS Plus software (2017) include all edits and all data that submitted to database, 2) Case records that fail to meet a standardized set of requirements are identified and returned to the hospital, 3) Check each hospital data by Commission on Cancer surveyors once every three years to ensure of data quality |
| HBCR, Japan (Higashi et al., 2013; Anazawa et al., 2015) | 1) Appropriate training of tumor registrars, 2) Consistency-checking software with HosCanR, 3) Additional support provided by the National Cancer Center staff. |
| The SSWAHS Clinical Registry, Australia (Health, 2014) | Not reported |
| KKCR, Thailand (University, 2013) | Linkage with national statistical data set, personal contact, and networking |
| SisRHC database, Brazil, (Pinheiro Zina Reis et al., 2008) | 1) Entering validated data through software, 2) Internal quality control systems, 3) logical checks through registry program, 4) Visual checks |
| Healthcare Quality Registries, Sweden (Emilsson et al., 2015) | 1) Automated checks to prevent the input of incorrect data, 2) linkage and compare data with government administrated registries, 3) comparison with patient charts, 4) manual check with health care personals |
| Michigan HBCR, USA (Hendren et al., 2014) | Comparing hospital medical record and tumor registry data. |
| 19 HBCRs, Nigeria (Jedy-Agba Elima E. et al., 2012) | Internal consistency ckeck between variables by software |
| Hacettepe HBCR, Turkey (Kutluk et al., 2013) | 1) Reviewing by research assistant to check accuracy of gender, age, histologic and morphologic diagnosis of the patients according to ICD-O, 2) Crosschecks by research assistant to increase the consistency of the database, 3) Revise and record of inaccurate data |
| India: National HBCRs program (Swaminathan Soumya, 2016) | 1) Consistency checks (comparing the values of certain variables against others) by software, 2) The various range, consistency, unlikely combinations, and duplicate checks by the coordinating unit of the National Cancer Registry Programme of Indian Council of Medical Research |
| Colombia (Cuervo Luis Gabriel et al., 1999) | Questionnaire that developed with Delphi method surveyed the function of the hospital director, the registry coordinator, and the registrar (data manager) about data quality. |
| FMRI, India (Institue, 2013) | Validation of data by using quality control programs/tools of International Agency for Research on Cancer (IARC) for avoiding duplication and any unlikely combination of age, sex, site and morphology and other factors in the database. |
Among the others, the National Cancer Database (NCDB) that is a joint program of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society was the most comprehensive and largest program (Sergeons, 1996-2017). It is a nationwide database containing approximately 34 million records from more than 1800 hospital cancer registries. NCDB has different objectives, including Community Assessment, Quality Improvement, and Cancer Program Administration. Its data included patient characteristics, cancer staging and tumor histological characteristics, type of first-course treatment administered and outcomes information. The NCDB does provide some web-based applications to data registry and quality control of cancer data and cancer care. While “Abstract Plus” is software that usually used for registry and “GenEdit Plus” is applied for validity checks and quality control measures (Sergeons, 1996-2017). Abstract Plus allows for registration of an extensive list of data items (more than 80) on patients identification, tumor diagnosis, stage and prognostic factors, different treatment types (i.e. surgery, chemotherapy, radiotherapy), and follow-up (Table 4) ((NPCR), 2016).
Table 4.
List of Data Items (N=83) Used in the Abstract Plus Software for HBCR in the US to Be Submitted to the National Cancer Data Base (NCDB)
| Patient ID (N=10) | Name-Last, Name-First, Name-Middle, Name-Maiden, Name-Alias, Social Security Number, Address at DX (No Street, Supplemental, City, state, Postal code), County at Dx, Race, Spanish/Hispanic Origin |
| Demographic information (N=6) | Date Of Birth, Birth place (state-county), Sex, Text-usual occupation, Occupation source, Text-usual industry, Industry source |
| Cancer identification (N=10) | Date of diagnosis, Age at diagnosis, Primary site, laterality, histologic type ICD-O-3, Behavior Code ICD-O-3, Grade, Grade path value, Grade path system, Diagnostic Confirmation |
| Stage/ prognostic factors (N=22) | Tumor size, Tumor extension, Tumor size/extension evaluation, Lymph nodes, Lymph nodes evaluation, Regional nodes positive, Regional nodes examined, Lymph-vascular Invasion, Metastasis at diagnosis, Metastasis evaluation, Site-specific factor 1- 25, Derived AJCC 6-T, Derived AJCC 6-N, Derived AJCC 6-M, Derived AJCC 6-stage group, Derived AJCC 7-T, Derived AJCC 7-N, Derived AJCC 7-M, Derived AJCC 7-stage group, |
| Hospital Specific (N=7) | Reporting facility, Type of reporting source, Medical record number, Primary payer at DX, Sequence number-hospital, Date of 1st contact, Class of case, |
| Treatment-1st course (20) | Treatment status, Date of 1st course, Date surgery, Summary-surgery primary site, Reason for no surgery, Date radiation, Radiation regional modality, Reason for no radiation, Surgery/radiation sequence, Date chemotherapy, Summary-chemotherapy, Date hormone, Summary-hormone, Date-BRM, Date other, Summary other, Summary-scope regional lymph node surgery, Surgery other regional, Summary-transplant/endocrine, Summary-systemic/surgery sequence |
| Follow-up /recurrence /death (N=8) | Date of last contact, Vital status, Follow-up source, Cause of death, ICD revision number, Place of death-state, Place of death-country, Physician-follow up, NPI-physician-follow-up |
In conclusion, the basic components of cancer control are prevention, early detection, diagnosis and treatment, and palliative care. Note that, diagnosis, treatment and care of cancer impose large cost to the community (Organization, 2007). HBCRs are important resource for planning and monitoring of cancer control program. In particular they play important role for the improvement of quality of care of cancer patients. In this study, authors tried to perform a systematic review of literature and retrieved information for 14 selected HBCRs. We found that improving quality of care were the most important platform to abstract high quality information about cancer patients, tumors and diagnosis, stage and prognostic factors, treatment and follow-up (Bhurgri, 2004; Ruiz and Facio, 2004; Bray and Parkin, 2009; Messenger et al., 2012; Posada and del Otero, 2014). Although the minimum data set for HBCRs was almost similar in different registries and covered all aspects of quality of care, the number of data items varied from country to country. Most registries applied either manual or electronic procedures for quality control of HBCR (Teppo et al., 1994; Shin et al., 2004; Pezzi, 2014), including training of the registrars, developing appropriate registration manuals, consistency checks by software performing surveys and serious supervision of the registry processes.
The limitation of our research was that number of publications about HBCRs was very low, and there was no standard recommendation for reporting the results from a HBCR. Therefore we tried to obtain our necessary information from various peer reviewed articles (Cuervo Luis Gabriel et al., 1999; Sibuea et al., 2000; Bhurgri, 2004; Jedy-Agba Elima E. et al., 2012; Higashi et al., 2013; Kutluk et al., 2013; Hendren et al., 2014; Anazawa et al., 2015; Emilsson et al., 2015), reports (Pinheiro Zina Reis et al., 2008; University, 2013; Swaminathan Soumya, 2016) and web sites (Sergeons, 1996-2017; Institue, 2013; Health, 2014). In addition, authors only studied the papers/documents that were available only in English language. International collaboration between HBCRs may increase the understanding about these programs and also improve the standards of reporting the results from HBCRs in the international peer reviewed journals.
Unlike PBCRs that are used for epidemiological studies and public health surveillance, the most important aims of HBCRs are improvement of quality of care and addressing administrating challenges in hospitals (Young, 1991; Ruiz and Facio, 2004). Although some registries did not mention “improving quality of care” in their objectives and highlighted other objectives in their program, including the monitoring of cancer control program, research and education (Cuervo Luis Gabriel et al., 1999; Aziz et al., 2003; Pinheiro Zina Reis et al., 2008; Institue, 2013; Kutluk et al., 2013), improvement of quality of care seems to be inherent objective of all HBCRs.
One of the important issues in registry programs is the number of variables and data items that are defined (Zachary et al., 2015). The tendency is to choose the least, but necessary information in each registry (Zachary et al., 2015). While the number of variables in the PBCRs are limited and hardly reach to 20 variables ((IARC)), the HBCRs dataset is a bit more extensive as it should cover clinical and administrative details, including patients data, administrative information, diagnosis and tumor information, treatment and care, and follow-up (Surgeons, 2016). Therefore, number of variables increase to more than 50-100 variables (Surgeons, 2016). We reported that the number of minimal data set in the Abstract Plus software, which is used in the US for establishment of the National Cancer Database (NCDB) was more than 80 data items ((NPCR), 2016). The large number of variables in different aspect of diagnosis and treatment create several issues. First, the detail information for all of the variables is not usually available in the patient charts. Second, the registrar should pass appropriate training to be able understand and abstract the information from medical records. Furthermore, the registration processes would be timely and would increase the workload and budget of the registry. Using electronic medical records have recently improved the efficiency of the registry program (Houser et al., 2012).
To reach the HBCRs objective(s), it would be important to make sure about quality of data and results from the registry program. Therefore, quality control measures should be inevitable part and be integrated in the HBCR program (Kim et al., 2010). Consistency checks by the registry software or linkage of different data sources would improve the validity and completeness of the registry program. (Tagliabue et al., 2006). HBCRs with high data quality can be used for administrating and clinical aspects including: resource allocation, planning and policymaking in the area of cancer care and cancer control program (Jedy-Agba Elima E. et al., 2012). Therefore, managers of the cancer hospitals should prioritize improvement of data quality through training of the registrars, developing appropriate registration manuals, control of the inconsistencies between different data, using appropriate software, and serious supervision of the registry processes. While quality control solutions are available for population-based cancer registries; there are no similar programs for HBCRs (Ruiz and Facio, 2004). We suggest further collaboration between HBCRs and sharing the experience, expertise, and protocols to enhance the quality of the HBCRs worldwide.
In conclusion, although HBCRs are important tools for monitoring and evaluating the quality of care, the design and performance of the HBCRs are very heterogeneous in different countries. Although diagnosis and treatment are important components of the cancer control programs, and all the countries suffer from the high cost of cancer treatment, there is no international coordination for the design and implementation of the standards of HBCRs. Results of this study highlighted the limitations of the HBCRs in the world and call for international programs to standardize the methods and publication of the HBCRs. It is important to support countries to set up this type of registries at least in the public hospitals and use appropriate methodology for quality control measures in these registries. Investment on HBCRs would improve quality of care and thereby would decrease the mortality and burden of cancer. In particular, it is important to launch HBCRs in the low and middle-income countries that are going to face an increasing trend in the incidence and mortality of cancer in the near future.
References
- Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American college of surgeon. Facility oncology registry data standards (FORDS): Revised for 2016. Commission on cancer. 2016 [Google Scholar]
- American college of surgeons. About the national cancer database [Online] 2017. Available: https://www.facs.org/quality-programs/cancer/ncdb/about [2017]
- Anazawa T, Miyata H, Gotoh M. Cancer registries in Japan: National Clinical Database and site-specific cancer registries. Int J Clin Oncol. 2015;20:5–10. doi: 10.1007/s10147-014-0757-4. [DOI] [PubMed] [Google Scholar]
- Aziz Z, Sana S, Saeed S, Akram M. Institution based tumor registry from Punjab: Five year data based analysis. JPMA. J Pak Med Assoc. 2003;53:350–3. [PubMed] [Google Scholar]
- Bhurgri Y. Karachi cancer registry data--implications for the national cancer control program of pakistan. Asian Pac J Cancer Prev. 2004;5:77–82. [PubMed] [Google Scholar]
- Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–90. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Parkin DM. Evaluation of data quality in the cancer registry: principles and methods. Part I: comparability, validity and timeliness. Eur J Cancer. 2009;45:747–55. doi: 10.1016/j.ejca.2008.11.032. [DOI] [PubMed] [Google Scholar]
- Bray F, Znaor A, Cueva P, et al. Planning and developing population-based cancer registration in low-and middle-income settings. Geneva: WHO Press; 2014. pp. 3–7. [PubMed] [Google Scholar]
- Claxton K, Martin S, Soares M, et al. Methods for the estimation of the national institute for health and care excellence cost-effectiveness threshold. Health Technol Assess. 2015;19:1–542. doi: 10.3310/hta19140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo LG, Sandra R, Rodríguez MN, et al. Evaluation of institutional cancer registries in Colombia. Rev Panam Salud Publica/Pan Am J Public Healt. 1999;6:202–6. doi: 10.1590/s1020-49891999000800008. [DOI] [PubMed] [Google Scholar]
- Ekanem I-OA, Island W. History of cancer resgistration in the world and in Nigeria. Abuja: Institute of human virology; 2009. Apr, pp. 7–10. [Google Scholar]
- Emilsson L, Lindahl B, Köster M, Lambe M, Ludvigsson JF. Review of 103 Swedish healthcare quality registries. J Int Med. 2015;277:94–136. doi: 10.1111/joim.12303. [DOI] [PubMed] [Google Scholar]
- Evelyn M, Shambaugh Mildred A, Weiss AF. U.S. department of health and human services-public health service-national institutes of health. NIH publication; 1999. Self-instructional manual for cancer registrars; pp. 16–21. [Google Scholar]
- Ferlay J, Bray F, Steliarova-Foucher E, Forman D. IARC cancer base. France: International agency for research on cancer 2014; 2014. Cancer incidence in five continents, CI5plus; pp. 4–6. [Google Scholar]
- Globocan. All cancers (excluding non-melanoma skin cancer) estimated incidence, mortality and prevalence world wide in 2012 [Online] 2012. Available: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. 2016] [DOI] [PubMed]
- Goldman DP, Schoenbaum M, Kilgore ML, et al. Using hospital tumor registries to identify research subjects. Health Serv Outcomes Res Methodol. 2006;2:67–76. [Google Scholar]
- Goss PE, Lee BL, Badovinac-Crnjevic T, et al. Planning cancer control in latin America and the Caribbean. Lancet Oncol. 2013;14:391–436. doi: 10.1016/S1470-2045(13)70048-2. [DOI] [PubMed] [Google Scholar]
- Health NMo. The SSWAHS clinical cancer registry [Online] 2014. Available: http://www.swslhd.nsw.gov.au/cancer/ccr.html. 2017]
- Heins MJ, de Jong JD, Spronk I, et al. Adherence to cancer treatment guidelines: influence of general and cancer-specific guideline characteristics. Eur J Pub Health. 2016;27:616–20. doi: 10.1093/eurpub/ckw234. [DOI] [PubMed] [Google Scholar]
- Hendren S, McKeown E, Morris AM, et al. Implementation of a hospital-based quality assessment program for rectal cancer. J Oncol Pract. 2014;10:e120–e9. doi: 10.1200/JOP.2014.001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi T, Nakamura F, Shibata A, Emori Y, Nishimoto H. The national database of hospital-based cancer registries: a nationwide infrastructure to support evidence-based cancer care and cancer control policy in Japan. Jpn J Clin Oncol. 2013;44:2–8. doi: 10.1093/jjco/hyt013. [DOI] [PubMed] [Google Scholar]
- Houser SH, Colquitt S, Clements K, Hart-Hester S. Impact of electronic health record usage on cancer registry systems in Alabama. Perspect Health Inf Manag. 2012;9:1–15. [PMC free article] [PubMed] [Google Scholar]
- (IARC) International Agency for Research on Cancer. IARC-Technical Report-Sources of information for the population-based cancer registry. International Agency for Research on Cancer (IARC); [Google Scholar]
- Institue FMR. Hospiatl based cancer registry 2013 [Online] Fortis memorial research institue; 2013. Available: https://www.fmri.in/hospital-cancer-registry-2013. 2017] [Google Scholar]
- Jedy-Agba EE, Curado MP, Oga E, et al. The role of hospital-based cancer registries in low and middle income countries – the Nigerian case study. Cancer Epidemiol. 2012;36:430–5. doi: 10.1016/j.canep.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel K. Development of cancer treatment guidelines. Alexandria Med J. 2011;47:11–4. [Google Scholar]
- Kim HJ, Cho JH, Lyu Y, et al. Construction and validation of hospital-based cancer registry using various health records to detect patients with newly diagnosed cancer: experience at Asan medical center. J Prev Med Public Health. 2010;43:257–64. doi: 10.3961/jpmph.2010.43.3.257. [DOI] [PubMed] [Google Scholar]
- Kutluk T, Mutlu HK, Kilickap S, et al. Increasing performance of a hospital-based cancer registry: Hacettepe University hospitals experience. J BUON. 2013;18:1088. [PubMed] [Google Scholar]
- Messenger JC, Ho KK, Young CH, et al. The national cardiovascular data registry (NCDR) data quality brief: the NCDR data quality program in 2012. J Am Coll Cardiol. 2012;60:1484–8. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- Mock V, Atkinson A, Barsevick A, et al. NCCN practice guidelines for cancer-related fatigue. Oncology (Williston Park, NY) 2000;14:151–61. [PubMed] [Google Scholar]
- Mohammadzadeh N, Safdari R, Rahimi A. Multi-agent systems: effective approach for cancer care information management. Asian Pac J Cancer Prev. 2013;14:7757–9. doi: 10.7314/apjcp.2013.14.12.7757. [DOI] [PubMed] [Google Scholar]
- (NPCR) NPoCR. Abstract Plus [Online]. Center of Disease Control and Prevention. 2016. Available: https://www.cdc.gov/cancer/npcr/tools/registryplus/ap_tech_info.htm. 2017]
- Organization WHO. Cancer control: knowledge into action: WHO guide for effective programmes. World Health Organization; 2007. [PubMed] [Google Scholar]
- Pezzi CM. Big data and clinical research in oncology: the good, the bad, the challenges, and the opportunities. Ann Surg Oncol. 2014;21:1506–7. doi: 10.1245/s10434-014-3519-7. [DOI] [PubMed] [Google Scholar]
- Pinheiro ZR, Gonçalves AA, Leitão AR. Integrated hospital-based cancer registry system, in international conference on information systems and technology management: São Paulo – Brasil. 2008:3459–69. [Google Scholar]
- Posada M, del Otero L, Villaverde A, et al. Data quality, validation and data source integration in rare disease registries. WP 7 deliverable EPIRARE project. 2014 [Google Scholar]
- Rouhollahi MR, Mohagheghi MA, Mohammadrezai N, et al. Situation analysis of the National Comprehensive Cancer Control Program in the IR of Iran;assessment and recommendations based on the IAEA imPACT mission. Arch Iran Med. 2014;17:222. [PubMed] [Google Scholar]
- Ruiz A, Facio Á. Hospital-based cancer registry: a tool for patient care, management and quality. A focus on its use for quality assessment. Clin Transl Oncol. 2004;6:104–13. [Google Scholar]
- Ryerson AB, Massetti GM. Peer reviewed: CDC's public health surveillance of cancer. Prev Chronic Dis. 2017:14. doi: 10.5888/pcd14.160480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEER. Hospital-based registries [Online] 2017. Available: https://training.seer.cancer.gov/registration/types/hospital.html. 2017]
- Sergeons ACo. National cancer database [Online] 1996-2017. Available: https://www.facs.org/quality%20programs/cancer/ncdb. 2017]
- Shiki N, Ohno Y, Fujii A, Murata T, Matsumura Y. Unified modeling language (UML) for hospital-based cancer registration processes. Asian Pac J Cancer Prev. 2008;9:789–96. [PubMed] [Google Scholar]
- Shin H-R, Jung K-W, Won Y-J, Park J-G. 2002 annual report of the Korea central cancer registry: based on registered data from 139 hospitals. Breast Cancer Res. 2004;36:103–14. doi: 10.4143/crt.2004.36.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibuea WH, Mangunkusumo RR, Akbar N, et al. Hospital based cancer registry in Cipto Mangunkusumo hospital Jakarta. Med J Indonesia. 2000;9:181. [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Smith RA, von Eschenbach AC, Wender R, et al. American cancer society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers: Also: update 2001-testing for early lung cancer detection. CA Cancer J Clin. 2001;51:38–75. doi: 10.3322/canjclin.51.1.38. [DOI] [PubMed] [Google Scholar]
- Somerfield MR, Einhaus K, Hagerty KL, et al. American society of clinical oncology clinical practice guidelines: opportunities and challenges. J Clin Oncol. 2008;26:4022–6. doi: 10.1200/JCO.2008.17.7139. [DOI] [PubMed] [Google Scholar]
- Stoffel EM, Mangu PB, Gruber SB, et al. Hereditary colorectal cancer syndromes: American society of clinical oncology clinical practice guideline endorsement of the familial risk–colorectal cancer: European society for medical oncology clinical practice guidelines. J Clin Oncol. 2014;33:209–17. doi: 10.1200/JCO.2014.58.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S. Consolidated report of hospital based cancer registries. Indian J Med Resvol. 2012-2014;1:7–13. and 145-50. [Google Scholar]
- Swan J, Wingo P, Clive R, et al. Cancer surveillance in the US. Cancer. 1998;83:1282–91. doi: 10.1002/(sici)1097-0142(19981001)83:7<1282::aid-cncr3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Tagliabue G, Maghini A, Fabiano S. Consistency and accuracy of diagnostic cancer codes generated by automated registration: comparison with manual registration. Popul Health Metr. 2006;4:10. doi: 10.1186/1478-7954-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry: experience in Finland. Acta Oncol. 1994;33:365–9. doi: 10.3109/02841869409098430. [DOI] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- University Khon Kaen. Hospital-based tumor registry;Srinagarind hospital, Khon Kaen university. Cancer unit, faculty of medicine, Khon Kaen University. 2013 [Google Scholar]
- Vineis P, Wild CP. Global cancer patterns: causes and prevention. Lancet. 2014;383:549–57. doi: 10.1016/S0140-6736(13)62224-2. [DOI] [PubMed] [Google Scholar]
- Young J. The hospital-based cancer registry. Cancer resgistration: Principles and methods. 1991;95:177–84. [PubMed] [Google Scholar]
- Zachary I, Boren SA, Simoes E, et al. Information management in cancer registries: Evaluating the needs for cancer data collection and cancer research. Online J Public Health Inform. 2015;7:1–10. doi: 10.5210/ojphi.v7i2.5664. [DOI] [PMC free article] [PubMed] [Google Scholar]


